Application of Immersive Virtual Reality for Assessment and Intervention in Psychosis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Sources and Search Terms
2.2. Eligibility and Inclusion Criteria
2.3. Data Extraction
2.4. Risk of Bias in Individual Articles
3. Results
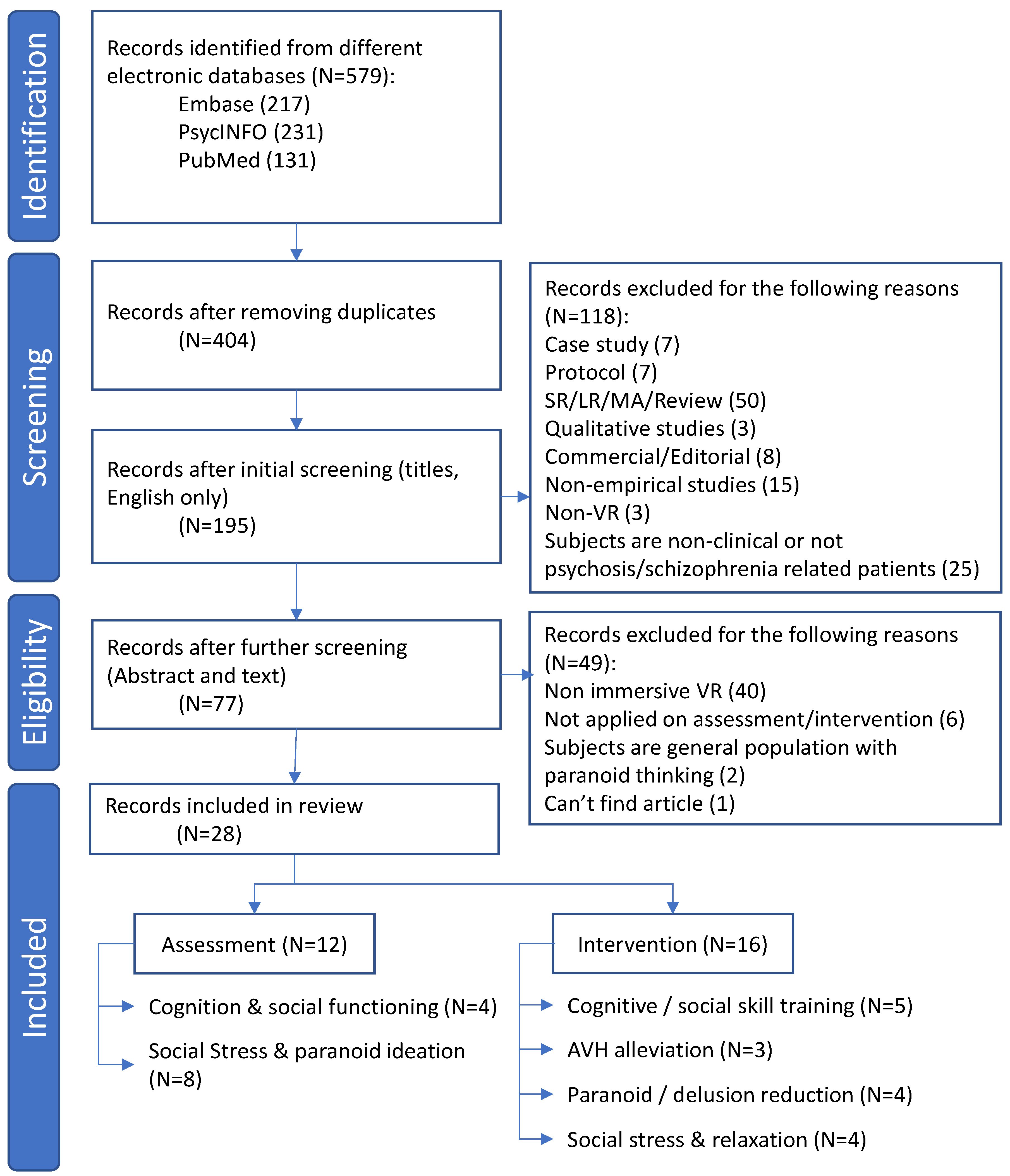
3.1. Search Results and Summary of the Studies
| Authors/Year/Country | Study Design | Study Population | VR Equipment | Interventions | Sessions | Baseline/Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|---|
| Assessment: cognition & social functioning | |||||||
| Dietrichkeit et al., 2020 [30] Germany | Pilot study | 39 PP (M 34.72, SD 8.68, male 62%) and 20 HC (M 30.55, SD 8.54, male 50%); N = 59 | HMD (Oculus Rift DK2) | Two VR scenes: a street and a metro station (social tasks)/a beach and a campground (object tasks). Half of the subjects provided feedback on the recollection task. | 4 (baseline assessment, one task each and a post-diagnostic assessment) | MINI, PANSS, BCIS, VR task performance (number of correct recollections) | Patients reported comparable performance on the object task, with only the social tasks showing lower performance. Feedback had little effect on patients’ cognitive insight. |
| Han et al., 2014 [31] Korea | Pilot study | 23 SZ (M 28.9, SD 3.4, male 43%) and 22 HC (M 27, SD 3.6, male 41%); N = 45 | HMD (eMagin Z800 3DVisor) with a head tracker and an eye tracker | Conversations with two avatars sitting at the same table with the participants in four scenarios, each of which contained both listening and speaking components. | 1 | RPM, TMT-B, PANSS, PQ (after the experiment) and VREQ (after the task) | Patients had active avoidance of eye contact during three-party conversations. Patients took longer pauses before speaking, while both subject groups demonstrated a longer time to begin speaking in unpleasant scenarios. Useful measurement tool for social behaviors. |
| Miskowiak et al., 2022 [32] Denmark | Validation study | 40 MD (M 35.3, SD 11.7, male 40%), 41 PSD (M 24.2, SD 3.9, male 58.5%), and 40 HC (M 30.4, SD 9.6, male 35%); N = 121 | Standalone HMD (Oculus Go 32 GB, LCD display of 5.5 inches, 1280 × 1440, 72hz refresh rate) & hand-held controller | CAVIR measures subjects’ verbal memory, processing speed, executive functioning, attention and working memory through five tasks performed in a simulated kitchen to make a meal. | 1 (self-administrated with a 15 min. duration) | OTS, SWM, RVP, RAVLT, WAIS-III, RBANS Coding, DS, VFT, TMT-A&B, DART, FAST, UPSA-B, PQ, VRSSQ, CAVIR, HDRS-17 + YMRS for MD/HC; SAPS + SANS for PSD | CAVIR is a sensitive and valid tool for assessing cognitive impairments in MD and PSD that occur in daily life. The results of the CAVIR and the neuropsychological tests showed strong correlations. CAVIR is also cost-effective due to its self-administration and usage of inexpensive equipment (Oculus Go; priced at $200). |
| Souto et al., 2013 [33] Portugal | Pilot study | 12 SZ (M 36.25, SD 5.754, male 75%) and 12 HC (M 36.25, SD 5.754, male 75%); N = 24 | A screen (4 × 3 m), 2 multimedia projectors with passive polarized glasses, and a surround-sound system | RV-REF (3D avatars and virtual reality environment) with a task to identify the six fundamental facial expressions, including sadness, happiness, fear, anger, disgust, and surprise. | 1 (25 min, including putting in and taking out electrodes) | MMSE, GAF, PSP, RV-REF (correct and error data), EEG (F3 and F4) | While SZ patients showed lower scores, the difference was not statistically significant. Happiness and anger received higher accuracy, and major difficulties came from fear and disgust recognition for both groups. The anger and disgust stimuli caused alterations in patients’ alpha frontal activity. |
| Assessment: paranoid ideation | |||||||
| Counotte et al., 2017 [21] Netherlands | Cross-sectional, between-subjects | 44 ROP (M27, male 79.5%), 17 UHR (M22, male 41.2%), 37 SPP (M27, male 59.5%), and 45 HC (M 24, male 46.7%); N = 170 (143 HR available) | Vizard software (CleVR), Logitech F310 Gamepad, Sony HMD headset model HMZ-T1, 1280 × 720, 51.6 FOV, and a 3DOF tracker | Virtual bar with 3 stressors (avatar density/ethnic density/hostility): (1) No stress (6 avatars, >80% Dutch, neutral expression); (2) 40 avatars; (3) 40 majority non-self ethnicity; (4) 40 angry/hostile expression; (5) 3 stressors. | 1 (exposed to VR-induced social stress in 5 settings lasting 4 min each) | Before and after/during VR task: SSQ, HR, SCL | Heart rate, HF, LF/HF, and skin conductance level were all significantly impacted by the quantity of virtual social stressors in all groups. Instead of higher autonomic responsiveness to social stresses, psychosis liability was associated with lower parasympathetic activity in virtual social contexts, which implies generally high levels of arousal. |
| Geraets et al., 2018 [22] Netherlands | Cross-sectional, between-subjects | 50 ROP (M26, SD 4.6, male 80%), 19 UHR (M24.3, SD 4.4, male 36.8%), 40 SPP (M26.5, SD 4.8, male 55%), and 47 HC (M 24.3, SD 4.3, male 46.8%); N = 170 (156 completed data) | Vizard software (CleVR), Logitech F310 Gamepad, Sony HMD headset model HMZ-T1, 1280 × 720, 51.6 FOV, and a 3DOF tracker | Five virtual café visits with various social stressors (crowdedness, ethnicity, and hostility) | 1 (each VR visit lasted 4 min with a 5 min break between experimental blocks) | Baseline: CAARMS, SUD, SOFAS, SERS. During VR tasks: IPD by the VR software. Outcome: SSPS | When social stresses were given in the VR settings, interpersonal distance (IPD) widened. IPD regulation between the groups did not differ. IPD may be impacted by more general states such as psychological stress, which are common but not unique to psychosis. |
| Hesse et al., 2017 [34] Germany | Randomized, controlled cross-over | 26 PP (M34.52, SD 8.7, male 71%) and 20 HC (M 32.3, SD 8.5, male 65%); N = 46 (including 5 drop-outs from PP) | HMD and Gamepad were used without further details | An open-plan office was simulated in VR. Participants were asked to complete two tasks with two types of social feedback—co-operative or rejective—from 5 avatar colleagues. | 3 (one for clinical interview, trait, and questionnaire and two for VR tasks) weekly | PANSS-DS, PSYRATS-DS, CDSS, SSPS, IPQ, GAF. Neurocognition: VLMT (learning/recall), TMT-A/B, MWT-B | PP and HC started with large baseline differences. Statistical trends indicated that social rejection increased paranoid ideations for PP. Additionally, PP displayed a greater sense of presence than HC. High convergent validity was demonstrated between state assessments of paranoid ideations and traditional delusion measures. |
| Jongeneel et al., 2018 [23] Netherlands | Cross-sectional, between-subjects | 94 HC + SPP (M 25.4, SD 4.6, male 51%) and 75 UHR + ROP (M 25.4, SD 4.7, male 65.3%); N = 169 | CleVR software; Logitech Chillstream Gamepad, HMD (Emagin Z800 3D Visor) featuring SVGA 800 × 600 24 bit, 40 FOV, and a 3DOF tracker | Five virtual café visits with various social stressors (crowdedness, ethnicity, and hostility) | 1 (five experiments of 4 min each) | CAARMS, SOFAS for UHR, SERS, SSPS, SUD | Negative self-esteem, rather than positive self-esteem, was linked to the subjects’ momentary paranoia, peak subjective distress, or reactivity to social stressors. All subject groups reported experiencing more paranoia as the quantity of social stressors rose. Negative self-esteem had a greater effect on stress reactivity in those with lower psychosis liability than in those with higher liability. |
| Pot-Kolder et al., 2017 [24] Netherlands | Cross-sectional, between-subjects | 55 ROP (M 26, SD 5, male 76.4%), 20 UHR (M 24 SD 4 Male 35%), 42 SPP (M 26, SD 5, male 54.8%), and 53 HC (M 25, SD 4, male 47.2%); N = 170 | Same as Jongeneel et al., 2018 [21] | CAARMS, SOFAS for UHR, CASH, SCAN, DACOBS, DOG, GPTS. Outcome: SSPS | Higher intensity of (1) cognitive biases attention to threat, (2) belief inflexibility, and (3) external attribution was linked to higher psychosis liability. The number of cognitive biases present was correlated with an increase in paranoid response. Further, attention to threat bias and external attribution bias both strengthened the impact of social environmental stressors on paranoid ideation. | ||
| Veling et al., 2014 [20] Netherlands | Pilot study | 17 FEP (M 27.3, SD 5.5, male 82.4%) and 24 HC (M 29, SD 9.2, male 83.3%); N = 41 | CleVR software; Logitech Chillstream Gamepad, HMD (Emagin Z800 3D Visor) SVGA 800 × 600 24 bit, 40 FOV, and built-in 3DOF tracker | In a VR café, subjects were asked to locate the numbered avatars & memorize the number under four scenarios: 2 ethnic appearances (own or other) × 2 population density (low/high) of avatars. | 1 (3.5 min for HC and 4 min for FEP for each task × 4) | Baseline: SIAS, GPTS, DACOBS, SSQ, SERS. During VR: GSR, HR, SSPS. Outcome: SSQ, IPQ | In comparison to HC, FEP showed considerably more paranoid thoughts, cognitive biases, more subjective distress, and poorer self-esteem. For both groups, there was a significant correlation between paranoid thoughts regarding avatars in VR and paranoia in the real world. Galvanic skin reaction was noticeably stronger in VR with avatars of other ethnicities in FEP but not in HC. |
| Veling et al., 2016a [25] Netherlands | Cross-sectional, between-subjects | 75 ROP + UHR (M 25.4, male 65.3%) and 95 SPP + HC (M 25.4, male 50.5%); N = 170 | CleVR software, Logitech F310 Gamepad, HMD (Sony HMZ-T1) featuring 1280 × 720, 51.6 FOV, and a 3DOF tracker | Same as Jongeneel et al., 2018 [21] | SIAS, GPTS, CTQ-SF, CAPE, SUD, SSPS | When the total number of virtual environmental stressors rose, the effects of childhood trauma on paranoia and subjective distress were noticeably larger across all subjects. The impact of childhood trauma on peak subjective distress and stress reactivity during experiments was amplified by higher psychosis liability. | |
| Veling et al., 2016b [13] Netherlands | Cross-sectional, between-subjects | 55 ROP (M 26, SD 4.7, male 76.4%), 20 UHR (M 24, SD 4.5, male 35%), 42 SPP (M 26.4, SD 4.8, male 54.8%), and 53 HC (M 24.6, SD 4.4, male 47.2%); N = 170 | Same as Veling et al., 2016a [23] | CAPE, GPTS, SSPS, SIAS, SUD | Along with increasing levels of social stress in the environment, paranoia and subjective distress rose. Population density had a significant positive impact on both paranoia and distress. Subject distress was not significantly correlated with hostility, but paranoia was. There was no correlation between paranoia and distress and ethnic density. The degree of paranoia and distress in response to social stress was positively impacted by psychosis liability and pre-existing symptoms. | ||
| Intervention: cognitive/social skill training | |||||||
| La Paglia et al., 2013 [35] Italy | Non-randomized CT, pilot study | 6 VR-CRT (M 31, SD 14.6) and 6 IPT (M 35, SD 9.9); N = 12 SZ | Neuro-VR vers. 2.0 software, no details on HMD | Virtual reality environment for daily life tasks; the VR attention training program involved hierarchical task sequences arranged in 4 different VR environments (park, valley, beach, and supermarket) | 10 (VR-CRT weekly individual sessions with each lasting ~90 min. IPT was in-group on a weekly basis, 60 min) | Pre–post measures: MMSE, FAB, TMT, ToL, WCST, and SCWT | Significant improvement in the assessed cognitive dimensions by VR training. VR training and IPT both led to better results on activities requiring divided attention. VR training was linked to fewer cognitive deficiencies and better planning. |
| La Paglia et al., 2016 [36] Italy | Non-randomized CT, pilot study | 9 VR-CRT (M 29, SD 12.05, male 66.6%) and 6 IPT (M 35, SD 9.9, male 100%); N = 15 SZ | Neuro-VR vers. 2.0 software, no details on HMD | Same as La Pagilia et al., 2013 [33] | Pre–post measures: MMSE, ToL, TMT (A/B), FAB, WCST | VR training and IPT both led to better performance on sustained attention tasks. VT training was also linked to gains in divided attention and planning. | |
| Rus-Calafell et al., 2013 [26] Spain | Single arm, uncontrolled pilot study | 12 SZ + SZA (M 36.5, SD 6.01, male 58.3%); N = 12 | A laptop, 3D glasses, and headphones. | Soskitrain: patients practiced social interactions with virtual avatars in everyday settings (e.g. a supermarket or bar) and experienced progressive learning in the social skills repertoire. | 16 (twice weekly over eight weeks, individual basis with each session lasting ~60 min) | Baseline: SCIP, WMT, VLT-I/II, VFT, PST, CPT II. Outcome: SUS | Post-treatment results showed strong presence sense and good verisimilability of the virtual environment. Acceptability scores were similarly high. Between deficiencies in verbal learning and processing speed and sensation of presence, a substantial negative association was discovered. |
| Rus-Calafell et al., 2014 [27] Spain | Single arm, uncontrolled pilot study with 4- month follow-up | 12 SZ + SZA (M 36.5, SD 6.01, male 58.3%); N = 12 (3 dropouts) | Same as Rus-Calafell et al. 2013 [24] | PANSS, AI, SSIT, SADS, SFS, qualitative and VR acceptance assessment | Positive symptoms, psychopathology, social anxiety and discomfort, avoidance, and social skill mastery significantly improved in the participants. The patient’s ability to apply new abilities to daily tasks was aided by the VR training. FU: 4 months; all gains maintained | ||
| Vass et al., 2021 [37] Hungary | Randomized pilot study with 3-month follow-up | 9 VR-ToMIS (M 38.6, SD 13.49, male 55.5%) and 8 VR (M 48.8, SD 8.87, male 37.5%); N = 21 SZ (4 dropouts) | Samsung’s Gear VR, a Samsung S7 smartphone, and a Samsung Simple Controller. Environment (vTime) and avatar by Temporal Disc Controller (TDC) | VR-ToMIS: Each session had 3 consecutive steps: (1) simulated social interactions with an avatar for a conversation, (2) a task to visualize the inferred emotions of the avatar, and (3) discussion with a therapist | 9 (weekly individual sessions of 50 min; 8 VR sessions and one preliminary pre-briefing session) | PANSS, RBANS, WCST-64, faux pas test and cartoon stories task, BCMET, the Hungarian metaphor & irony test, LQoLP, SSQ | Positive symptoms, one neurocognitive domain (immediate memory), ToM, and pragmatic language abilities all improved as a result of the VR training, but there was no appreciable increase in quality of life. Modest to significant therapeutic effects were linked to significant alterations in the VR-ToMIS group. FU: 3-month (no data was shown) |
| Intervention: AVH alleviation | |||||||
| Dellazizzo et al., 2020 [38] Canada | Single-arm study with 3-month follow-up | 10 SZ/SZA with AVH (M 43.4, SD 14.6, male 80%); N = 10 | Samsung GearVR HMD set; avatar face (Morph3D character system) and voice (Roland AIRA VT-3), BehaVR software with Unity game engine | CBT for AVH included goal-setting and learning about AVH, diverse attributional mechanisms, mindfulness exercises, use of alternative explanation and relapse prevention, etc. VRT included one session for creating an avatar and eight sessions for therapy. | 18 by combining CBT + VRT (9 individual, weekly, 1 h sessions with either CBT for AVH or VRT) | BAVQ-R, PSYRATS-AH, PANSS, BDI-II, Q-LES-Q-SF | Auditory verbal hallucinations, depressive symptoms, schizophrenia symptoms (both negative and positive), beliefs about voices, and QoL all showed significant improvement. Effects of CBT + VRT on symptoms of schizophrenia and depression were greater than those demonstrated by either intervention alone. FU: 3-month FU on CBT (T3) and 3-month FU on VRT (T5); most gains were maintained or further improved at T5, but beliefs about voices at T5 retreated closer to baseline level. |
| Dellazizzo et al., 2021 [39] Canada | Randomized parallel comparative pilot trial with follow-ups at 3, 6, and 12 months | 37 VRT (M 43.6, SD 12, male 78.4%) and 37 CBT (M 41.4, SD 13.4, male 73%); N = 74 (11 dropouts) | Same as Dellazizzo et al., 2020 [36] | 9 (individual weekly 1 h sessions with either CBT for AVH and VRT) | PSYRATS, BAVQ-R; secondary outcomes: BDI-II, PANSS, Q-LES-Q-SF, semi-structured interview | Both interventions resulted in substantial reductions in the severity of AVH and depressive symptoms. While VRT did not statistically significantly outperform CBT for AVH, it did have a greater impact on AVH overall and on affective symptoms. A significant impact from VRT was also seen in QoL and persecutory beliefs. FU: 3, 6, and 12 months; effects maintained for up to 1 year | |
| du Sert et al., 2018 [40] Spain | Randomized, partial cross-over pilot trial, with a follow-up of 3 months | 7 VRT and 7 TAU (TAU received a delayed 7 weeks of VRT) (M 42.9, SD 12.4, male 66.7%); N = 19 SZ with AVH (4 dropouts) | Samsung GearVR HMD set, avatar face (Morph3D character system) and voice (Roland AIRA VT-3), BehaVR software | VRT consisted of one avatar-creation session and eight therapeutic sessions with each containing pre-immersion, immersion, and post-immersion debriefing. | 7 (weekly sessions including one avatar-creation session and six sessions of 45 min therapy). | PSYRATS, BAVQ-R, PANSS, BDI-II, QLESQ-SF, fear and anxiety scale (after each VRT) | Substantial improvements in AVH, depressive symptoms, and QoL were achieved with VRT. VRT had notably strong therapeutic benefits on the distress brought on by the voices. Participants gave high credibility to their avatars, feeling as though their persecutors were present there. FU: 3 months; improvements remained significantly |
| Intervention: paranoia/delusion reduction | |||||||
| Freeman et al., 2016 [41] UK | RCT | 15 VR with cognitive therapy (M 42.1, SD 13.4, male 67%) and 15 VR (M 40.6, SD14.4, male 40%); N= 30 PPD | nVisor SX111 HDM 1280 × 1024, 60 hz refresh rate, a computer and tracking system (Intersense) | Virtual reality cognitive therapy; patients were exposed to two VR places (train and lift), gradually building up with more avatars each time. Total of 7 VR scenarios, 5 min each. | 1 (~60–90 min in VR lab) | PANSS, PSYRATS-DS, SBQ-PB, BAI, BDI, VAS | Large reductions in delusional conviction (a 22% reduction) were seen in the VR experimental group, and they also showed a 19.6% reduction of distress in the real world. VR-based cognitive therapy proved to be efficient in reducing delusions. |
| Geraets et al., 2020 [8] Netherlands | RCT with 6-month follow-up | 43 VR-CBT (M 38, SD 10, male 67.4%) and 48 TAU (M 40.9, SD 10, male 70.8%); N = 91 PP | Logitech F310 Gamepad, Vizard software, Sony HMZ-T1/T2/T3 HMD, 1280 × 720, 51·6 FOV, and a 3DOF tracker. | VR-CBT; the first two sessions presented the VR system and set personal goals. Remaining sessions consisted of 40 min practicing time in VR (a street, bus, café, and supermarket) and 20 min to plan and reflect on exercises. | 16 (1 h one-on-one therapy sessions) | ESM by PsyMate | Subjects who participated in VR-CBT, compared to those in TAU, saw greater improvement in paranoia level (feeling hurt and disliked) and negative affect (insecure and down), but not in positive affect. Treatment had no effect on the way that emotional states and paranoia interacted. FU: 6 months; the change in paranoia and negative affect were maintained or further enhanced at FU |
| Pot-Kolder et al., 2018 [29] Netherlands | Single blind RCT with 6-month follow-up | 58 VR-CBT group (M 36.5, SD 10, male 69%) and 58 TAU (M 39.5, SD 10, male 72%); N = 116 PPD (13 dropouts) | Logitech F310 Gamepad, Vizard software, Sony HMZ-T1/T2/T3 HMD, 1280 × 720, 51·6 FOV, and a 3DOF tracker | VR-CBT; to match the patient’s paranoid fears, therapists could change the avatars’ attributes (including sex and ethnicity), as well as their number (0–40) and how they responded to the patient | 16 (one-hour individual therapy sessions). | Primary: time spent with others, ESM by PsyMate. Secondary: SBQ-PD, GPTS, SIAC, BDI, SOFAS, MANSA, DACOBS, ISMI, BCSS, BARS, IPQ, SSQ | Momentary anxiety and paranoid ideation were dramatically decreased in the VR-CBT group. However, the time spent with others (i.e., social participation) did not considerably increase after VR-CBT. Social cognition issue and a decrease in safety behavior were found to be the mediators of a shift in paranoid ideation. FU: 6 months; improvement maintained with further gains in social functioning and self-stigmatization |
| Pot-Kolder et al., 2020 [28] Netherlands | Single blind RCT with 6-month follow-up | 58 VR-CBT (M 36.5, SD 10, male 69%) and 58 TAU (M 39.5, SD 10, male 72%); N = 116 PPD (13 dropouts) | Same as Pot-Kolder et al. 2018 [27] | Outcome measure: QALY (by GPTS) and social participation. Cost measures: (1) direct medical cost + VR costs; (2) direct travel costs; (3) indirect cost from productivity loss. | Between-group comparison showed statistically significant differences on all measures except monetary anxiety and indirect cost of productivity. The ICER for VR-CBT treatment was €48,868 per QALY gained compared to the €80,000 willingness to pay. Offering VR-CBT to patients with paranoid delusions is a financially viable strategy for cost-effectively enhancing patients’ health. FU: 6 months; there were 68 hospitalization days for TAU vs no psychiatric admission for VR-CBT | ||
| Intervention: social stress & relaxation | |||||||
| Freeman et al., 2022 [42] UK | Parallel-group, single-blind RCT with 6-month follow-up | 174 gameChange (M 36.6, SD 12.8, male 67%) and 172 TAU (M 37.8, SD 12.2, male 67%); N = 346 SSD with agoraphobic (9 dropouts) | Standalone VR headset | gameChange: designed to specifically target everyday situational anxiety. Six situations, including leaving the house, being in a café, shop, doctor’s office, or pub, as well as boarding a bus, in which patients practice various tasks. Each scenario had five levels of difficulty. | 6 (over 6 weeks, each including 30 min in VR) | Primary: O-AS, O-BAT. Secondary: AMI-AS, C-SSRS, GPTS, PWQ, PHQ-9, EQ-5D, ReQoL, O-CDQ, BHS, BESAA | Agoraphobic avoidance and distress in everyday situations significantly decreased in the gameChange VR therapy group. Most secondary outcomes showed no significant changes, and the overall treatment effect sizes were small. However, the benefits of treatment increased with the degree of anxious fears and avoidance. FU: at 6-month FU, moderate-to-large improvements in agoraphobic avoidance were maintained |
| Rault et al., 2022 [43] France | Single-arm pilot study | 13 SZ/SZA (M 43, SD 11.5, male 77%); N = 13 | HMD (Oculus Rift) with a 360° view | Relaxation therapy used “C2 Hypno” category, which included 4 pre-selected landscapes in VR | 5 (30 min duration for each session with 10 min of VR exposure) | PANSS, ITQ, CDS, SSQ, VASA, CAS | Anxiolytic effects were demonstrated in the COVI and VAS scales. The VR relaxation therapy was not aimed to improve psychotic symptoms, but the PANSS tended to improve. |
| Tan et al., 2021 [12] Singapore | Single-blinded pilot RCT | 19 VR relaxation (male 26.3%) and 20 WL (male 52.4%); N = 40 PP/BD/SZ | HMD (iTVGoggles Wide View 3D+), videos developed by Klainin-Yobas et al. (2015) | V-DESSERTS; comprised psychoeducation (20 min) and virtual screen-based relaxation practice (20 min). WL were brought into the same quiet room for 40 min to read health pamphlets | Twice-daily individual-based sessions | PSS, NSRS, HR, BP and ST, PRS, KSMMQ | The intervention group reported significantly increased levels of perceived relaxation and knowledge, but results on subjective and objective stress were inconclusive. |
| Veling et al., 2021 [44] Netherlands | Crossover RCT | 25 VRelax and 25 standard relaxation; then crossover to other group after 10 days (M 41.6, SD 14.2, male 34%); N = 50 BD/PP (1 dropout) | Samsung Gear VR set with the 360° videos developed by The Dolphin Swim Club, VIEMR, and Atmospheres | VRelax used immersive 360° videos of nature with some added interactive components. Subjectss were free to choose the video within the app at home. Standard relaxation subjects used audio tracks of progressive relaxation exercises and guided meditation | 10 (at least once daily for 10 min during a 10-day period) | Primary: VAS. Secondary: PSS, IDS-SR, BAI, GPTS, SSQ | Based on the improvements in all VAS categories, both groups reported a statistically significant decrease in negative affective states and an increase in positive affective states. Compared to relaxation exercise, VRelax had a greater positive impact on negative affective states, especially when it came to feeling down, cheerful, or anxious. Ten-day measurements of psychiatric symptoms showed some improvement in both conditions. |
3.2. Sample Size, Participants and Study Design
3.3. VR Equipment and System Used
3.4. VR Environments and Tasks
3.5. Duration and Structure of the VR-Based Assessment or Intervention
3.6. Measurements
3.7. Follow-Up and Dropout of Intervention Studies
3.8. Validity of Using VR as an Assessment Tool
3.8.1. Cognitive and Social Functioning
3.8.2. Social Stress and Paranoid Ideation
3.9. Effectiveness of Using VR as an Intervention
3.9.1. Cognitive and Social Skill Training
3.9.2. AVH Alleviation
3.9.3. Paranoia/Delusion Reduction
3.9.4. Social Stress and Relaxation
3.10. Feasibility or Acceptance of the Use of VR
4. Discussion
4.1. Implications for VR-Based Assessments for Psychosis
4.2. Implications for VR-Based Interventions in Psychosis
4.3. Limitations
4.4. Future Direction and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 1996, 153, 321–330. [Google Scholar] [CrossRef]
- Perlick, D.A.; Rosenheck, R.A.; Kaczynski, R.; Bingham, S.; Collins, J. Association of symptomatology and cognitive deficits to functional capacity in schizophrenia. Schizophr. Res. 2008, 99, 192–199. [Google Scholar] [CrossRef]
- Arciniegas, D.B. Psychosis. Contin. Minneap Minn 2015, 21, 715–736. [Google Scholar] [CrossRef]
- Schroeder, A.H.; Bogie, B.J.M.; Rahman, T.T.; Thérond, A.; Matheson, H.; Guimond, S. Feasibility and Efficacy of Virtual Reality Interventions to Improve Psychosocial Functioning in Psychosis: Systematic Review. JMIR Ment. Health 2022, 9, e28502. [Google Scholar] [CrossRef]
- Thompson, A.; Elahi, F.; Realpe, A.; Birchwood, M.; Taylor, D.; Vlaev, I.; Leahy, F.; Bucci, S. A Feasibility and Acceptability Trial of Social Cognitive Therapy in Early Psychosis Delivered through a Virtual World: The VEEP Study. Front. Psychiatry 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Calafell, M.D.M.R.; Garety, P.; Sason, E.; Craig, T.J.K.; Valmaggia, L.R. Virtual reality in the assessment and treatment of psychosis: A systematic review of its utility, acceptability and effectiveness. Psychol. Med. 2018, 48, 362–391. [Google Scholar] [CrossRef]
- Martens, M.A.; Antley, A.; Freeman, D.; Slater, M.; Harrison, P.J.; Tunbridge, E.M. It feels real: Physiological responses to a stressful virtual reality environment and its impact on working memory. J. Psychopharmacol. 2019, 33, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Geraets, C.N.; Snippe, E.; van Beilen, M.; Pot-Kolder, R.M.; Wichers, M.; van der Gaag, M.; Veling, W. Virtual reality based cognitive behavioral therapy for paranoia: Effects on mental states and the dynamics among them. Schizophr. Res. 2020, 222, 227–234. [Google Scholar] [CrossRef]
- Veling, W.; Moritz, S.; van der Gaag, M. Brave New Worlds—Review and Update on Virtual Reality Assessment and Treatment in Psychosis. Schizophr. Bull. 2014, 40, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-M.; Ku, J.; Choi, S.-H.; Jang, H.-J.; Park, J.-Y.; Kim, S.I.; Kim, J.-J. A virtual reality application in role-plays of social skills training for schizophrenia: A randomized, controlled trial. Psychiatry Res. 2011, 189, 166–172. [Google Scholar] [CrossRef]
- O’Hanlon, P.; Aref-Adib, G.; Fonseca, A.; Lloyd-Evans, B.; Osborn, D.; Johnson, S. Tomorrow’s World: Current Developments in the Therapeutic Use of Technology for Psychosis. BJPsych Adv. 2016, 22, 301–310. [Google Scholar] [CrossRef]
- Tan, H.L.E.; Chng, C.M.L.; Lau, Y.; Klainin-Yobas, P. Investigating the Effects of a Virtual Reality-based Stress Management Programme on Inpatients with Mental Disorders: A Pilot Randomised Controlled Trial. Int. J. Psychol. 2021, 56, 444–453. [Google Scholar] [CrossRef]
- Veling, W.; Pot-Kolder, R.; Counotte, J.; van Os, J.; van der Gaag, M. Environmental Social Stress, Paranoia and Psychosis Liability: A Virtual Reality Study. Schizophr. Bull. 2016, 42, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Makransky, G.; Lilleholt, L. A structural equation modeling investigation of the emotional value of immersive virtual reality in education. Educ. Technol. Res. Dev. 2018, 66, 1141–1164. [Google Scholar] [CrossRef]
- Valmaggia, L.R.; Latif, L.; Kempton, M.J.; Rus-Calafell, M. Virtual reality in the psychological treatment for mental health problems: An systematic review of recent evidence. Psychiatry Res. 2016, 236, 189–195. [Google Scholar] [CrossRef]
- Riches, S.; Pisani, S.; Bird, L.; Rus-Calafell, M.; Garety, P.; Valmaggia, L. Virtual reality-based assessment and treatment of social functioning impairments in psychosis: A systematic review. Int. Rev. Psychiatry 2021, 33, 337–362. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Veling, W.; Brinkman, W.-P.; Dorrestijn, E.; Van Der Gaag, M. Virtual Reality Experiments Linking Social Environment and Psychosis: A Pilot Study. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 191–195. [Google Scholar] [CrossRef]
- Counotte, J.; Pot-Kolder, R.; van Roon, A.M.; Hoskam, O.; van der Gaag, M.; Veling, W. High psychosis liability is associated with altered autonomic balance during exposure to Virtual Reality social stressors. Schizophr. Res. 2017, 184, 14–20. [Google Scholar] [CrossRef]
- Geraets, C.N.W.; van Beilen, M.; Pot-Kolder, R.; Counotte, J.; van der Gaag, M.; Veling, W. Social Environments and Inter-personal Distance Regulation in Psychosis: A Virtual Reality Study. Schizophr. Res. 2018, 192, 96–101. [Google Scholar] [CrossRef]
- Jongeneel, A.; Pot-Kolder, R.; Counotte, J.; van der Gaag, M.; Veling, W. Self-esteem moderates affective and psychotic responses to social stress in psychosis: A virtual reality study. Schizophr. Res. 2018, 202, 80–85. [Google Scholar] [CrossRef]
- Pot-Kolder, R.; Veling, W.; Counotte, J.; van der Gaag, M. Self-reported Cognitive Biases Moderate the Associations between Social Stress and Paranoid Ideation in a Virtual Reality Experimental Study. Schizophr. Bull. 2017, 44, 749–756. [Google Scholar] [CrossRef]
- Veling, W.; Counotte, J.; Pot-Kolder, R.; van Os, J.; van der Gaag, M. Childhood trauma, psychosis liability and social stress reactivity: A virtual reality study. Psychol. Med. 2016, 46, 3339–3348. [Google Scholar] [CrossRef]
- Rus-Calafell, M.; Gutiérrez-Maldonado, J.; Ribas-Sabaté, J. Neurocognition, Presence and Acceptance of a VR Programme for Psychotic Patients: A Correlational Study. Stud. Health Technol. Inform. 2013, 191, 141–145. [Google Scholar] [CrossRef]
- Rus-Calafell, M.; Gutiérrez-Maldonado, J.; Ribas-Sabaté, J. A virtual reality-integrated program for improving social skills in patients with schizophrenia: A pilot study. J. Behav. Ther. Exp. Psychiatry 2014, 45, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Pot-Kolder, R.; Veling, W.; Geraets, C.; Lokkerbol, J.; Smit, F.; Jongeneel, A.; Ising, H.; van der Gaag, M. Cost-Effectiveness of Virtual Reality Cognitive Behavioral Therapy for Psychosis: Health-Economic Evaluation within a Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e17098. [Google Scholar] [CrossRef] [PubMed]
- Pot-Kolder, R.M.C.; Geraets, C.N.W.; Veling, W.; van Beilen, M.; Staring, A.B.P.; Gijsman, H.J.; Delespaul, P.A.E.G.; van der Gaag, M. Virtual-reality-based cognitive behavioural therapy versus waiting list control for paranoid ideation and social avoidance in patients with psychotic disorders: A single-blind randomised controlled trial. Lancet Psychiatry 2018, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Dietrichkeit, M.; Grzella, K.; Nagel, M.; Moritz, S. Using Virtual Reality to Explore Differences in Memory Biases and Cognitive Insight in People with Psychosis and Healthy Controls. Psychiatry Res. 2020, 285, 112787. [Google Scholar] [CrossRef]
- Han, K.; Shin, J.; Yoon, S.Y.; Jang, D.-P.; Kim, J.-J. Deficient gaze pattern during virtual multiparty conversation in patients with schizophrenia. Comput. Biol. Med. 2014, 49, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Jespersen, A.E.; Kessing, L.V.; Aggestrup, A.S.; Glenthøj, L.B.; Nordentoft, M.; Ott, C.V.; Lumbye, A. Cognition Assessment in Virtual Reality: Validity and feasibility of a novel virtual reality test for real-life cognitive functions in mood disorders and psychosis spectrum disorders. J. Psychiatr. Res. 2021, 145, 182–189. [Google Scholar] [CrossRef]
- Souto, T.; Baptista, A.; Tavares, D.; Queirós, C.; António, M. Facial emotional recognition in schizophrenia: Preliminary results of the virtual reality program for facial emotional recognition. Arch. Clin. Psychiatry 2013, 40, 129–134. [Google Scholar] [CrossRef]
- Hesse, K.; Schroeder, P.A.; Scheeff, J.; Klingberg, S.; Plewnia, C. Experimental variation of social stress in virtual reality—Feasibility and first results in patients with psychotic disorders. J. Behav. Ther. Exp. Psychiatry 2017, 56, 129–136. [Google Scholar] [CrossRef] [PubMed]
- La Paglia, F.; Serino, S.; Gaggioli, A.; Albani, G.; Riva, G. Cognitive Rehabilitation of Schizophrenia through NeuroVr Training. In Annual Review of Cybertherapy and Telemedicine; Wiederhold, B.K., Riva, G., Eds.; Ebook; IOS Press: Amsterdam, The Netherlands, 2013; Volume 191, pp. 158–162. [Google Scholar]
- La Paglia, F.; La Cascia, C.; Rizzo, R.; Sanna, M.; Cangialosi, F.; Sideli, L.; Francomano, A.; Riva, G.; La Barbera, D. Virtual Reality Environments to Rehabilitation Attention Deficits in Schizophrenic Patients. Annu. Rev. Cyber Ther. Telemed. 2016, 14, 143–148. [Google Scholar]
- Vass, E.; Simon, V.; Fekete, Z.; Lencse, L.; Ecseri, M.; Kis, B.; Simon, L. A Novel Virtual Reality-based Theory of Mind Intervention for Outpatients with Schizophrenia: A Proof-of-concept Pilot Study. Clin. Psychol. Psychother. 2021, 28, 727–738. [Google Scholar] [CrossRef]
- Dellazizzo, L.; Potvin, S.; Phraxayavong, K.; Dumais, A. Exploring the Benefits of Virtual Reality-Assisted Therapy Following Cognitive-Behavioral Therapy for Auditory Hallucinations in Patients with Treatment-Resistant Schizophrenia: A Proof of Concept. J. Clin. Med. 2020, 9, 3169. [Google Scholar] [CrossRef] [PubMed]
- Dellazizzo, L.; Potvin, S.; Phraxayavong, K.; Dumais, A. One-year randomized trial comparing virtual reality-assisted therapy to cognitive–behavioral therapy for patients with treatment-resistant schizophrenia. Schizophrenia 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- du Sert, O.P.; Potvin, S.; Lipp, O.; Dellazizzo, L.; Laurelli, M.; Breton, R.; Lalonde, P.; Phraxayavong, K.; O’Connor, K.; Pelletier, J.-F.; et al. Virtual reality therapy for refractory auditory verbal hallucinations in schizophrenia: A pilot clinical trial. Schizophr. Res. 2018, 197, 176–181. [Google Scholar] [CrossRef]
- Freeman, D.; Bradley, J.; Antley, A.; Bourke, E.; DeWeever, N.; Evans, N.; Černis, E.; Sheaves, B.; Waite, F.; Dunn, G.; et al. Virtual reality in the treatment of persecutory delusions: Randomised controlled experimental study testing how to reduce delusional conviction. Br. J. Psychiatry 2016, 209, 62–67. [Google Scholar] [CrossRef]
- Freeman, D.; Lambe, S.; Kabir, T.; Petit, A.; Rosebrock, L.; Yu, L.-M.; Dudley, R.; Chapman, K.; Morrison, A.; O’Regan, E.; et al. Automated Virtual Reality Therapy to Treat Agoraphobic Avoidance and Distress in Patients with Psychosis (Game-Change): A Multicentre, Parallel-Group, Single-Blind, Randomised, Controlled Trial in England with Mediation and Moderation Analyses. Lancet Psychiatry 2022, 9, 375–388. [Google Scholar] [CrossRef]
- Rault, O.; Lamothe, H.; Pelissolo, A. Therapeutic use of virtual reality relaxation in schizophrenia: A pilot study. Psychiatry Res. 2022, 309, 114389. [Google Scholar] [CrossRef]
- Veling, W.; Lestestuiver, B.; Jongma, M.; Hoenders, H.J.R.; van Driel, C. Virtual Reality Relaxation for Patients with a Psy-chiatric Disorder: Crossover Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e17233. [Google Scholar] [CrossRef] [PubMed]
- Geraets, C.N.W.; Wallinius, M.; Sygel, K. Use of Virtual Reality in Psychiatric Diagnostic Assessments: A Systematic Review. Front. Psychiatry 2022, 13, 828410. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Thier, P.; Leube, D.T.; Schlotterbeck, P.; Lindner, A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain 2010, 133, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, S.; Krebs, M.-O.; Olié, J.-P.; Committeri, G.; van der Meer, E.; Berthoz, A.; Amado, I. Real world referencing and schizophrenia: Are we experiencing the same reality? Neuropsychologia 2010, 48, 2922–2930. [Google Scholar] [CrossRef]
- DeRosse, P.; Karlsgodt, K.H. Examining the Psychosis Continuum. Curr. Behav. Neurosci. Rep. 2015, 2, 80–89. [Google Scholar] [CrossRef]
- Oestreich, L.K.; Mifsud, N.G.; Ford, J.M.; Roach, B.J.; Mathalon, D.H.; Whitford, T.J. Cortical suppression to delayed selfinitiated auditory stimuli in schizotypy: Neurophysiological evidence for a continuum of psychosis. Clin. EEG Neurosci. 2016, 47, 3–10. [Google Scholar] [CrossRef]
- Di Plinio, S.; Ebisch, S.J.H. Probabilistically Weighted Multilayer Networks disclose the link between default mode network instability and psychosis-like experiences in healthy adults. Neuroimage 2022, 257, 119291. [Google Scholar] [CrossRef]
- Freeman, D.; Evans, N.; Lister, R.; Antley, A.; Dunn, G.; Slater, M. Height, social comparison, and paranoia: An immersive virtual reality experimental study. Psychiatry Res. 2014, 218, 348–352. [Google Scholar] [CrossRef]
- Bohil, C.; Alicea, B.; Biocca, F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 2011, 12, 752–762. [Google Scholar] [CrossRef] [PubMed]
| Therapies | Studies | Session Schedule and Duration |
|---|---|---|
| VR Cognitive Rehabilitation Therapy | La Paglia et al., 2013 & 2016 [35,36] | 10 weekly individual sessions of app. 90 min duration each, guided by a predefined protocol |
| Soskitrain | Rus-Calafell et al., 2013 & 2014 [26,27] | 16 twice-weekly individual sessions over eight weeks of app. 60 min each, including two parts: (1) 30 min to discuss content and (2) reminding practice with the VR program |
| VR-ToMIS | Vass et al., 2021 [37] | 9 individual weekly sessions of 50 min each, comprising 8 virtual simulation-based sessions and one pre-briefing session |
| CBT + VRT | Dellazizzo et al., 2020 & 2021 [38,39] | 9 individual weekly one-hour sessions for CBT and VRT, separately (total of 18 sessions) |
| VRT | du Sert et al., 2018 [40] | 7 individual weekly sessions, including one session for creating an avatar and six 45 min therapeutic sessions |
| VR-CBT | Geraets et al., 2020; Pot-Kolder et al., 2018 & 2020 [8,28,29] | 16 one-hour individual therapy sessions, with each session spending 40 min on virtual program and 20 min on discussion with therapists |
| gameChange | Freeman et al., 2022 [42] | 6 weekly sessions each involving 30 min of VR |
| VR relaxation therapy | Rault et al., 2014 [43] | 5 sessions of 30 min each, including 10 min VR exposure followed by psychometric assessment |
| V-DESSERTS | Tan et al., 2021 [12] | 2 sessions within the same day, each consisting of 20 min of psychoeducation and 20 min of virtual screen-based relaxation practice |
| VRelax | Veling et al., 2021 [44] | At least once daily for 10 min over a 10-day period |
| Dimensions | No. of Studies | Standardized Psychometric Instruments |
|---|---|---|
| Diagnosis/symptoms | 15 | PANSS, PSYRATS, CAARMS, CAPE, SCAN, BAVQ-R, SAPS, SANS |
| Neuropsychiatric or cognitive functioning | 16 | MINI, RPM, BRANS, BCIS, MMSE, FAB, TMT-A/B, ToL, SCWT, WCST, VLMT (learning/recall), MWT B Vocabulary test, OTS, SWM, RVP, RAVLT, WAIS-III, SCIP, WMT, VLT-I/II, DS, VFT, PST, CPT II, ToM (BCMET, faux pas test and cartoon stories task, the Hungarian metaphor and irony test), DACOBS, DOG, CASH |
| Paranoia/delusion | 13 | GPTS, SSPS, CDS, SBQ-PD, C-SSRS, PWQ |
| Mood | 8 | CDSS, BDI, BAI, IDS-SR, BHS, HDRS-17, YMRS |
| Social anxiety/distress | 12 | IPD, SUD, SIAS, AI, SSIT, SADS, VASA, CAS, O-AS and O-BAT, AMI-AS, O-CDQ, PSS, NSRS |
| Global/daily functioning | 5 | FAST, UPSA-B, GAF, ESM |
| Social functioning | 3 | PSP, SFS, SOFAS |
| Quality of Life | 6 | LQoLP, Q-LES-Q-SF, MANSA, EQ-5D, ReQoL |
| VR experience | 11 | PQ, SSQ, IPQ, ITQ SUS |
| Self-concept | 3 | SERS, BCSS, BESAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.C.-S.; Hui, C.L.-M.; Suen, Y.-N.; Lee, E.H.-M.; Chang, W.-C.; Chan, S.K.-W.; Chen, E.Y.-H. Application of Immersive Virtual Reality for Assessment and Intervention in Psychosis: A Systematic Review. Brain Sci. 2023, 13, 471. https://doi.org/10.3390/brainsci13030471
Chan KC-S, Hui CL-M, Suen Y-N, Lee EH-M, Chang W-C, Chan SK-W, Chen EY-H. Application of Immersive Virtual Reality for Assessment and Intervention in Psychosis: A Systematic Review. Brain Sciences. 2023; 13(3):471. https://doi.org/10.3390/brainsci13030471
Chicago/Turabian StyleChan, Karen Chui-Shan, Christy Lai-Ming Hui, Yi-Nam Suen, Edwin Ho-Ming Lee, Wing-Chung Chang, Sherry Kit-Wa Chan, and Eric Yu-Hai Chen. 2023. "Application of Immersive Virtual Reality for Assessment and Intervention in Psychosis: A Systematic Review" Brain Sciences 13, no. 3: 471. https://doi.org/10.3390/brainsci13030471
APA StyleChan, K. C.-S., Hui, C. L.-M., Suen, Y.-N., Lee, E. H.-M., Chang, W.-C., Chan, S. K.-W., & Chen, E. Y.-H. (2023). Application of Immersive Virtual Reality for Assessment and Intervention in Psychosis: A Systematic Review. Brain Sciences, 13(3), 471. https://doi.org/10.3390/brainsci13030471







