Neural Processing of Sexist Comments: Associations between Perceptions of Sexism and Prefrontal Activity
Abstract
:1. Introduction
1.1. Neural Processing of Sexist Events
1.2. Neural Processes of Sexism and Gender-Related Stereotypes
1.3. Significance and Aim of the Present Study
2. Method
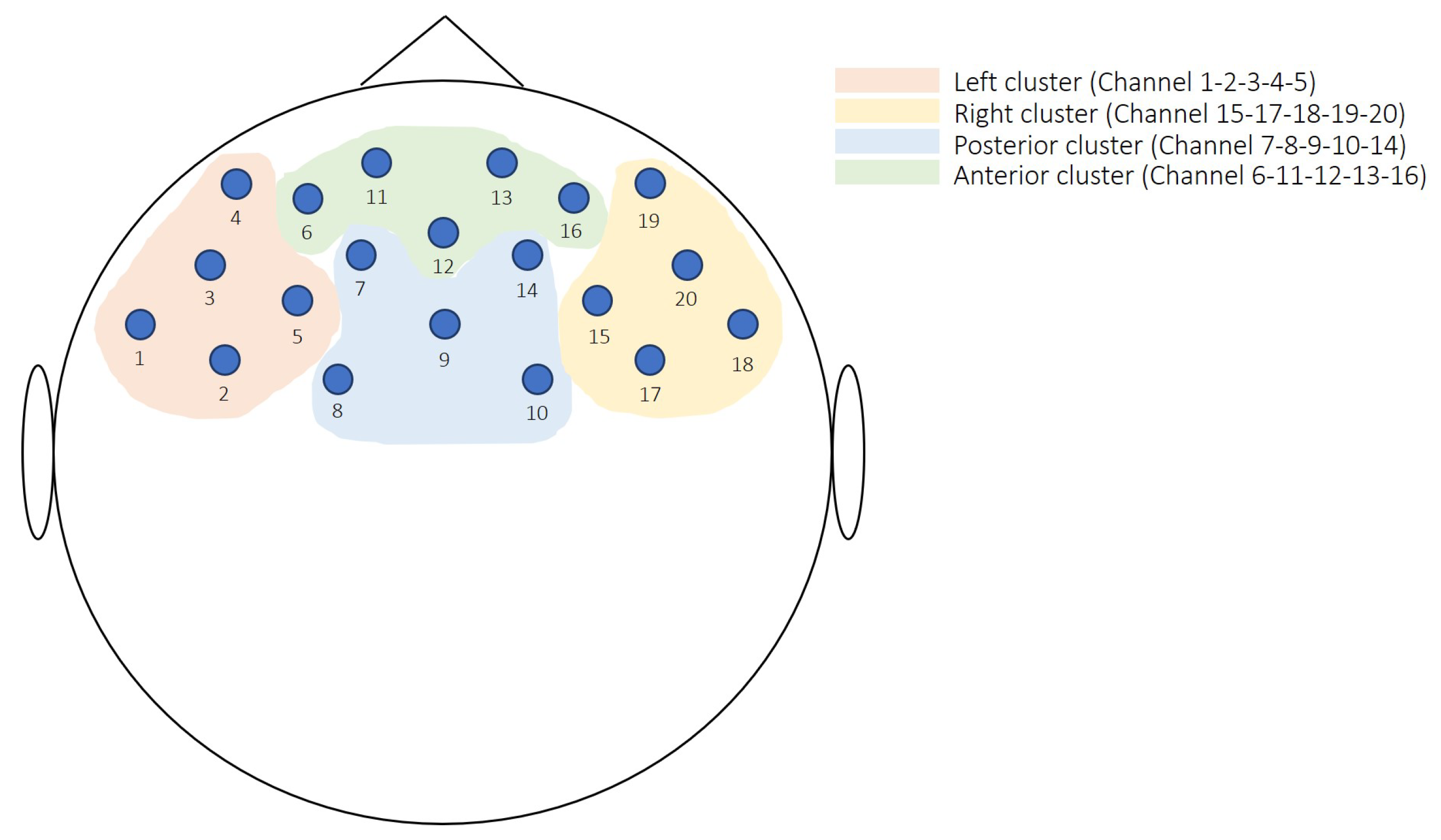
2.1. Participants
2.2. Experimental Procedure
2.2.1. Experimental Stimuli
2.2.2. fNIRS Recording
2.3. fNIRS Preprocessing
2.4. Data Analysis Plan
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klonoff, E.A.; Landrine, H. The Schedule of Sexist Events: A measure of lifetime and recent sexist discrimination in women’s lives. Psychol. Women Q. 1995, 19, 439–470. [Google Scholar] [CrossRef]
- Kuchynka, S.L.; Salomon, K.; Bosson, J.K.; El-Hout, M.; Kiebel, E.; Cooperman, C.; Toomey, R. Hostile and benevolent sexism and college women’s STEM outcomes. Psychol. Women Q. 2018, 42, 72–87. [Google Scholar] [CrossRef]
- Swim, J.K.; Hyers, L.L.; Cohen, L.L.; Ferguson, M.J. Everyday sexism: Evidence for its incidence, nature, and psychological impact from three daily diary studies. J. Soc. Issues 2001, 57, 31–53. [Google Scholar] [CrossRef]
- Glick, P.; Fiske, S.T. The Ambivalent Sexism Inventory: Differentiating hostile and benevolent sexism. J. Personal. Soc. Psychol. 1996, 70, 491. [Google Scholar] [CrossRef]
- Glick, P.; Fiske, S.T. An ambivalent alliance: Hostile and benevolent sexism as complementary justifications for gender inequality. Am. Psychol. 2001, 56, 109. [Google Scholar] [CrossRef]
- Oswald, D.L.; Baalbaki, M.; Kirkman, M. Experiences with benevolent sexism: Scale development and associations with women’s well-being. Sex Roles 2019, 80, 362–380. [Google Scholar] [CrossRef] [Green Version]
- Bosson, J.K.; Pinel, E.C.; Vandello, J.A. The emotional impact of ambivalent sexism: Forecasts versus real experiences. Sex Roles 2010, 62, 520–531. [Google Scholar] [CrossRef]
- Barreto, M.; Ellemers, N. The perils of political correctness: Men’s and women’s responses to old-fashioned and modern sexist views. Soc. Psychol. Q. 2005, 68, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.C.; Wright, S.C. Yet another dark side of chivalry: Benevolent sexism undermines and hostile sexism motivates collective action for social change. J. Personal. Soc. Psychol. 2011, 101, 62. [Google Scholar] [CrossRef]
- Schmitt, M.T.; Branscombe, N.R. The internal and external causal loci of attributions to prejudice. Personal. Soc. Psychol. Bull. 2002, 28, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Siegle, G.J.; Dahl, R.E.; Hooley, J.M.; Silk, J.S. Neural responses to maternal criticism in healthy youth. Soc. Cogn. Affect. Neurosci. 2015, 10, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Gusnard, D.A.; Akbudak, E.; Shulman, G.L.; Raichle, M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 4259–4264. [Google Scholar] [CrossRef] [Green Version]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Siegle, G.J. Common and distinct brain networks underlying explicit emotional evaluation: A meta-analytic study. Soc. Cogn. Affect. Neurosci. 2012, 7, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Dannlowski, U.; Ohrmann, P.; Bauer, J.; Kugel, H.; Arolt, V.; Heindel, W.; Suslow, T. Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res. Neuroimaging 2007, 154, 13–20. [Google Scholar] [CrossRef]
- Sinke, C.B.; Sorger, B.; Goebel, R.; de Gelder, B. Tease or threat? Judging social interactions from bodily expressions. Neuroimage 2010, 49, 1717–1727. [Google Scholar] [CrossRef]
- Eippert, F.; Veit, R.; Weiskopf, N.; Erb, M.; Birbaumer, N.; Anders, S. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007, 28, 409–423. [Google Scholar] [CrossRef]
- Kim, S.H.; Hamann, S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007, 19, 776–798. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Bunge, S.A.; Gross, J.J.; Gabrieli, J.D. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002, 14, 1215–1229. [Google Scholar] [CrossRef] [Green Version]
- Phan, K.L.; Wager, T.; Taylor, S.F.; Liberzon, I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002, 16, 331–348. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.S.; Hamann, S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia 2012, 50, 1578–1593. [Google Scholar] [CrossRef]
- Lang, P.J.; Greenwald, M.K.; Bradley, M.M.; Hamm, A.O. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology 1993, 30, 261–273. [Google Scholar] [CrossRef]
- Vigil, J.M. A socio-relational framework of sex differences in the expression of emotion. Behav. Brain Sci. 2009, 32, 375–390. [Google Scholar] [CrossRef]
- Lithari, C.; Frantzidis, C.; Papadelis, C.; Vivas, A.B.; Klados, M.; Kourtidou-Papadeli, C.; Pappas, C.; Ioannides, A.; Bamidis, P. Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topogr. 2010, 23, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Domes, G.; Schulze, L.; Böttger, M.; Grossmann, A.; Hauenstein, K.; Wirtz, P.H.; Heinrichs, M.; Herpertz, S.C. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp. 2010, 31, 758–769. [Google Scholar] [CrossRef] [Green Version]
- Shirao, N.; Okamoto, Y.; Okada, G.; Ueda, K.; Yamawaki, S. Gender differences in brain activity toward unpleasant linguistic stimuli concerning interpersonal relationships: An fMRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 255, 327–333. [Google Scholar] [CrossRef]
- Hofer, A.; Siedentopf, C.M.; Ischebeck, A.; Rettenbacher, M.A.; Verius, M.; Felber, S.; Fleischhacker, W.W. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychol. Med. 2007, 37, 109–119. [Google Scholar] [CrossRef]
- Schirmer, A.; Zysset, S.; Kotz, S.A.; Von Cramon, D.Y. Gender differences in the activation of inferior frontal cortex during emotional speech perception. NeuroImage 2004, 21, 1114–1123. [Google Scholar] [CrossRef]
- Whittle, S.; Simmons, J.G.; Allen, N.B. Emotion and gender-specific neural processing in men and women. In Principles of Gender-Specific Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–201. [Google Scholar]
- McRae, K.; Ochsner, K.N.; Mauss, I.B.; Gabrieli, J.J.; Gross, J.J. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Process. Intergroup Relations 2008, 11, 143–162. [Google Scholar] [CrossRef] [Green Version]
- Mak, A.K.; Hu, Z.g.; Zhang, J.X.; Xiao, Z.; Lee, T.M. Sex-related differences in neural activity during emotion regulation. Neuropsychologia 2009, 47, 2900–2908. [Google Scholar] [CrossRef]
- Amodio, D.M. The neuroscience of prejudice and stereotyping. Nat. Rev. Neurosci. 2014, 15, 670–682. [Google Scholar] [CrossRef]
- Quadflieg, S.; Turk, D.J.; Waiter, G.D.; Mitchell, J.P.; Jenkins, A.C.; Macrae, C.N. Exploring the neural correlates of social stereotyping. J. Cogn. Neurosci. 2009, 21, 1560–1570. [Google Scholar] [CrossRef] [Green Version]
- Beer, J.S.; Heerey, E.A.; Keltner, D.; Scabini, D.; Knight, R.T. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. J. Personal. Soc. Psychol. 2003, 85, 594. [Google Scholar] [CrossRef] [Green Version]
- Beer, J.S.; Stallen, M.; Lombardo, M.V.; Gonsalkorale, K.; Cunningham, W.A.; Sherman, J.W. The Quadruple Process model approach to examining the neural underpinnings of prejudice. NeuroImage 2008, 43, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Bechara, A.; Damasio, H.; Damasio, A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 2000, 10, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Amodio, D.M.; Kubota, J.T.; Harmon-Jones, E.; Devine, P.G. Alternative mechanisms for regulating racial responses according to internal vs external cues. Soc. Cogn. Affect. Neurosci. 2006, 1, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Van Overwalle, F. Social cognition and the brain: A meta-analysis. Hum. Brain Mapp. 2009, 30, 829–858. [Google Scholar] [CrossRef]
- Krueger, F.; Barbey, A.K.; Grafman, J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn. Sci. 2009, 13, 103–109. [Google Scholar] [CrossRef]
- Frith, C.D.; Frith, U. The neural basis of mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Adolphs, R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001, 11, 231–239. [Google Scholar] [CrossRef]
- Adolphs, R. Cognitive neuroscience of human social behaviour. Nat. Rev. Neurosci. 2003, 4, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.N.; Heatherton, T.F.; Kelley, W.M. A self less ordinary: The medial prefrontal cortex and you. In The Cognitive Neurosciences; Gazzaniga, M.S., Ed.; MIT Press: Cambridge, MA, USA, 2004; pp. 1067–1075. [Google Scholar]
- Mason, M.F.; Macrae, C.N. Categorizing and individuating others: The neural substrates of person perception. J. Cogn. Neurosci. 2004, 16, 1785–1795. [Google Scholar] [CrossRef]
- Haxby, J.V.; Gobbini, M.I.; Montgomery, K. Spatial and Temporal Distribution of Face and Object Representations in the Human Brain. In The Cognitive Neurosciences; Gazzaniga, M.S., Ed.; Boston Review: Cambridge, MA, USA, 2004. [Google Scholar]
- Ochsner, K.N.; Knierim, K.; Ludlow, D.H.; Hanelin, J.; Ramachandran, T.; Glover, G.; Mackey, S.C. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004, 16, 1746–1772. [Google Scholar] [CrossRef]
- Castelli, F.; Happé, F.; Frith, U.; Frith, C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. In Social Neuroscience; Psychology Press: New York, NY, USA, 2013; pp. 155–169. [Google Scholar]
- Frith, U.; Frith, C. The biological basis of social interaction. Curr. Dir. Psychol. Sci. 2001, 10, 151–155. [Google Scholar] [CrossRef]
- Saxe, R.; Wexler, A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia 2005, 43, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Macrae, C.N.; Banaji, M.R. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron 2006, 50, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Knutson, K.M.; Mah, L.; Manly, C.F.; Grafman, J. Neural correlates of automatic beliefs about gender and race. Hum. Brain Mapp. 2007, 28, 915–930. [Google Scholar] [CrossRef]
- Milne, E.; Grafman, J. Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. J. Neurosci. 2001, 21, RC150. [Google Scholar] [CrossRef]
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Knutson, K.M.; Wood, J.N.; Spampinato, M.V.; Grafman, J. Politics on the brain: An fMRI investigation. Soc. Neurosci. 2006, 1, 25–40. [Google Scholar] [CrossRef]
- Koechlin, E.; Ody, C.; Kouneiher, F. The architecture of cognitive control in the human prefrontal cortex. Science 2003, 302, 1181–1185. [Google Scholar] [CrossRef] [Green Version]
- Aron, A.R. The neural basis of inhibition in cognitive control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Ames, D.L.; Jenkins, A.C.; Banaji, M.R. Neural correlates of stereotype application. J. Cogn. Neurosci. 2009, 21, 594–604. [Google Scholar] [CrossRef]
- MacLeod, A.; Buckner, R.; Miezin, F.; Petersen, S.; Raichle, M. Right anterior prefrontal cortex activation during semantic monitoring and working memory. Neuroimage 1998, 7, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Reber, P.; Stark, C.; Squire, L. Cortical areas supporting category learning identified using functional MRI. Proc. Natl. Acad. Sci. USA 1998, 95, 747–750. [Google Scholar] [CrossRef] [Green Version]
- Banaji, M.R.; Hardin, C.D. Automatic stereotyping. Psychol. Sci. 1996, 7, 136–141. [Google Scholar] [CrossRef]
- Cacciari, C.; Padovani, R. Further evidence of gender stereotype priming in language: Semantic facilitation and inhibition in Italian role nouns. Appl. Psycholinguist. 2007, 28, 277–293. [Google Scholar] [CrossRef]
- Carreiras, M.; Garnham, A.; Oakhill, J.; Cain, K. The use of stereotypical gender information in constructing a mental model: Evidence from English and Spanish. Q. J. Exp. Psychol. Sect. A 1996, 49, 639–663. [Google Scholar] [CrossRef]
- Irmen, L.; Roßberg, N. Gender markedness of language: The impact of grammatical and nonlinguistic information on the mental representation of person information. J. Lang. Soc. Psychol. 2004, 23, 272–307. [Google Scholar] [CrossRef]
- Kennison, S.M.; Trofe, J.L. Comprehending pronouns: A role for word-specific gender stereotype information. J. Psycholinguist. Res. 2003, 32, 355–378. [Google Scholar] [CrossRef]
- Proverbio, A.M.; Alberio, A.; De Benedetto, F. Neural correlates of automatic beliefs about gender stereotypes: Males are more prejudicial. Brain Lang. 2018, 186, 8–16. [Google Scholar] [CrossRef]
- Proverbio, A.M.; La Mastra, F.; Zani, A. How negative social bias affects memory for faces: An electrical neuroimaging study. PLoS ONE 2016, 11, e0162671. [Google Scholar] [CrossRef] [Green Version]
- Fourie, M.M.; Thomas, K.G.; Amodio, D.M.; Warton, C.M.; Meintjes, E.M. Neural correlates of experienced moral emotion: An fMRI investigation of emotion in response to prejudice feedback. Soc. Neurosci. 2014, 9, 203–218. [Google Scholar] [CrossRef]
- Freeman, J.B.; Schiller, D.; Rule, N.O.; Ambady, N. The neural origins of superficial and individuated judgments about ingroup and outgroup members. Hum. Brain Mapp. 2010, 31, 150–159. [Google Scholar] [CrossRef]
- Ibañez, A.; Manes, F. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology 2012, 78, 1354–1362. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, Z.; Mattavelli, G.; Platania, E.; Papagno, C. The role of the prefrontal cortex in controlling gender-stereotypical associations: A TMS investigation. NeuroImage 2011, 56, 1839–1846. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Guo, W.; Ye, H.; Lu, X.; Luo, J.; Zheng, H. Gender difference in gender bias: Transcranial direct current stimulation reduces male’s gender stereotypes. Front. Hum. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef] [Green Version]
- Lane, K.A.; Goh, J.X.; Driver-Linn, E. Implicit science stereotypes mediate the relationship between gender and academic participation. Sex Roles 2012, 66, 220–234. [Google Scholar] [CrossRef]
- Nosek, B.A.; Banaji, M.R.; Greenwald, A.G. Math= male, me= female, therefore math≠ me. J. Personal. Soc. Psychol. 2002, 83, 44. [Google Scholar] [CrossRef]
- Nosek, B.A.; Smyth, F.L.; Sriram, N.; Lindner, N.M.; Devos, T.; Ayala, A.; Bar-Anan, Y.; Bergh, R.; Cai, H.; Gonsalkorale, K.; et al. National differences in gender–science stereotypes predict national sex differences in science and math achievement. Proc. Natl. Acad. Sci. USA 2009, 106, 10593–10597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plante, I.; Theoret, M.; Favreau, O.E. Student gender stereotypes: Contrasting the perceived maleness and femaleness of mathematics and language. Educ. Psychol. 2009, 29, 385–405. [Google Scholar] [CrossRef]
- Plante, I.; O’Keefe, P.A.; Aronson, J.; Fréchette-Simard, C.; Goulet, M. The interest gap: How gender stereotype endorsement about abilities predicts differences in academic interests. Soc. Psychol. Educ. 2019, 22, 227–245. [Google Scholar] [CrossRef]
- Steffens, M.C.; Jelenec, P. Separating implicit gender stereotypes regarding math and language: Implicit ability stereotypes are self-serving for boys and men, but not for girls and women. Sex Roles 2011, 64, 324–335. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. Functional near-infrared spectroscopy (fNIRS) for assessing cerebral cortex function during human behavior in natural/social situations: A concise review. Organ. Res. Methods 2019, 22, 46–68. [Google Scholar] [CrossRef]
- Bizzego, A.; Battisti, A.; Gabrieli, G.; Esposito, G.; Furlanello, C. pyphysio: A physiological signal processing library for data science approaches in physiology. SoftwareX 2019, 10, 100287. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433. [Google Scholar] [CrossRef] [Green Version]
- Bizzego, A.; Neoh, M.; Gabrieli, G.; Esposito, G. A machine learning perspective on fnirs signal quality control approaches. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2292–2300. [Google Scholar] [CrossRef]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259. [Google Scholar] [CrossRef]
- Brigadoi, S.; Ceccherini, L.; Cutini, S.; Scarpa, F.; Scatturin, P.; Selb, J.; Gagnon, L.; Boas, D.A.; Cooper, R.J. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage 2014, 85, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Rodd, J.M.; Vitello, S.; Woollams, A.M.; Adank, P. Localising semantic and syntactic processing in spoken and written language comprehension: An Activation Likelihood Estimation meta-analysis. Brain Lang. 2015, 141, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 2009, 19, 2767–2796. [Google Scholar] [CrossRef] [PubMed]
- Belyk, M.; Brown, S.; Lim, J.; Kotz, S.A. Convergence of semantics and emotional expression within the IFG pars orbitalis. Neuroimage 2017, 156, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.R. Differential perceptions of sexism. Women Ther. 1992, 12, 187–200. [Google Scholar] [CrossRef]
- Schmitt, M.T.; Branscombe, N.R.; Kobrynowicz, D.; Owen, S. Perceiving discrimination against one’s gender group has different implications for well-being in women and men. Personal. Soc. Psychol. Bull. 2002, 28, 197–210. [Google Scholar] [CrossRef]
- Klonoff, E.A.; Landrine, H.; Campbell, R. Sexist discrimination may account for well-known gender differences in psychiatric symptoms. Psychol. Women Q. 2000, 24, 93–99. [Google Scholar] [CrossRef]
- Fairchild, K.M. Everyday Stranger Harassment: Frequency and Consequences. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2007. [Google Scholar]
- Fairchild, K. Context effects on women’s perceptions of stranger harassment. Sex Cult 2010, 14, 191–216. [Google Scholar] [CrossRef]
- Desouza, E.R.; Pryor, J.B.; Hutz, C.S. Reactions to sexual harassment charges between North Americans and Brazilians. Sex Roles 1998, 39, 913–928. [Google Scholar] [CrossRef]
- Luthar, V.K.; Luthar, H.K. Using Hofstede’s cultural dimensions to explain sexually harassing behaviours in an international context. Int. J. Hum. Resour. Manag. 2002, 13, 268–284. [Google Scholar] [CrossRef]
- Yee, M.W.; Alagappar, P.N.; Ngeow, Y.M. Differences in the perception of sexual harassment by gender and ethnicity among selected Malaysian undergraduates. Gender Technol. Dev. 2015, 19, 204–230. [Google Scholar] [CrossRef]

| N | Spearman’s r | W | p | ||
|---|---|---|---|---|---|
| Anterior | Female | 36 | −0.114 (0.469) | 551.5 | 0.149 |
| Male | 16 | 0.034 (0.391) | |||
| Left | Female | 32 | −0.188 (0.527) | 335.5 | 0.013 |
| Male | 16 | −0.085 (0.399) | |||
| Posterior | Female | 15 | −0.307 (0.476) | 23.0 | 0.019 |
| Male | 3 | 0.022 (0.074) | |||
| Right | Female | 34 | −0.327 (0.399) | 279.0 | 0.001 |
| Male | 16 | 0.042 (0.433) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neoh, M.J.Y.; Bizzego, A.; Teng, J.H.; Gabrieli, G.; Esposito, G. Neural Processing of Sexist Comments: Associations between Perceptions of Sexism and Prefrontal Activity. Brain Sci. 2023, 13, 529. https://doi.org/10.3390/brainsci13040529
Neoh MJY, Bizzego A, Teng JH, Gabrieli G, Esposito G. Neural Processing of Sexist Comments: Associations between Perceptions of Sexism and Prefrontal Activity. Brain Sciences. 2023; 13(4):529. https://doi.org/10.3390/brainsci13040529
Chicago/Turabian StyleNeoh, Michelle Jin Yee, Andrea Bizzego, Jia Hui Teng, Giulio Gabrieli, and Gianluca Esposito. 2023. "Neural Processing of Sexist Comments: Associations between Perceptions of Sexism and Prefrontal Activity" Brain Sciences 13, no. 4: 529. https://doi.org/10.3390/brainsci13040529






