Cerebellar TMS Induces Motor Responses Mediating Modulation of Spinal Excitability: A Literature Review
Abstract
1. Introduction
2. Search Strategy
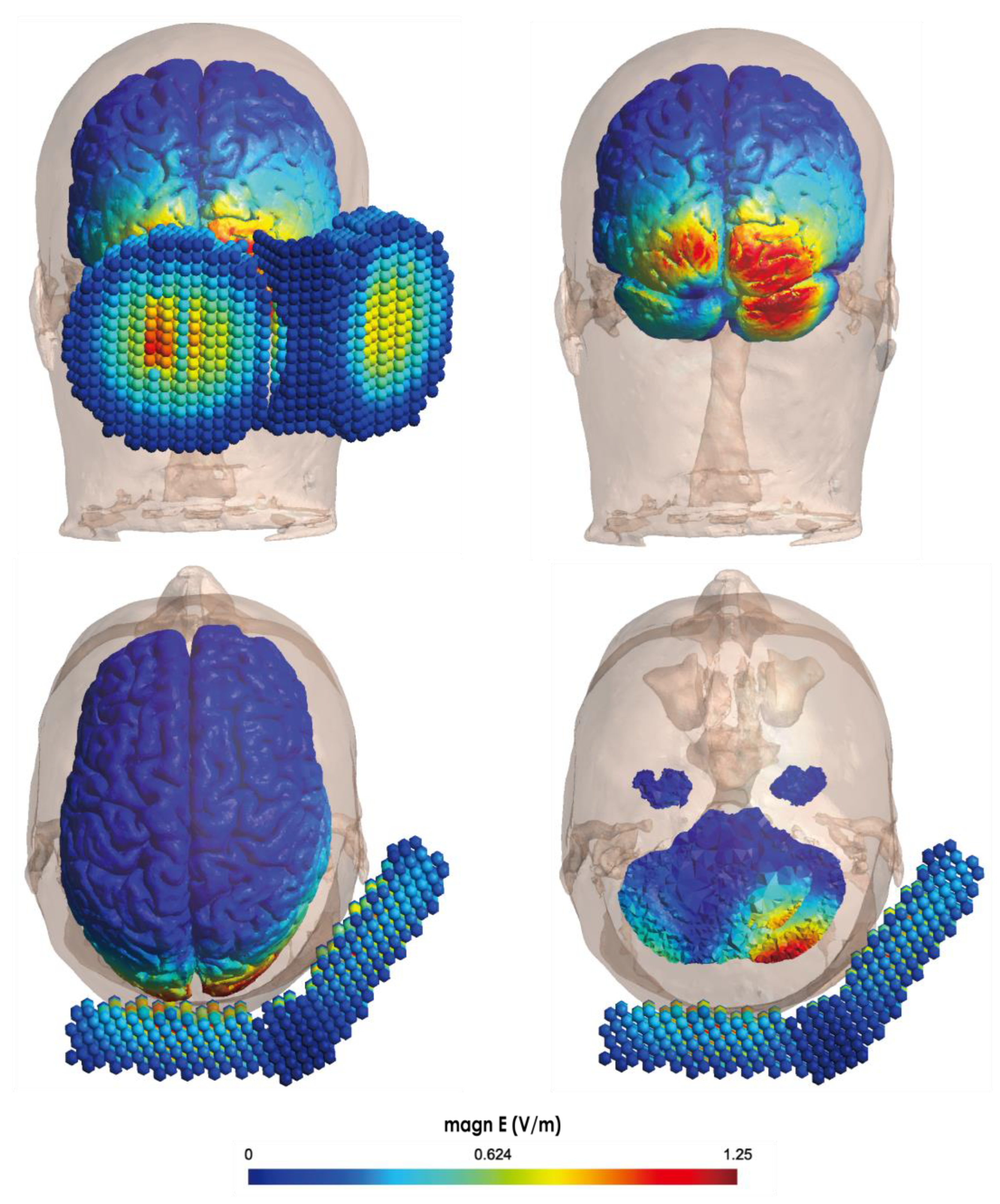
3. Cerebellar TMS
4. Short-Latency Motor Responses following TMS over the Posterior Fossa
5. Long-Latency Motor Responses following Cerebellar TMS
| Author | Year | Coil | Target Muscle | Outcome | Latency | Findings |
|---|---|---|---|---|---|---|
| Sakihara et al. [33] | 2003 | F8 | Soleus | Motor response | about 100 ms | Dependency of the stimulation site |
| Sakihara et al. [32] | 2007 | DC | Soleus | Motor response | about 100 ms | Modulation by optokinetic stimulation |
| Hiraoka et al. [34] | 2010 | DC | Rt-FDI | Motor response | about 90 ms | Task dependency |
| Matsugi et al. [35] | 2012 | DC | Rt-FDI | Motor response | about 90 ms | No dependency of the stimulation site in the cerebellum |
| Matsugi et al. [31] | 2013 | DC | Rt-FDI | Motor response | about 80 ms | Appearance depended on the task |
| Hosokawa et al. [36] | 2014 | DC | Bilateral ECR | Motor response | Ipsilateral 60 ms, contralateral 70 ms | Affected by postural control, drowsiness |
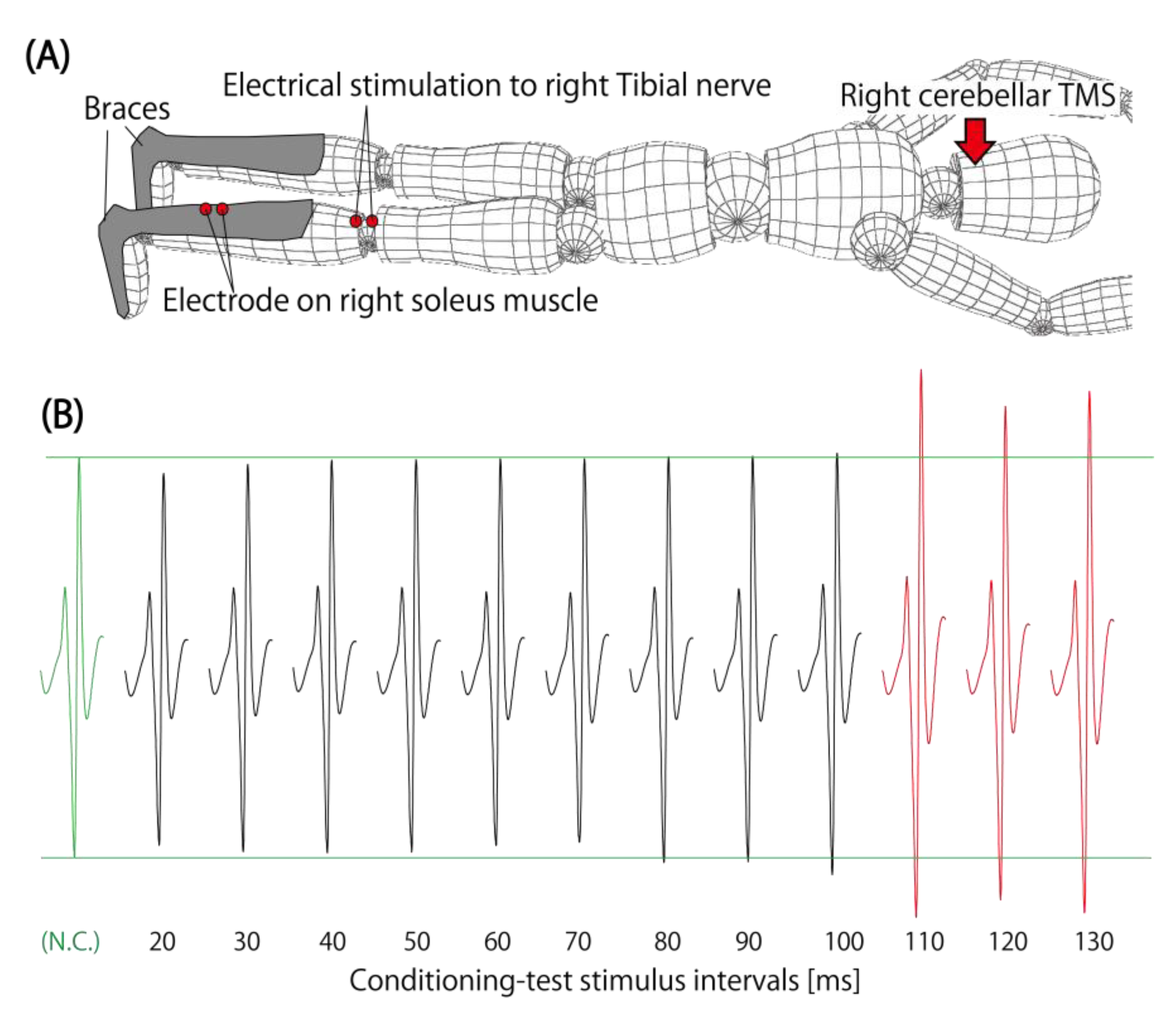
6. Modulation of Spinal Excitability after Cerebellar TMS
| Author | Year | Coil | Target Muscle | Outcome | Latency | Findings |
|---|---|---|---|---|---|---|
| Matsugi et al. [46] | 2014 | DC | Rt-Soleus | Modulation of H-reflex | ISI 110–130 ms | Time course of ISI, task dependency |
| Matsugi et al. [48] | 2015 | DC | Rt-Soleus | Modulation of H-reflex | ISI 110 ms | Mediation of PSI, not of RI |
| Matsugi [49] | 2018 | DC | Rt-Soleus | Modulation of H-reflex | ISI 110 ms | No dependency of stimulation site in the cerebellum |
| Matsugi et al. [21] | 2018 | DC | Rt-Soleus | Modulation of H-reflex | ISI 110 ms | Facilitation in SCD with CBI-absent |
| Matsugi et al. [47] | 2020 | DC | Rt-Soleus | Modulation of H-reflex | ISI 110 ms | Dependency of tDCS-polarity |
7. Possible Pathway
8. Future Outlook
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manto, M. The underpinnings of cerebellar ataxias. Clin. Neurophysiol. Pract. 2022, 7, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Bodranghien, F.; Bastian, A.; Casali, C.; Hallett, M.; Louis, E.D.; Manto, M.; Marien, P.; Nowak, D.A.; Schmahmann, J.D.; Serrao, M.; et al. Consensus Paper: Revisiting the Symptoms and Signs of Cerebellar Syndrome. Cerebellum 2016, 15, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J. Sensory integration for human balance control. Handb. Clin. Neurol. 2018, 159, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Udo, M.; Mano, N.; Kawai, N. Synaptic action of the fastigiobulbar impulses upon neurones in the medullary reticular formation and vestibular nuclei. Exp. Brain Res. 1970, 11, 29–47. [Google Scholar] [CrossRef]
- Rispal-Padel, L.; Cicirata, F.; Pons, C. Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio). Exp. Brain Res. 1982, 47, 365–380. [Google Scholar] [CrossRef]
- Schultz, W.; Montgomery, E.B., Jr.; Marini, R. Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain 1979, 102, 127–146. [Google Scholar] [CrossRef]
- Ashida, R.; Walsh, P.; Brooks, J.C.W.; Cerminara, N.L.; Apps, R.; Edwards, R.J. Sensory and motor electrophysiological mapping of the cerebellum in humans. Sci. Rep. 2022, 12, 177. [Google Scholar] [CrossRef]
- Ugawa, Y.; Uesaka, Y.; Terao, Y.; Hanajima, R.; Kanazawa, I. Magnetic stimulation over the cerebellum in humans. Ann. Neurol. 1995, 37, 703–713. [Google Scholar] [CrossRef]
- Gutierrez, M.I.; Poblete-Naredo, I.; Mercado-Gutierrez, J.A.; Toledo-Peral, C.L.; Quinzanos-Fresnedo, J.; Yanez-Suarez, O.; Gutierrez-Martinez, J. Devices and Technology in Transcranial Magnetic Stimulation: A Systematic Review. Brain Sci. 2022, 12, 1218. [Google Scholar] [CrossRef]
- Daskalakis, Z.J.; Paradiso, G.O.; Christensen, B.K.; Fitzgerald, P.B.; Gunraj, C.; Chen, R. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. 2004, 557, 689–700. [Google Scholar] [CrossRef]
- Matsugi, A.; Nishishita, S.; Yoshida, N.; Tanaka, H.; Douchi, S.; Bando, K.; Tsujimoto, K.; Honda, T.; Kikuchi, Y.; Shimizu, Y.; et al. Impact of Repetitive Transcranial Magnetic Stimulation to the Cerebellum on Performance of a Ballistic Targeting Movement. Cerebellum 2022. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Mawase, F.; Celnik, P. Learning Similar Actions by Reinforcement or Sensory-Prediction Errors Rely on Distinct Physiological Mechanisms. Cereb. Cortex 2018, 28, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Hosomi, K.; Mano, T.; Takeya, Y.; Tagami, S.; Mori, N.; Matsugi, A.; Jono, Y.; Harada, H.; Yamada, T.; et al. Randomized, sham-controlled, clinical trial of repetitive transcranial magnetic stimulation for patients with Alzheimer’s dementia in Japan. Front. Aging Neurosci. 2022, 14, 993306. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Hosomi, K.; Nishi, A.; Matsugi, A.; Dong, D.; Oshino, S.; Kishima, H.; Saitoh, Y. Exploratory study of optimal parameters of repetitive transcranial magnetic stimulation for neuropathic pain in the lower extremities. Pain Rep. 2021, 6, e964. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.M.; Lai, H.M.; Baker, M.R.; Baker, S.N. Corticospinal activation confounds cerebellar effects of posterior fossa stimuli. Clin. Neurophysiol. 2009, 120, 2109–2113. [Google Scholar] [CrossRef]
- Gomez, L.J.; Dannhauer, M.; Peterchev, A.V. Fast computational optimization of TMS coil placement for individualized electric field targeting. Neuroimage 2021, 228, 117696. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Lesage, E.; Miall, R.C. Cerebellar Transcranial Magnetic Stimulation: The Role of Coil Geometry and Tissue Depth. Brain Stimul. 2014, 7, 643–649. [Google Scholar] [CrossRef]
- Fernandez, L.; Major, B.P.; Teo, W.P.; Byrne, L.K.; Enticott, P.G. The Impact of Stimulation Intensity and Coil Type on Reliability and Tolerability of Cerebellar Brain Inhibition (CBI) via Dual-Coil TMS. Cerebellum 2018, 17, 540–549. [Google Scholar] [CrossRef]
- Matsugi, A.; Okada, Y. Cerebellar transcranial static magnetic field stimulation transiently reduces cerebellar brain inhibition. Funct. Neurol. 2017, 32, 77–82. [Google Scholar] [CrossRef]
- Shirota, Y.; Hanajima, R.; Shimizu, T.; Terao, Y.; Tsuji, S.; Ugawa, Y. Quantitative Evaluation of Cerebellar Function in Multiple System Atrophy with Transcranial Magnetic Stimulation. Cerebellum 2022, 21, 219–224. [Google Scholar] [CrossRef]
- Matsugi, A.; Kikuchi, Y.; Kaneko, K.; Seko, Y.; Odagaki, M. Cerebellar transcranial magnetic stimulation facilitates excitability of spinal reflex, but does not affect cerebellar inhibition and facilitation in spinocerebellar ataxia. Neuroreport 2018, 29, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.A.; Celnik, P.A.; Rothwell, J.C. Cerebellar-Motor Cortex Connectivity: One or Two Different Networks? J. Neurosci. 2020, 40, 4230–4239. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsugi, A.; Okada, Y. The effects of imaginary voluntary muscle contraction and relaxation on cerebellar brain inhibition. Neurosci. Res. 2017, 133, 15–20. [Google Scholar] [CrossRef]
- Galea, J.M.; Jayaram, G.; Ajagbe, L.; Celnik, P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J. Neurosci. 2009, 29, 9115–9122. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, A.; Yoshida, N.; Nishishita, S.; Okada, Y.; Mori, N.; Oku, K.; Douchi, S.; Hosomi, K.; Saitoh, Y. Cerebellum-mediated trainability of eye and head movements for dynamic gazing. PLoS ONE 2019, 14, e0224458. [Google Scholar] [CrossRef]
- Shemesh, A.A.; Zee, D.S. Eye Movement Disorders and the Cerebellum. J. Clin. Neurophysiol. 2019, 36, 405–414. [Google Scholar] [CrossRef]
- Taylor, J.L. Stimulation at the cervicomedullary junction in human subjects. J. Electromyogr. Kinesiol. 2006, 16, 215–223. [Google Scholar] [CrossRef]
- Ugawa, Y.; Uesaka, Y.; Terao, Y.; Hanajima, R.; Kanazawa, I. Magnetic stimulation of the descending and ascending tracts at the foramen magnum level. Electroencephalogr. Clin. Neurophysiol. 1997, 105, 128–131. [Google Scholar] [CrossRef]
- Tsuyama, S.; Hyodo, A.; Sekino, M.; Hayami, T.; Ueno, S.; Iramina, K. The numeric calculation of eddy current distributions in transcranial magnetic stimulation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 4286–4289. [Google Scholar] [CrossRef]
- McNeil, C.J.; Butler, J.E.; Taylor, J.L.; Gandevia, S.C. Testing the excitability of human motoneurons. Front. Hum. Neurosci. 2013, 7, 152. [Google Scholar] [CrossRef]
- Matsugi, A.; Iwata, Y.; Mori, N.; Horino, H.; Hiraoka, K. Long latency electromyographic response induced by transcranial magnetic stimulation over the cerebellum preferentially appears during continuous visually guided manual tracking task. Cerebellum 2013, 12, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sakihara, K.; Hirata, M.; Nakagawa, S.; Fujiwara, N.; Sekino, M.; Ueno, S.; Ihara, A.; Yorifuji, S. Late response evoked by cerebellar stimuli: Effect of optokinetic stimulation. Neuroreport 2007, 18, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Sakihara, K.; Yorifuji, S.; Ihara, A.; Izumi, H.; Kono, K.; Takahashi, Y.; Imaoka, H.; Ogino, S. Transcranial magnetic stimulation over the cerebellum evokes late potential in the soleus muscle. Neurosci. Res. 2003, 46, 257–262. [Google Scholar] [CrossRef]
- Hiraoka, K.; Horino, K.; Yagura, A.; Matsugi, A. Cerebellar TMS evokes a long latency motor response in the hand during a visually guided manual tracking task. Cerebellum 2010, 9, 454–460. [Google Scholar] [CrossRef]
- Matsugi, A.; Kamata, N.; Tanaka, T.; Hiraoka, K. Long latency fluctuation of the finger movement evoked by cerebellar TMS during visually guided manual tracking task. Indian J. Physiol. Pharmacol. 2012, 56, 193–200. [Google Scholar]
- Hosokawa, S.; Hirata, M.; Goto, T.; Yanagisawa, T.; Sugata, H.; Araki, T.; Okamura, Y.; Hasegawa, Y.; Shinshi, M.; Yorifuji, S. Cerebellar-related long latency motor response in upper limb musculature by transcranial magnetic stimulation of the cerebellum. Neuroreport 2014, 25, 353–357. [Google Scholar] [CrossRef]
- Brownstone, R.M.; Chopek, J.W. Reticulospinal Systems for Tuning Motor Commands. Front. Neural Circuits 2018, 12, 30. [Google Scholar] [CrossRef]
- Vantomme, G.; Osorio-Forero, A.; Luthi, A.; Fernandez, L.M.J. Regulation of Local Sleep by the Thalamic Reticular Nucleus. Front. Neurosci. 2019, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Miall, R.C.; Imamizu, H.; Miyauchi, S. Activation of the cerebellum in co-ordinated eye and hand tracking movements: An fMRI study. Exp. Brain Res. 2000, 135, 22–33. [Google Scholar] [CrossRef]
- Honda, T.; Nagao, S.; Hashimoto, Y.; Ishikawa, K.; Yokota, T.; Mizusawa, H.; Ito, M. Tandem internal models execute motor learning in the cerebellum. Proc. Natl. Acad. Sci. USA 2018, 115, 7428–7433. [Google Scholar] [CrossRef]
- Baizer, J.S. Unique features of the human brainstem and cerebellum. Front. Hum. Neurosci. 2014, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Austin, D.; Hannah, R.; Rothwell, J.C. Non-invasive brain stimulation as a tool to study cerebellar-M1 interactions in humans. Cerebellum Ataxias 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Gilio, F.; Conte, A.; Vanacore, N.; Frasca, V.; Inghilleri, M.; Berardelli, A. Excitatory and inhibitory after-effects after repetitive magnetic transcranial stimulation (rTMS) in normal subjects. Exp. Brain Res. 2007, 176, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M. The H-reflex as a probe: Pathways and pitfalls. J. Neurosci. Methods 2008, 171, 1–12. [Google Scholar] [CrossRef]
- Misiaszek, J.E. The H-reflex as a tool in neurophysiology: Its limitations and uses in understanding nervous system function. Muscle Nerve 2003, 28, 144–160. [Google Scholar] [CrossRef]
- Matsugi, A.; Mori, N.; Uehara, S.; Kamata, N.; Oku, K.; Mukai, K.; Nagano, K. Task dependency of the long-latency facilitatory effect on the soleus H-reflex by cerebellar transcranial magnetic stimulation. Neuroreport 2014, 25, 1375–1380. [Google Scholar] [CrossRef]
- Matsugi, A.; Okada, Y. Cerebellar transcranial direct current stimulation modulates the effect of cerebellar transcranial magnetic stimulation on the excitability of spinal reflex. Neurosci. Res. 2020, 150, 37–43. [Google Scholar] [CrossRef]
- Matsugi, A.; Mori, N.; Uehara, S.; Kamata, N.; Oku, K.; Okada, Y.; Kikuchi, Y.; Mukai, K.; Nagano, K. Effect of cerebellar transcranial magnetic stimulation on soleus Ia presynaptic and reciprocal inhibition. Neuroreport 2015, 26, 139–143. [Google Scholar] [CrossRef]
- Matsugi, A. Do changes in spinal reflex excitability elicited by transcranial magnetic stimulation differ based on the site of cerebellar stimulation? Somatosens. Mot. Res. 2018, 35, 80–85. [Google Scholar] [CrossRef]
- Teune, T.M.; van der Burg, J.; van der Moer, J.; Voogd, J.; Ruigrok, T.J. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog. Brain Res. 2000, 124, 141–172. [Google Scholar] [CrossRef]
- Ito, M. Neurophysiological aspects of the cerebellar motor control system. Int. J. Neurol. 1970, 7, 162–176. [Google Scholar] [PubMed]
- Daniel, H.; Angaut, P.; Batini, C.; Billard, J.M. Topographic organization of the interpositorubral connections in the rat. A WGA-HRP study. Behav. Brain Res. 1988, 28, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Tadi, P. Neuroanatomy, Pons. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsugi, A. Cerebellar TMS Induces Motor Responses Mediating Modulation of Spinal Excitability: A Literature Review. Brain Sci. 2023, 13, 531. https://doi.org/10.3390/brainsci13040531
Matsugi A. Cerebellar TMS Induces Motor Responses Mediating Modulation of Spinal Excitability: A Literature Review. Brain Sciences. 2023; 13(4):531. https://doi.org/10.3390/brainsci13040531
Chicago/Turabian StyleMatsugi, Akiyoshi. 2023. "Cerebellar TMS Induces Motor Responses Mediating Modulation of Spinal Excitability: A Literature Review" Brain Sciences 13, no. 4: 531. https://doi.org/10.3390/brainsci13040531
APA StyleMatsugi, A. (2023). Cerebellar TMS Induces Motor Responses Mediating Modulation of Spinal Excitability: A Literature Review. Brain Sciences, 13(4), 531. https://doi.org/10.3390/brainsci13040531







