Electrophysiological Characterization of Cerebellar Responses during Exploration and Grooming Behaviors in a Rat Model of Parkinsonism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. Study Groups
2.3. Grooming
2.4. Exploratory Behavior
2.5. Electrode Implant and Recording
2.6. Electrolytic Lesion and Tissue Processing
2.7. Statistical Analysis
3. Results
3.1. Grooming
3.2. Horizontal Exploration
3.3. Vertical Exploration
4. Discussion
4.1. Summary and Contributions
4.2. Strength and Limitations
4.3. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| µm | Micrometer |
| ANOVA | Nested analysis of variance |
| AP | Anteroposterior |
| BG | Basal ganglia |
| CCU1 | Constant Current Unit |
| Cm | Centimeter |
| Crus II | Anxiform lobe 2 |
| DA | Dopamine |
| DLNT | Dorsolateral nucleus of the trigeminal nerve |
| DN | Deep Dentate Nucleus |
| DV | Dorsoventral |
| GCUAL | Guide for Care and Use of Laboratory Animals |
| GLM | Generalized Linear Model |
| GPe | External globus pallidus |
| Gpi | Internal globus pallidus |
| kg | Kilograms |
| mA | Milliamps |
| mg | Milligrams |
| ML | Mediolateral |
| ml | Milliliters |
| ms | Milliseconds |
| MUA | Multiunit electrical activity |
| mV | Millivolts |
| MΩ | Milliohm |
| PD | Parkinson’s disease |
| RF | Reticular formation |
| SC | Superior culliculus |
| SE | Standard Error |
| Sim B | Lobes Simple B |
| SIU | Stimulus Isolation Unit |
| SNc | Substantia nigra pars compacta |
| STN | Subthalamic nucleus |
| TJMs | Tremulous jaw movements |
| VLS | Ventrolateral striatum |
References
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Gasca-Salas, C.C.; Sánchez-Ferro, Á.; Ángel Obeso, J. Actualización En La Enfermedad de Parkinson. Rev. Méd. Clín. Las Condes 2016, 27, 363–379. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, J.; Kim, E.; Choi, J.H.; Rah, J.-C.; Choi, J.-W. Dopamine Depletion Can Be Predicted by the Aperiodic Component of Subthalamic Local Field Potentials. Neurobiol. Dis. 2022, 168, 105692. [Google Scholar] [CrossRef] [PubMed]
- Haumesser, J.K.; Beck, M.H.; Pellegrini, F.; Kühn, J.; Neumann, W.J.; Altschüler, J.; Harnack, D.; Kupsch, A.; Nikulin, V.V.; Kühn, A.A.; et al. Subthalamic Beta Oscillations Correlate with Dopaminergic Degeneration in Experimental Parkinsonism. Exp. Neurol. 2021, 335, 113513. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, E.; Tremblay, L.; Féger, J.; Carras, P.L.; Strick, P.L. The Cerebellum Communicates with the Basal Ganglia. Nat. Neurosci. 2005, 8, 1491–1493. [Google Scholar] [CrossRef]
- Bostan, A.C.; Strick, P.L. The Basal Ganglia and the Cerebellum: Nodes in an Integrated Network. Nat. Rev. Neurosci. 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Doya, K. Complementary Roles of Basal Ganglia and Cerebellum in Learning and Motor Control. Curr. Opin. Neurobiol. 2000, 10, 732–739. [Google Scholar] [CrossRef]
- Yu, H.; Sternad, D.; Corcos, D.M.; Vaillancourt, D.E. Role of Hyperactive Cerebellum and Motor Cortex in Parkinson’s Disease. Neuroimage 2007, 35, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Milardi, D.; Quartarone, A.; Bramanti, A.; Anastasi, G.; Bertino, S.; Basile, G.; Buonasera, P.; Pilone, G.; Celeste, G.; Rizzo, G.; et al. The Cortico-Basal Ganglia-Cerebellar Network: Past, Present and Future Perpectives. Front. Syst. Neurosci. 2019, 13, 61. [Google Scholar] [CrossRef]
- Vásquez-Celaya, L.; Marín, G.; Hernández, M.E.; Carrillo, P.; Pérez, C.A.; Coria-Avila, G.A.; Manzo, J.; Miquel, M.; García, L.I. Functional Correlation between Cerebellum and Basal Ganglia: A Parkinsonism Model. Neurologia 2022, 33, 215–226. [Google Scholar] [CrossRef]
- Herrera-Meza, G.; Manzo, J.; Hernández, M.E.; Miquel, M.; García, L.I. Induction of Mandibular Tremor Using Electrolytic Lesion of the Ventrolateral Striatum or Using Subchronic Haloperidol Therapy in Male Rats: An Electromyographic Comparison. Neurología (Engl. Ed.) 2014, 29, 416–422. [Google Scholar] [CrossRef]
- Kosmowska, B.; Ossowska, K.; Wardas, J. Blockade of Adenosine A2A Receptors Inhibits Tremulous Jaw Movements as Well as Expression of Zif-268 and GAD65 MRNAs in Brain Motor Structures. Behav. Brain Res. 2022, 417, 113585. [Google Scholar] [CrossRef]
- Salamone, J.D.; Johnson, C.J.; McCullough, L.D.; Steinpreis, R.E. Lateral Striatal Cholinergic Mechanisms Involved in Oral Motor Activities in the Rat. Psychopharmacology 1990, 102, 529–534. [Google Scholar] [CrossRef]
- Voorn, P.; Vanderschuren, L.J.M.J.; Groenewegen, H.J.; Robbins, T.W.; Pennartz, C.M.A. Putting a Spin on the Dorsal-Ventral Divide of the Striatum. Trends Neurosci. 2004, 27, 468–474. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of Rodent Self-Grooming and Its Value for Translational Neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Lynn, D.A.; Brown, G.R. The Ontogeny of Exploratory Behavior in Male and Female Adolescent Rats (Rattus Norvegicus). Dev. Psychobiol. 2009, 51, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Gharbawie, O.A.; Whishaw, P.A.; Whishaw, I.Q. The Topography of Three-Dimensional Exploration: A New Quantification of Vertical and Horizontal Exploration, Postural Support, and Exploratory Bouts in the Cylinder Test. Behav. Brain Res. 2004, 151, 125–135. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Rodríguez, A.T.; Vásquez-Celaya, L.; Coria-Avila, G.A.; Pérez, C.A.; Aranda-Abreu, G.E.; Carrillo, P.; Manzo, J.; García, L.I. Changes in Multiunit Activity Pattern in Cerebellar Cortex Associated to Olfactory Cues during Sexual Learning in Male Rats. Neurosci. Lett. 2018, 687, 241–247. [Google Scholar] [CrossRef]
- Hernández-Briones, Z.S.; García-Bañuelos, P.; Hernández, M.E.; López, M.-L.; Chacón, A.M.; Carrillo, P.; Coria-Avila, G.; Manzo, J.; García, L.I. Olfactory Stimulation Induces Cerebellar Vermis Activation during Sexual Learning in Male Rats. Neurobiol. Learn Mem. 2017, 146, 31–36. [Google Scholar] [CrossRef]
- Lazic, S.E. The problem of pseudoreplication in neuroscientific studies: Is it affecting your analysis? BMC Neurosci. 2010, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Jwair, S.; Coulon, P.; Ruigrok, T.J.H. Disynaptic Subthalamic Input to the Posterior Cerebellum in Rat. Front. Neuroanat. 2017, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-M.; Wei, P.-H.; Shan, Y.; Han, M.; Zhang, M.; Liu, H.; Gao, J.-H.; Lu, J. Identifying and Characterizing Projections from the Subthalamic Nucleus to the Cerebellum in Humans. Neuroimage 2020, 210, 116573. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Mayorga, A.J.; Trevitt, J.T.; Cousins, M.S.; Conlan, A.; Nawab, A. Tremulous Jaw Movements in Rats:A Model of Parkinsonian Tremor. Prog. Neurobiol. 1998, 56, 591–611. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Meza, G.; Aguirre-Manzo, L.; Coria-Avila, G.A.; Lopez-Meraz, M.L.; Toledo-Cárdenas, R.; Manzo, J.; Garcia, L.I.; Miquel, M. Beyond the Basal Ganglia: CFOS Expression in the Cerebellum in Response to Acute and Chronic Dopaminergic Alterations. Neuroscience 2014, 267, 219–231. [Google Scholar] [CrossRef]
- Hall, J.E. Tratado de Fisiología Medica Guyton y Hall, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. (Eds.) Kandel Ch43—The Basal Ganglia. In Principles of Neural Science; McGraw-hill: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Pong, M.; Horn, K.M.; Gibson, A.R. Pathways for Control of Face and Neck Musculature by the Basal Ganglia and Cerebellum. Brain Res. Rev. 2008, 58, 249–264. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. Evidence for Topographic Organization in the Cerebellum of Motor Control versus Cognitive and Affective Processing. Cortex 2010, 46, 831–844. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, I.; Shinoda, Y. Molecular, Topographic, and Functional Organization of the Cerebellar Cortex: A Study with Combined Aldolase C and Olivocerebellar Labeling. J. Neurosci. 2004, 24, 8771–8785. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, C.J.; Schmahmann, J.D. Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage 2009, 44, 489–501. [Google Scholar] [CrossRef]
- Kelly, R.M.; Strick, P.L. Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci. 2003, 23, 8432–8444. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, D.; Caparelli, E.C.; Chang, L.; Ernst, T. FMRI-Acoustic Noise Alters Brain Activation during Working Memory Tasks. Neuroimage 2005, 27, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Hallett, M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005, 10, 2250–2259. [Google Scholar] [CrossRef] [Green Version]




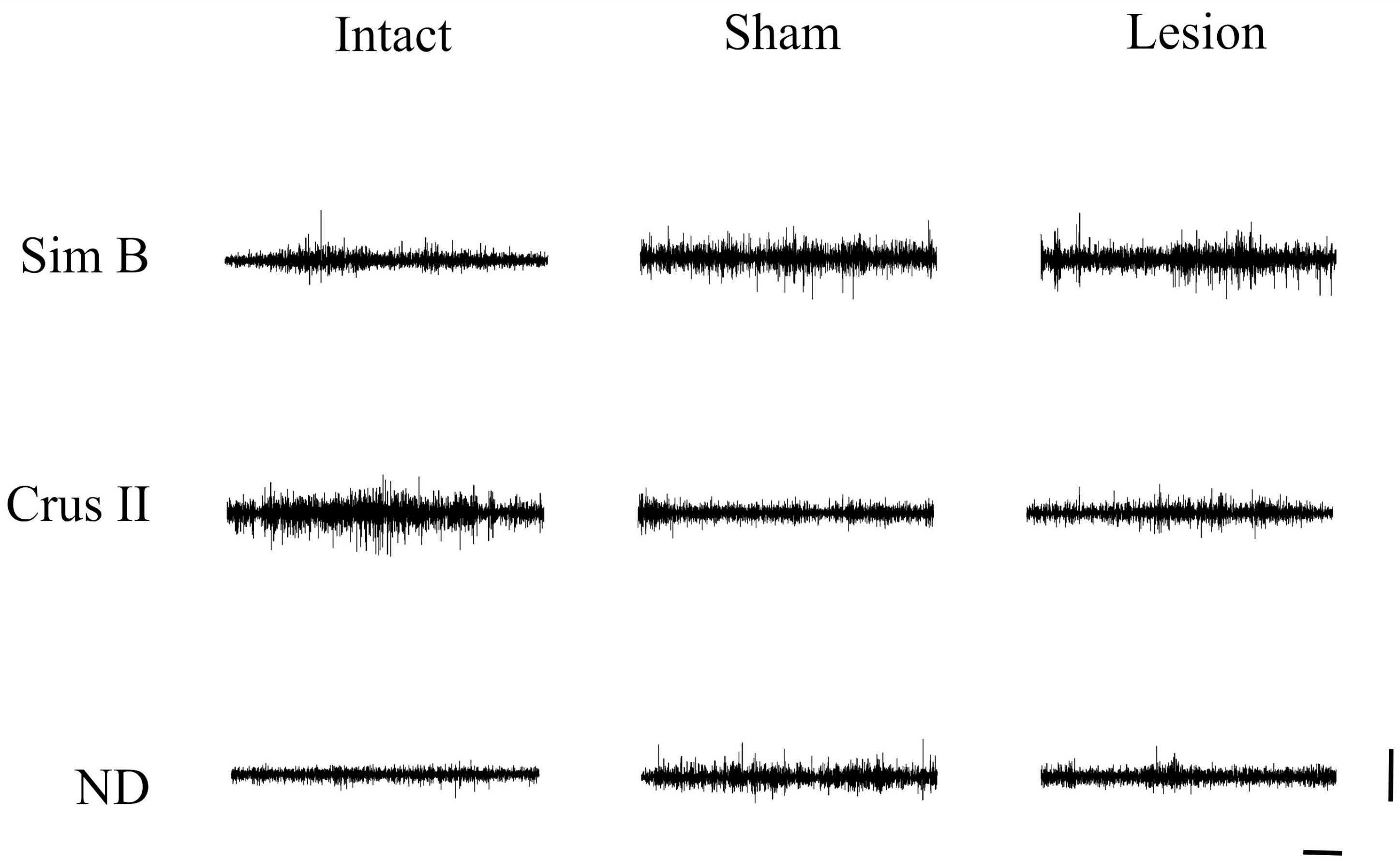
| Source | DF | Sum of Squares | Ratio F | p | Post Hoc |
|---|---|---|---|---|---|
| Group | 2 | 9.00 | 4.93 | 0.001 | I > S, L |
| Structure | 2 | 31.93 | 17.49 | <0.00001 | C > Si, D |
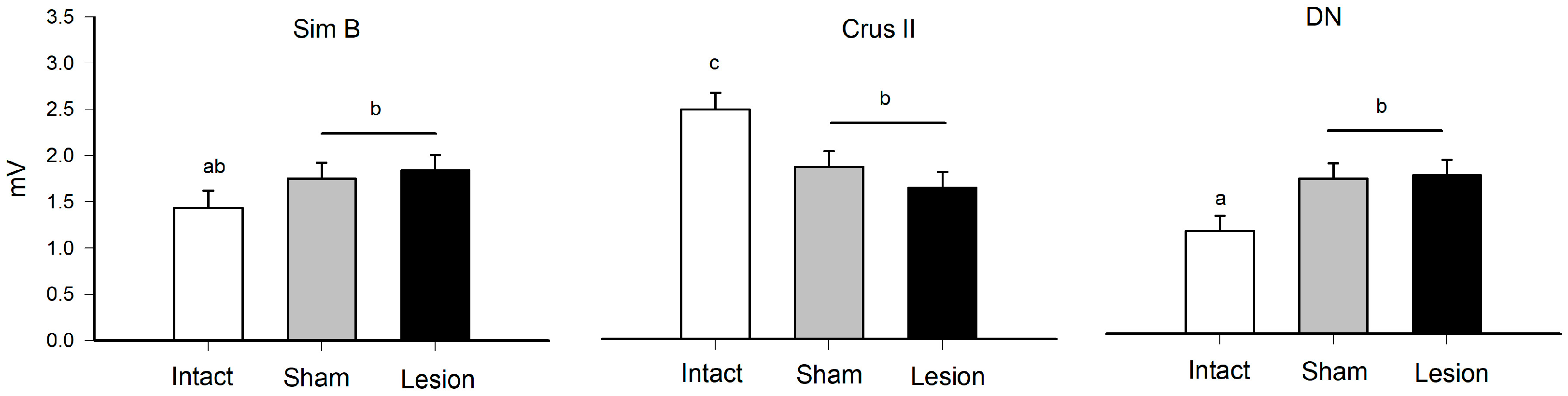
| Group × Structure | 4 | 26.78 | 7.33 | <0.0001 | see Figure 2 |
| Rat [Structure] | 9 | 83.07 | 10.11 | <0.0001 | |
| Traces [Rat] | 36 | 13.32 | 0.40 | NS |
| Source | DF | Sum of Squares | Ratio F | p | Post Hoc |
|---|---|---|---|---|---|
| Group | 2 | 22.15 | 8.50 | <0.0001 | I > S, L |
| Structure | 2 | 17.42 | 6.69 | <0.001 | C, Si > D |
| Group × Structure | 4 | 26.98 | 5.17 | <0.0001 | see Figure 4 |
| Rat [Structure] | 9 | 104.59 | 8.92 | <0.0001 | |
| Traces [Rat] | 36 | 29.01 | 0.61 | NS |
| Source | DF | Sum of Squares | Ratio F | p | Post hoc |
|---|---|---|---|---|---|
| Group | 2 | 0.47 | 0.20 | 0.81 | NS |
| Structure | 2 | 13.26 | 5.79 | <0.003 | C > Si, D |
| Group × Structure | 4 | 26.25 | 5.73 | <0.0001 | see Figure 6 |
| Rat [Structure] | 9 | 94.03 | 9.13 | <0.0001 | |
| Traces [Rat] | 36 | 27.03 | 0.65 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez-Celaya, L.; Marín-Márquez, G.; Manzo, J.; Carrillo-Castilla, P.; Martínez, A.J.; Ortiz Pulido, R.; Zempoalteca Ramírez, R.; Coria-Avila, G.A.; García, L.I. Electrophysiological Characterization of Cerebellar Responses during Exploration and Grooming Behaviors in a Rat Model of Parkinsonism. Brain Sci. 2023, 13, 537. https://doi.org/10.3390/brainsci13040537
Vásquez-Celaya L, Marín-Márquez G, Manzo J, Carrillo-Castilla P, Martínez AJ, Ortiz Pulido R, Zempoalteca Ramírez R, Coria-Avila GA, García LI. Electrophysiological Characterization of Cerebellar Responses during Exploration and Grooming Behaviors in a Rat Model of Parkinsonism. Brain Sciences. 2023; 13(4):537. https://doi.org/10.3390/brainsci13040537
Chicago/Turabian StyleVásquez-Celaya, Lizbeth, Gerardo Marín-Márquez, Jorge Manzo, Porfirio Carrillo-Castilla, Armando Jesús Martínez, Ricardo Ortiz Pulido, René Zempoalteca Ramírez, Genaro A. Coria-Avila, and Luis I. García. 2023. "Electrophysiological Characterization of Cerebellar Responses during Exploration and Grooming Behaviors in a Rat Model of Parkinsonism" Brain Sciences 13, no. 4: 537. https://doi.org/10.3390/brainsci13040537










