Cyclin-Dependent Kinase Inhibitor 2A/B Homozygous Deletion Prediction and Survival Analysis
Abstract
:1. Introduction
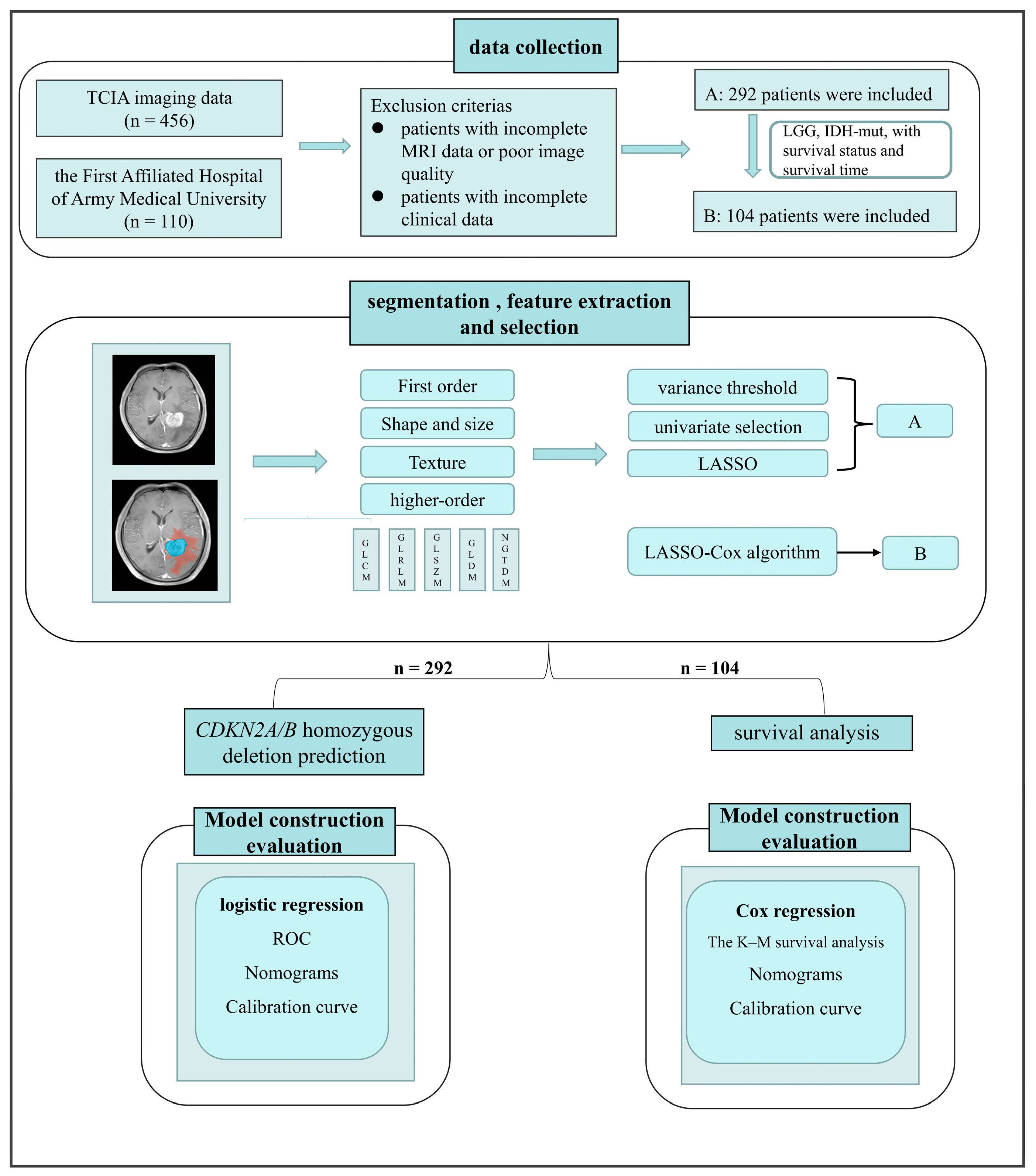
2. Materials and Methods
2.1. Study Population
2.2. CDKN2A/B Pathological Testing
2.3. MRI Acquisition
- (1)
- Trio TIM: 3T-MR and Gd-T1W were obtained at repetition times of 180–459 ms (echo time: 3 ms, section thickness: 2.0–5.0 mm). The repetition time for FLAIR was 7000 ms (echo time: 79 ms, section thickness: 5.0 mm).
- (2)
- Spectra: 3T-MR and Gd-T1W were obtained at repetition times of 300–400 ms (echo time: 3–4 ms, section thickness: 2.0–5.0 mm). The repetition time for FLAIR was 9000 ms (echo time: 81 ms, section thickness: 5.0 mm).
- (3)
- Avanto: 1.5T-MR and Gd-T1W were obtained at a repetition time of 204 ms (echo time: 5 ms, section thickness: 5.0 mm). The repetition time of FLAIR was 8460 ms (echo time: 134–136 ms, section thickness: 5.0 mm).
- (4)
- MAGNETOM_ESSENZA: 1.5T-MR and Gd-T1W were obtained at a repetition time of 250 ms (echo time: 5 ms, section thickness: 5.0 mm). The repetition time for FLAIR was 8000 ms (echo time: 84 ms, section thickness: 5.0 mm).
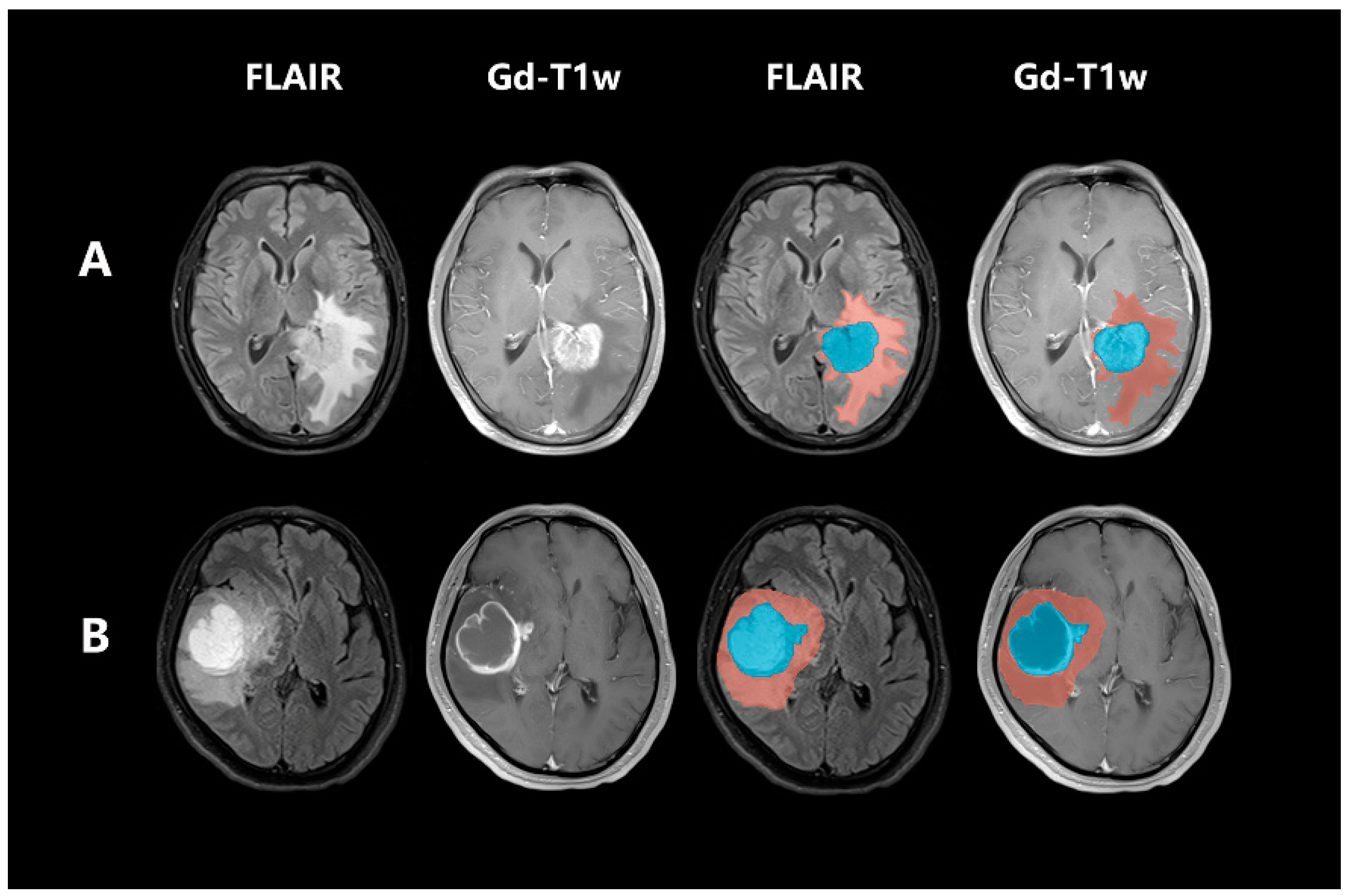
2.4. Post-Acquisition Preprocessing and Segmentation
2.5. Radiomic Feature Extraction and Radiomic Signature Construction
2.6. Clinical and Radiological Factors for Predicting CDKN2A/B
2.7. Prognostic Value Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics
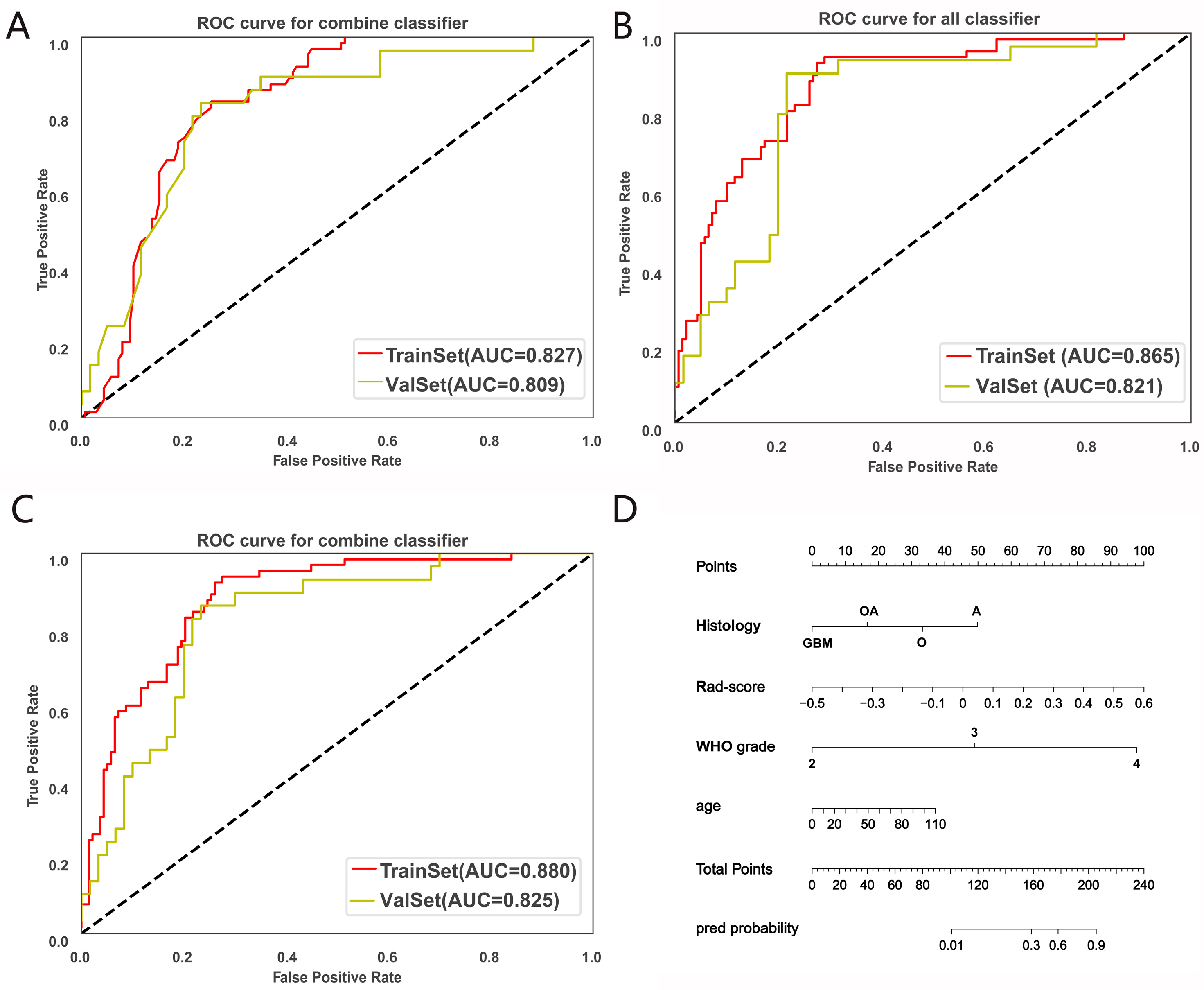
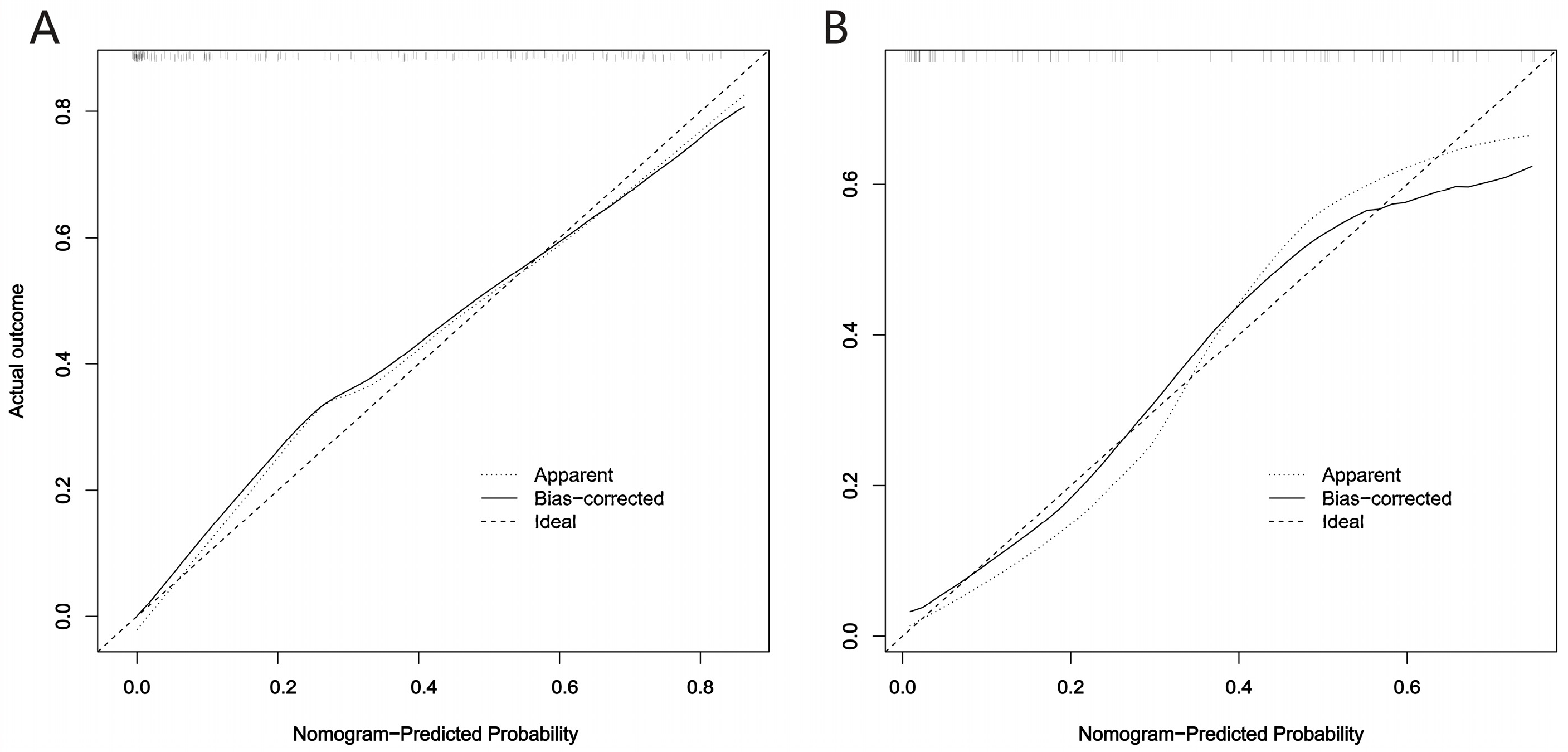
3.2. CDKN2A/B Predictive Model Construction and Evaluation
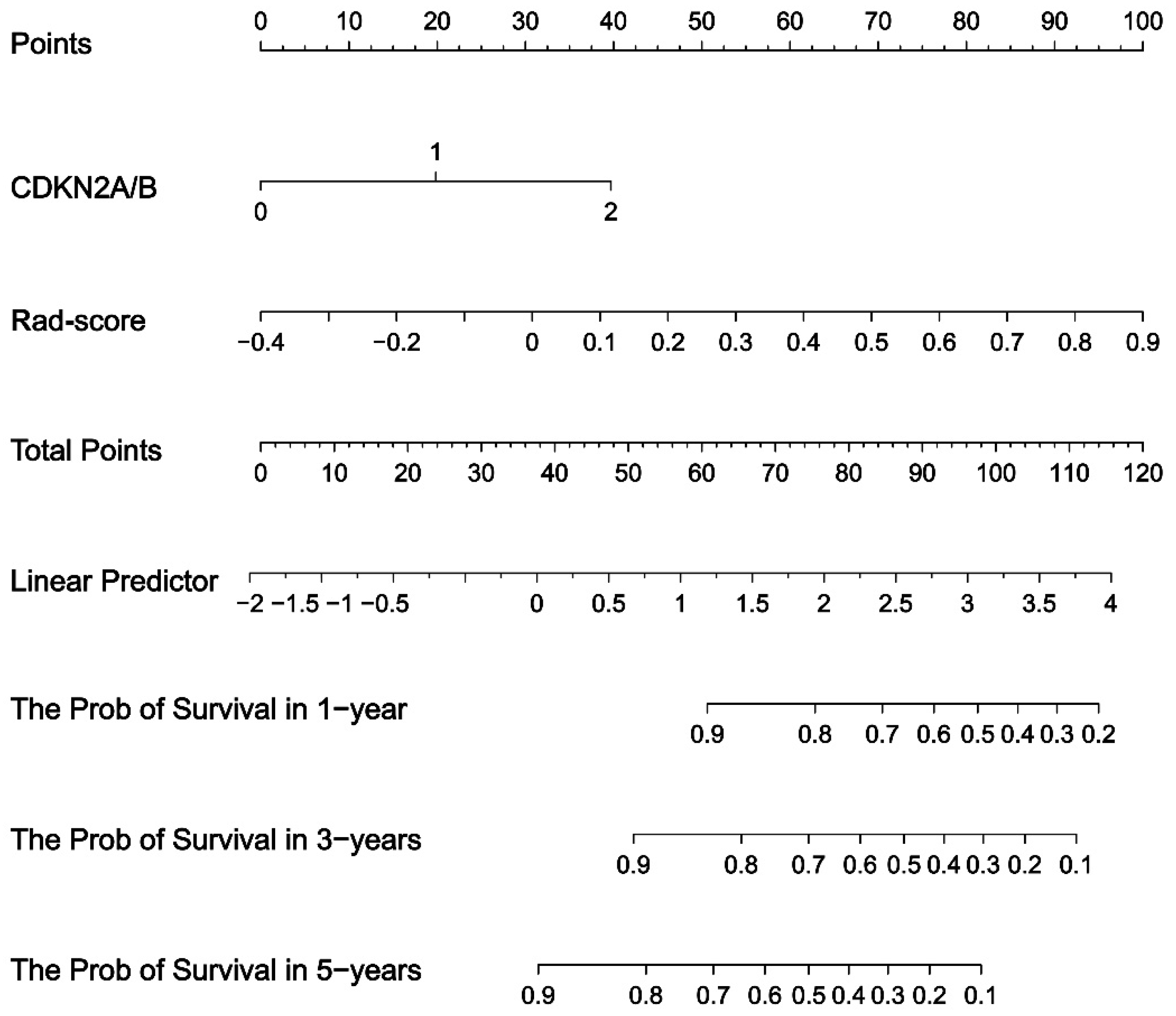
3.3. Prognostic Value of the Fusion Radiomics Signature and Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary Brain Tumours in Adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Prim. 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. Idh1 and Idh2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Alfaro-Munoz, K.D.; Heathcock, L.E.; van Thuijl, H.F.; Gilbert, M.R.; Armstrong, T.S.; Sulman, E.P.; Cahill, D.P.; Vera-Bolanos, E.; et al. Idh Mutation Status and Role of Who Grade and Mitotic Index in Overall Survival in Grade Ii-Iii Diffuse Gliomas. Acta Neuropathol. 2015, 129, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Alentorn, A.; Dehais, C.; Ducray, F.; Carpentier, C.; Mokhtari, K.; Figarella-Branger, D.; Chinot, O.; Cohen-Moyal, E.; Ramirez, C.; Loiseau, H.; et al. Allelic Loss of 9p21.3 Is a Prognostic Factor in 1p/19q Codeleted Anaplastic Gliomas. Neurology 2015, 85, 1325–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, A.; Leyland-Jones, B.; Dey, N.; De, P. A Combination of the Pi3k Pathway Inhibitor Plus Cell Cycle Pathway Inhibitor to Combat Endocrine Resistance in Hormone Receptor-Positive Breast Cancer: A Genomic Algorithm-Based Treatment Approach. Am. J. Cancer Res. 2018, 8, 2359–2376. [Google Scholar]
- Ichimura, K.; Schmidt, E.E.; Goike, H.M.; Collins, V.P. Human Glioblastomas with No Alterations of the Cdkn2a (P16ink4a, Mts1) and Cdk4 Genes Have Frequent Mutations of the Retinoblastoma Gene. Oncogene 1996, 13, 1065–1072. [Google Scholar]
- Liu, L.; Dilworth, D.; Gao, L.; Monzon, J.; Summers, A.; Lassam, N.; Hogg, D. Mutation of the Cdkn2a 5’ Utr Creates an Aberrant Initiation Codon and Predisposes to Melanoma. Nat. Genet. 1999, 21, 128–132. [Google Scholar] [CrossRef]
- Cimino, P.J.; Holland, E.C. Targeted Copy Number Analysis Outperforms Histologic Grading in Predicting Patient Survival for Who Grades Ii/Iii Idh-Mutant Astrocytomas. Neuro Oncol. 2019, 21, 819–821. [Google Scholar] [CrossRef]
- Aoki, K.; Nakamura, H.; Suzuki, H.; Matsuo, K.; Kataoka, K.; Shimamura, T.; Motomura, K.; Ohka, F.; Shiina, S.; Yamamoto, T.; et al. Prognostic Relevance of Genetic Alterations in Diffuse Lower-Grade Gliomas. Neuro Oncol. 2018, 20, 66–77. [Google Scholar] [CrossRef] [Green Version]
- Appin, C.L.; Brat, D.J. Biomarker-Driven Diagnosis of Diffuse Gliomas. Mol. Asp. Med. 2015, 45, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Appay, R.; Dehais, C.; Maurage, C.A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. Cdkn2a Homozygous Deletion Is a Strong Adverse Prognosis Factor in Diffuse Malignant Idh-Mutant Gliomas. Neuro Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Juratli, T.A.; Qin, N.; Cahill, D.P.; Filbin, M.G. Molecular Pathogenesis and Therapeutic Implications in Pediatric High-Grade Gliomas. Pharmacol. Ther. 2018, 182, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 Who Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Zhou, H.; Vallières, M.; Bai, H.X.; Su, C.; Tang, H.; Oldridge, D.; Zhang, Z.; Xiao, B.; Liao, W.; Tao, Y.; et al. Mri Features Predict Survival and Molecular Markers in Diffuse Lower-Grade Gliomas. Neuro Oncol. 2021, 19, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, A.; Zimmermann, M.; Kitzwögerer, M.; Oberndorfer, S.; Rössler, K.; Dörfler, A.; Buchfelder, M.; Heinz, G. Mr Imaging-Derived Oxygen Metabolism and Neovascularization Characterization for Grading and Idh Gene Mutation Detection of Gliomas. Radiology 2017, 283, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Lasocki, A.; Anjari, M.; Örs Kokurcan, S.; Thust, S.C. Conventional Mri Features of Adult Diffuse Glioma Molecular Subtypes: A Systematic Review. Neuroradiology 2021, 63, 353–362. [Google Scholar] [CrossRef]
- Rogers, W.; Seetha, S.T.; Refaee, T.A.G.; Lieverse, R.I.Y.; Granzier, R.W.Y.; Ibrahim, A.; Keek, S.A.; Sanduleanu, S.; Primakov, S.P.; Beuque, M.P.L.; et al. Radiomics: From Qualitative to Quantitative Imaging. Br. J. Radiol. 2020, 93, 20190948. [Google Scholar] [CrossRef]
- Kickingereder, P.; Neuberger, U.; Bonekamp, D.; Piechotta, P.L.; Götz, M.; Wick, A.; Sill, M.; Kratz, A.; Shinohara, R.T.; Jones, D.T.W.; et al. Radiomic Subtyping Improves Disease Stratification Beyond Key Molecular, Clinical, and Standard Imaging Characteristics in Patients with Glioblastoma. Neuro Oncol. 2018, 20, 848–857. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More Than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The Bridge between Medical Imaging and Personalized Medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The Process and the Challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kickingereder, P.; Bonekamp, D.; Nowosielski, M.; Kratz, A.; Sill, M.; Burth, S.; Wick, A.; Eidel, O.; Schlemmer, H.P.; Radbruch, A.; et al. Radiogenomics of Glioblastoma: Machine Learning-Based Classification of Molecular Characteristics by Using Multiparametric and Multiregional Mr Imaging Features. Radiology 2016, 281, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Nowak, J.; Jünger, S.T.; Huflage, H.; Seidel, C.; Hohm, A.; Vandergrift, L.A.; von Hoff, K.; Rutkowski, S.; Pietsch, T.; Warmuth-Metz, M. Mri Phenotype of Rela-Fused Pediatric Supratentorial Ependymoma. Clin. Neuroradiol. 2019, 29, 595–604. [Google Scholar] [CrossRef]

| CDKN2A/B Homozygous Deletion | |||||
|---|---|---|---|---|---|
| NO | YES | p-Value | SMD | ||
| N | 198 | 94 | |||
| Age (median [IQR]) | 41.50 [24.00, 57.75] | 60.00 [52.25, 66.00] | <0.001 | 0.856 | |
| Gender (%) | Female | 111 (56.1) | 61 (64.9) | 0.192 | 0.181 |
| Male | 87 (43.9) | 33 (35.1) | |||
| Histology | Astrocytoma | 43 (21.7) | 8 (8.5) | <0.001 | 1.249 |
| Oligoastrocytoma | 27 (13.6) | 2 (2.1) | |||
| Oligodendroglioma | 51 (25.8) | 1 (1.1) | . | ||
| GBM | 77 (38.9) | 83 (88.3) | |||
| WHO grade (%) | Ⅱ | 65 (32.8) | 1 (1.1) | <0.001 | 1.262 |
| Ⅲ | 56 (28.3) | 10 (10.6) | |||
| Ⅳ | 77 (38.9) | 83 (88.3) | |||
| Dataset | Model | AUC | 95%CI | ACC | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Training set | radomics | 0.865 | 0.820~0.909 | 0.793 | 0.631 | 0.87 |
| clinic | 0.824 | 0.776~0.871 | 0.783 | 0.723 | 0.812 | |
| radiomic + clinic | 0.88 | 0.840~0.918 | 0.793 | 0.662 | 0.855 | |
| Validation set | radiomic | 0.821 | 0.741~0.890 | 0.708 | 0.483 | 0.817 |
| clinic | 0.802 | 0.722~0.875 | 0.753 | 0.655 | 0.8 | |
| radiomic + clinic | 0.825 | 0.743~0.894 | 0.73 | 0.517 | 0.833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, L.; Luo, T.; Nie, C.; Fan, R.; Li, D.; Yang, R.; Zhou, C.; Li, Q.; Hu, X.; et al. Cyclin-Dependent Kinase Inhibitor 2A/B Homozygous Deletion Prediction and Survival Analysis. Brain Sci. 2023, 13, 548. https://doi.org/10.3390/brainsci13040548
Yang J, Li L, Luo T, Nie C, Fan R, Li D, Yang R, Zhou C, Li Q, Hu X, et al. Cyclin-Dependent Kinase Inhibitor 2A/B Homozygous Deletion Prediction and Survival Analysis. Brain Sciences. 2023; 13(4):548. https://doi.org/10.3390/brainsci13040548
Chicago/Turabian StyleYang, Jing, Lei Li, Tao Luo, Chengsong Nie, Rui Fan, Deqiang Li, Rui Yang, Changru Zhou, Qian Li, Xiaofei Hu, and et al. 2023. "Cyclin-Dependent Kinase Inhibitor 2A/B Homozygous Deletion Prediction and Survival Analysis" Brain Sciences 13, no. 4: 548. https://doi.org/10.3390/brainsci13040548





