Abstract
The paradigm of the framing of Parkinson’s disease (PD) has undergone significant revision in recent years, making this neurodegenerative disease a multi-behavioral disorder rather than a purely motor disease. PD affects not only the “classic” substantia nigra at the subthalamic nuclei level but also the nerve nuclei, which are responsible for sleep regulation. Sleep disturbances are the clinical manifestations of Parkinson’s disease that most negatively affect the quality of life of patients and their caregivers. First-choice treatments for Parkinson’s disease determine amazing effects on improving motor functions. However, it is still little known whether they can affect the quantity and quality of sleep in these patients. In this perspective article, we will analyze the treatments available for this specific clinical setting, hypothesizing a therapeutic approach in relation to neurodegenerative disease state.
1. Introduction
Parkinson’s disease affects 1–2% of the population over 60 years of age. Its incidence and prevalence are constantly increasing, especially in the last decades of life [1,2]. Due to the age of onset and the complexity and manifestation of symptoms, a comprehensive geriatric assessment is considered the best-suited approach for this disease.
Recently, Parkinson’s disease has been recognized as a multisystemic disorder, and while the inflammatory pathogenesis for years was only the prerogative of the cardiovascular system [3], more and more evidence indicates that even neurodegeneration is secondary to genetic causes and to alterations of the inflammatory state [4,5].
This is an important upgrade in comparison with preexisting theories focusing only on the dopaminergic neurons of the substantia nigra [6,7,8,9,10,11,12,13]. Thus, in the absence of treatments attenuating or reverting neurodegeneration, the symptomatic management of the disease, targeting both motor and nonmotor symptoms, has become very important. Over the years, the therapeutic approach to motor symptoms has produced surprising results in patients who fully respond to dopamine replacement therapy [14].
However, more remains to be done for non-motor symptoms. Given the growing number of elderly and multimorbid patients and the increasingly demanding management of chronic and advanced stages of the disease, this approach is particularly relevant to the quality of life of patients and their caregivers [15].
Therefore, regarding the plethora of drugs available for the treatment of Parkinson’s disease, the choice must be accurate and suited to the patient’s needs. Any treatment worsening the quality of life of patients or their caregivers should be avoided [16].
Under the umbrella of non-motor symptoms, sleep disturbances are among the most common and those with the greatest impact on a patient’s quality of life [17,18]. It is estimated that nearly half of the patients with Parkinson’s disease suffer from sleep disturbances. Surprisingly, many patients underreport the symptom simply because they do not consider it as part of the disease [17,18,19,20]. Thus, the correct classification of sleep disorders in Parkinson’s disease is relevant. The aim of this perspective article is to provide a correct framing of sleep disturbances in Parkinson’s disease in relation to the Braak’s scale. Finally, we will try to identify the most correct treatment in relation to the disease state.
2. Sleep Disorders in Parkinson’s Disease: A Motley Melting Pot
Symptom-wise, sleep disturbances in Parkinson’s occur in variegate ways. In this section, we will analyze the different ways in which sleep disorders can occur. Insomnia is the most frequent sleep disorder in Parkinson’s disease with the prevalence, ranging from 30% to 80% of affected patients, increasing as the disease progresses [21,22]. Insomnia is difficulty in initiating or maintaining sleep. In patients with Parkinson’s disease, difficulty in maintaining sleep (with early awakenings and sleep fragmentation) is more frequently described than difficulty in initiating sleep [23]. The sleep and circadian rhythm regulatory centers are affected by the neurodegeneration typical of Parkinson’s disease, which lays the pathophysiological basis for the development of insomnia [24]. This substrate combined with the presence of the off symptoms contributes to the development and aggravation of insomnia as the disease progresses [25,26].
As for the diagnosis, in addition to an accurate medical history, clinicians have at their disposal a series of questionnaires, some of these specifically validated for Parkinson’s disease (PDSS, PDSS-II, and SCOPA). In the most severe cases or the differential diagnosis of comorbidities, polysomnography is indicated [27,28]. Another way of presentation of sleep disturbances in Parkinson’s disease is restless legs syndrome (RLS). A meta-analysis clearly shows that this syndrome affects about 15% of Parkinsonian patients [29]. This disorder occurs with the urge to move the legs and is usually associated with leg discomfort. Symptoms generally begin in the late afternoon or during the night, causing a great deal of discomfort to the patient and her/his partner. Regarding the etiology, there are three pathogenetic hypotheses: (one) in relation to the response to dopaminergic supplementation, Parkinson’s disease and RLS share a common dopaminergic degeneration and a possible genetic connection [30]; (two) RLS in Parkinson’s disease has a different etiology than idiopathic RLS; (three) RLS and Parkinson’s disease are two different pathologies [31].
As evident from these hypotheses, there is also a type of RLS that occurs independently of Parkinson’s disease [31]. The diagnostic criteria of RLS are described in the International Classification of Sleep Disorders [32]. Particular attention is needed in the diagnosis of this syndrome since it is capable of imitating other common symptoms, especially in elderly patients such as myalgia, leg cramps, and arthritis. Another sleep disorder typical of Parkinson’s disease is rapid eye movement sleep behavior disorder (RBD). This disorder is parasomnia and consists of repeated vocalizations during sleep or complex motor behaviors during REM sleep. Polysomnographic studies have shown that the loss of muscle tone typical of the REM phase is lost in this disorder [32]. Approximately 24% of patients with Parkinson’s disease are affected by this disorder, compared with 3.4% of affected individuals in the general population [33]. Similar rates were also found in another study [34]. It is important to highlight how idiopathic RBD is considered a strong predictor of synucleinopathies. A multicenter study reported a conversion rate from RBD to Parkinson’s disease of approximately 6.3% annually and 73.5% after a 12-year follow-up period [35]. RBD precedes the onset of Parkinson’s disease by about 13 years [36]. As far as pathophysiology is concerned, RBD has been associated with dysfunction in the pontomedullary and other structures regulating REM sleep, in particular, the locus coeruleus [37]. Also, in this case, in addition to the diagnostic criteria of the International Classification of Sleep Disorders, a specific diagnostic questionnaire was drawn up [38].
A consequence of sleep disturbances in patients with Parkinson’s disease is excessive daytime sleepiness (EDS) which occurs in 20 to 75% of patients with Parkinson’s disease [39,40,41]. This disorder consists of difficulty staying awake and alert during the day [32]. An accredited etiopathogenetic hypothesis ascribes this disorder to hypothalamic neurodegeneration and different nuclei of the brain stem responsible for the sleep–wake cycle [42]. As regards the diagnostic process, the Epworth sleepiness scale (ESS) is generally used as a screening tool. It is important to exclude other diseases that can cause daytime sleepiness such as RLS, OSA, and RBD. Finally, sleep disorders related to respiratory problems, in particular obstructive sleep apnea, should be accounted giving the prevalence of 20–60% of patients with Parkinson’s disease [43,44]. In Parkinsonian patients, laryngopharyngeal motor dysfunction with occlusion of the upper respiratory tract is the cause of obstructive apneas [45]. As far as diagnosis is concerned, polysomnography is the gold standard exam, also validated in patients with Parkinson’s disease [46,47]. Sleep disorders in Parkinson’s disease are a heterogeneous melting pot of disorders. It is difficult to draw a guideline to guide clinicians in the treatment of these pathologies since many patients do not even ascribe the problem to Parkinson’s disease. Except for RBD, which can be framed as a prodrome of Parkinson’s disease, the rest of the sleep disorders generally present with a more advanced state of disease. It will be the task of the clinician who, with a careful history and a comprehensive assessment, will be able to diagnose and treat these disorders. An upgrade toward a comprehensive assessment of Parkinson’s disease patients cannot be postponed. There is increasing evidence that sleep disturbances not only correlate with a worse quality of life but also trigger a pathophysiological mechanism that exacerbates major depressive states [20]. Especially in the later stages of life with Parkinson’s disease, depression and nonmotor symptoms, rather than motor symptoms, have a greater impact on the quality of life of patients [48]. Therefore, patients with Parkinson’s require a comprehensive assessment to stop this vicious circle (neurodegenerative disease -> depression -> neurodegenerative disease) that, in the long term, leads to disability [49]. It will be interesting in the future to try to identify the primum movens of this vicious circle, also in consideration of its pathogenetic affinity with sleep disorders.
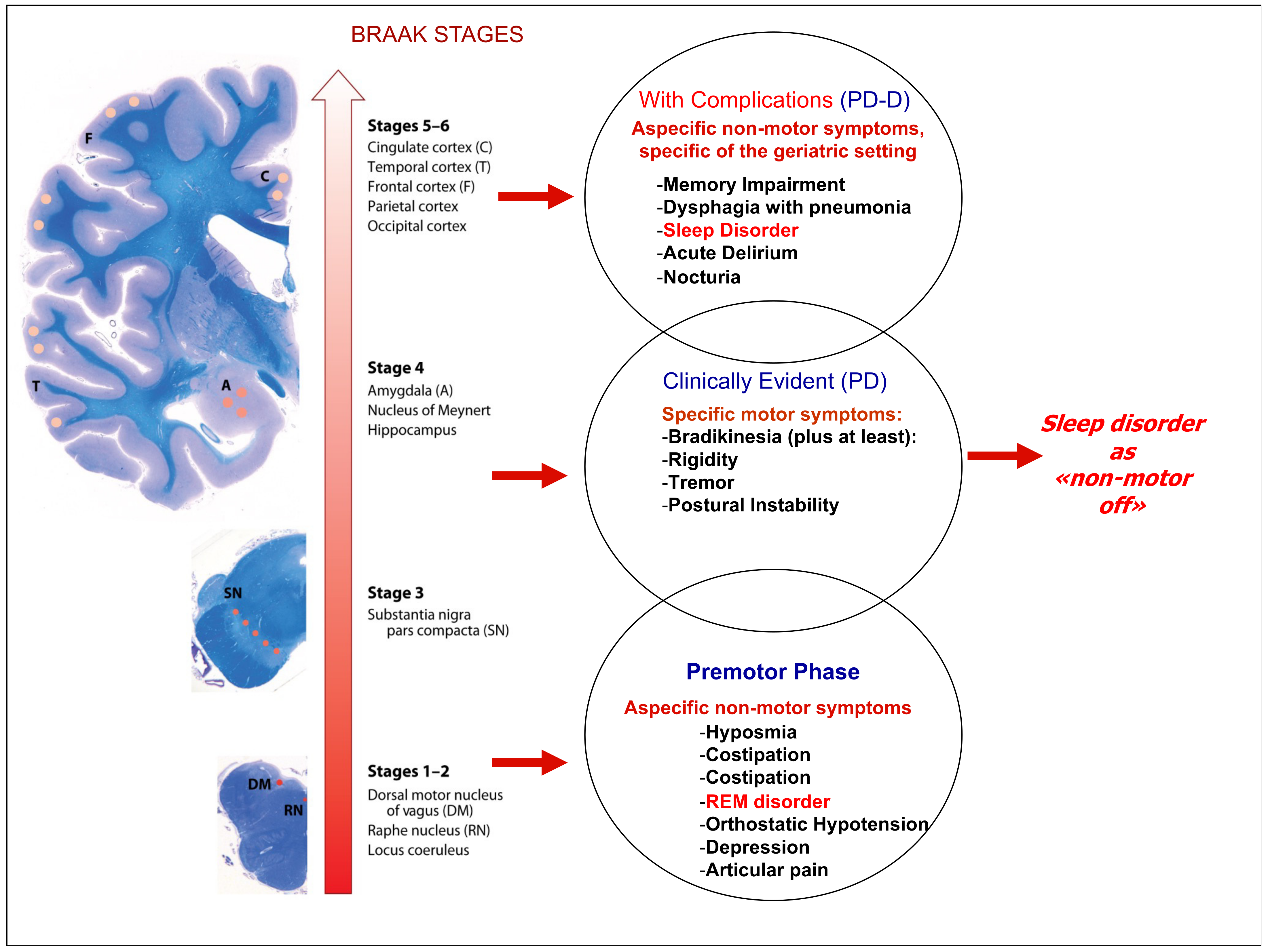
3. The Braak Scale: An Old Staging with a New Awareness
For more than 20 years, Braak and colleagues [42] have postulated the hypothesis of progressive neurodegeneration in the etiology of sporadic Parkinson’s disease, and although there are numerous scales for staging Parkinson’s [50], the Braak scale is the one that best explains the pathophysiology of the disease. Regardless of the underlying etiological cause, over time, this hypothesis has been examined in various clinical and preclinical settings and was recently confirmed [51]. The concept of progressive neurodegeneration that inexorably advances and affects more and more brain areas is supported by the clinical manifestations of the disease. Six microscopically additive disease stages are described, with typical histological lesions (Lewy neurites and Lewy bodies): (one) lesions in the dorsal IX/X motor nucleus and/or intermediate reticular zone; (two) lesions in caudal raphe nuclei, gigantocellular reticular nucleus, and coeruleus—subcoeruleus complex; (three) midbrain lesions, particularly in the pars compacta of the substantia nigra; (four) prosencephalic lesions. Cortical involvement is confined to the temporal mesocortex (transentorhinal region) and allocortex (CA2-plexus). The neocortex is unaffected; (five) lesions in high-order sensory association areas of the neocortex and prefrontal neocortex; (six) lesions in first-order sensory association areas of the neocortex and premotor areas, occasionally mild changes in primary sensory areas and the primary motor field.
It should be emphasized that the motor symptoms appear during the late phase of the disease progression, Braak stages 3–4 (39). The long prodromal phase corresponds to a neurodegeneration that remains in the subclinical state. RBD, which by many authors is considered a prodrome of Parkinson’s disease, is caused precisely by a degeneration at the level of the locus coeruleus which is affected in the initial stages of the disease (Braak stage two). Unlike RBD, other sleep disorders generally occur at or shortly after the onset of motor symptoms. The mode of presentation varies from patient to patient but with the progression of the disease and the worsening of motor symptoms less controlled by pharmacological treatment, there is a worsening presentation of sleep disturbances. This, almost schematic, trend must guide the clinical approach to stabilizing the sleep–wake cycle in the prodromal phases of the disease, to prevent dopaminergic neurodegeneration in the intermediate phases of the disease up to treatment with drugs that also target cognitive impairment associated-conditions during the final stages of the disease.
4. Clinical Implications and Available Treatments for Sleep Disorders in Parkinson’s Disease
The main clinical implication of sleep disorders is the major negative impact on the quality of life of patients with Parkinson’s disease. Reduced quality of life very often results in a greater tendency to develop a mood disorder. In recent years, clinicians have begun to focus their attention on treating non-motor symptoms, particularly depression. Recent evidence indicates that drugs such as SSRI and SNRI, in young subjects’ tricyclic antidepressants, dopamine agonists, and behavioral therapy have good efficacy in the treatment of depression in patients with Parkinson’s disease.
Insomnia and depression are closely related to Parkinson’s disease [23]. Patients with insomnia usually have a more advanced state of disease and are more prone to symptoms due to the wearing-off of the levodopa effect. They also show problems such as autonomic dysfunction, hallucinations, and postural instability [23,52]. The correct treatment of insomnia in Parkinson’s disease starts with the careful collection of patients’ histories. Insomnia can occur during the night as an end-of-dose effect of dopamine. For this reason, the use of an additional tablet of levodopa, prolonged-release levodopa, or a dopamine agonist finds more and more space in this clinical setting. Recent evidence indicates that drugs such as eszopiclone and melatonin also find their place in the treatment of insomnia related to Parkinson’s disease, especially in the early phase of the disease [53].
Related to insomnia or the underlying cause of insomnia, is restless leg syndrome (RLS). Dopamine agonists such as pramipexole, rotigotine, and ropinirole have their therapeutic rationale for treating it [54,55,56]. Careful attention is needed in the use of these drugs since, in some cases, they lead to a worsening of nocturnal symptoms after an initial benefit or could produce impulse compulsive disorders (ICDs) with nocturnal activity and sleep interruption [57]. In these cases, the treatment must be suspended and replaced either with a long-acting dopaminergic drug or with a drug acting beyond dopamine stimulation [58]. In this regard, good evidence of efficacy has been found with the use of gabapentin or pregabalin [59,60].
The clinical implications of rapid eye movement sleep behavior disorder (RBD) are very important because when this syndrome occurs as a comorbidity in Parkinson’s disease, it is associated with increased motor dysfunction, hallucinations, cognitive impairment, and autonomic dysfunction, especially in the advanced phase of the disease [61,62]. There is still little evidence of an effective treatment for RBD, though some evidence of efficacy has been obtained with melatonin or clonazepam [63,64].
Older age is characterized by a worse presentation of disease symptoms and is also associated with excessive daytime sleepiness (EDS). EDS is often also the consequence of the breathing disorders associated with Parkinson’s disease. Once again, a correct diagnosis is important since in OSAs independent of Parkinson’s disease, the treatment of choice is C-PAP, while in OSAs caused by Parkinson’s disease, the use of controlled release formulations of carbidopa/levodopa at bedtime may improve symptoms [65].
It is evident that there is a lack of effective treatments in most of the analyzed conditions. Furthermore, to set up a correct therapeutic procedure, a precise diagnosis is required, together with a good knowledge of the different ways in which sleep disorders can occur in relation to the degree of neurodegeneration. Finally, it is necessary to modulate the therapeutic treatments based on the caregiver’s feedback, especially in the most advanced stages of the disease. The application of these procedures allows the avoidance of pharmacological overshooting that can lead to a decrease in alertness with very serious consequences such as inhalations of ingests and consequent pneumonia.
5. Available Drugs and State of Art of Treatment
Although sleep disorders in Parkinson’s disease have been a clinical problem receiving interest from the scientific community for many years, no effective and lasting therapeutic approach has still been validated. Probably, the primary reason for the existing gap between clinical need and lack of adequate therapies is due to the heterogeneity, not only of the clinical manifestations but also of the type of patient in which these clinical manifestations appear. With the need to improve patients’ symptoms, numerous therapeutic approaches have been attempted, whose clinical efficacy is substantially anecdotal and not evidence-based. The complexity of the patients and the heterogeneous responses to the different pharmacological treatments must direct clinicians to have an excellent knowledge of the numerous drugs available to avoid exposing the patient to the sedative actions of these drugs. In this clinical setting, there is no “one drug fits all”, though only an adequate comprehensive assessment oriented to the patient and the living environment can help physicians towards a multidomain treatment, including prescribing or sometimes deprescribing certain medications.
Among the first drugs used in this clinical context are melatonin and its synthetic derivative (Ramelteon and Agomelatine), agonists of melatonin receptors. Melatonin is an agonist of the MT1, MT2, and MT3 receptors, has a half-life of about 4 h, and is one of the first drugs used in patients with insomnia. Experiences in patients with Parkinson’s disease indicate that melatonin can improve the quality of sleep [66,67]. We have previously found that in patients with Parkinson’s disease, insomnia does not manifest itself as difficulty falling asleep, but rather as difficulty staying asleep. In this regard, various therapeutic approaches are available, and one of these is the use of prolonged-release melatonin. In two recent studies, a 2 mg dose of prolonged-release melatonin was associated with significant improvements in night-time frequency and nocturnal voided volumes, and beneficial effects on sleep quality with improved nonmotor symptoms and quality of life in PD patients [68,69]. In patients in whom a coexistence between sleep disorder and depression of mood emerges at the visit, the use of agomelatine at a dosage of 12.5 mg, titrated up to 50 mg, could be useful [70]. Ramelteon is the synthetic derivative of melatonin mostly used in patients with Parkinson’s disease. It acts as an MT1 and MT2 receptor agonist and has a half-life of approximately 2.5 h. At an 8 mg dose, ramelteon was effective in the treatment of sleep disorders in Parkinson’s patients, particularly in RBD [71].
There is little data in the literature on the use of benzodiazepines in patients with Parkinson’s disease, although in clinical practice they are often used above all for the relief of depressive symptoms and for their hypnotic action. These drugs act as positive allosteric modulators of the GABA receptor and differ substantially in the length of half-life. In light of the scarcity of significant evidence, the use of benzodiazepines in patients with Parkinson’s disease should be weighed on a case-by-case basis, especially in relation to their side effects, one of which is inhalation pneumonia. A case-control study of over 550,000 patients found that benzodiazepine use is associated with an increased risk of pneumonia in elderly patients with Parkinson’s disease [72]. It is therefore essential that the indication for the use of these drugs, in this particular setting, should be managed by expert physicians. Among the various benzodiazepines, more consistent data have emerged on the use of clonazepam for RBD. Clonazepam is a benzodiazepine with a long half-life (30–40 h) and is indicated by many as the first-line treatment in RBD. The evidence for the use of clonazepam in RBD is supported by studies with small sample sizes, some of which did not reach statistical significance when compared to a placebo [73,74,75]. Considering the long half-life and the possibility of accumulation phenomena, especially in elderly patients, the use of clonazepam in this clinical setting requires careful attention. Among the antidepressants, the one with the greatest sedative action, trazodone, is often used off label as a hypnotic inducer in elderly patients. This molecule acts as an antagonist of the serotonin 5HT2a/c receptor, the stimulation of which has a known antidopaminergic effect. In relation to this function, trazodone improved depressive symptoms and motor function in patients with Parkinson’s disease [76]. In patients with Parkinson’s, there are few data concerning the use of trazodone as a hypnotic inducer. However, a very recent experience conducted on 31 patients demonstrated its efficacy and tolerability at a sedative hypnotic dosage (50 mg) in this clinical setting [77]. The efficacy of trazodone as a hypnotic inducer is probably also due to its biphasic half-life with a first phase of 3–6 h and a second phase of 5–6 h. This biphasic effect is particularly welcome in Parkinsonian patients where insomnia is mainly due to difficulty staying asleep.
Among the nonbenzodiazepine allosteric modulators of the GABA receptor, z-compounds are often used in clinical practice as hypnotic agents due to their reduced sedative effects and therapeutic handling [53,78]. Randomized controlled trials on the use of these drugs in the setting of our interest are scarce. More significant experiences have been made with eszoplicone, which has demonstrated excellent tolerance and good clinical efficacy as a hypnoinducer in Parkinson’s patients [79].
Among the nonbenzodiazepine allosteric modulators of the GABA receptor, drugs such as gabapentin and pregabalin have a more defined and codified therapeutic niche for sleep disorders in Parkinson’s patients. In fact, there are numerous pieces of evidence, especially for the long-release pharmaceutical formulation of gabapentin, of their effectiveness in restless leg syndrome. In geriatric patients, these drugs are well-tolerated, though they require careful evaluation of renal function before starting and during treatment [80,81,82].
Few randomized controlled trials are available for the use of antipsychotic drugs in patients with sleep disorders and Parkinson’s disease. The complexity of using these drugs is mainly due to the side effects, in particular sedation, and the need for clinical monitoring at the time of cardiac repolarization, exposing the patient to a greater risk of arrhythmias. There is no indication of these drugs in the initial stages of the disease, while their use will be more appropriate in the very advanced stage. Quetiapine is an atypical antipsychotic drug with low receptor specificity. Antagonized receptors include the histamine H1 receptor and serotonin 2A receptor. Consequently, sedation is intrinsic to the drug’s activity. Due to this receptor specificity, attempts have been made to use quetiapine for the treatment of insomnia, regardless of the presence of Parkinson’s disease, though the results due to the sedative effects, often in the presence of the other approved drugs for insomnia, are largely disappointing and the benefits of using quetiapine do not outweigh the risks [83]. The results of an open-label study have demonstrated that quetiapine can find its place in the treatment of insomnia in patients with Parkinson’s [84]. However, these results need to be confirmed with an appropriate study design that includes the comparison with placebo control or with drugs already approved for insomnia and using an adequate sample size. The effects of quetiapine in improving visual hallucinations in Parkinson’s patients are not related to a normalization of sleep architecture [85]. The effects of clozapine on sleep have not been specifically studied in patients with Parkinson’s disease, though its use may consolidate sleep in psychiatric patients [86].
In patients with Parkinson’s dementia, and consequent behavioral disturbances, low-dose clozapine may have a clinical indication, especially in patients where behavioral disturbances are particularly accentuated [87]. The use of other antipsychotic drugs in patients with sleep disorders and Parkinson’s disease has very little evidence-based validation in the literature and, consequently, their use must be weighed on a case-by-case basis. The use of antipsychotics in Parkinson’s disease is especially indicated in the treatment of psychosis in patients who have a very advanced state of the disease. However, compliance with therapy is low, and about one-third of patients prematurely terminate therapy due to both the side and antidopaminergic effects [88].
Pimavanserin is the most recently developed antipsychotic and has a peculiar mechanism of action that makes it substantially inactive on dopamine receptors. It acts as an antagonist and inverse agonist of serotonin 2A and 2C receptors. It finds indication above- all for hallucinations and delusions associated with psychoses related to Parkinson’s dementia, a fact now corroborated in the literature [89]. Already in phase 1/2 studies, some evidence indicated that pimavanserin could have an effect objectively assessed on the sleep rhythm [90]. Recent findings indicate that this new treatment may improve the quality of sleep both in patients treated for major depressive disorder and in those with psychosis in Parkinson’s disease [91,92].
Among the tricyclic antidepressants, doxepin has been shown to be effective in improving the quality of sleep in patients with Parkinson’s disease, though its use in this setting is not widespread [93].
Despite its efficacy in the treatment of primary insomnia and the prevention of delirium in hospitalized patients [94], Suvorexant has not yet found a validated clinical indication in patients with sleep disorders and Parkinson’s disease. Numerous scientific pieces of evidence justify the use of antidepressant drugs for Parkinson’s disease. However, it is still difficult in this setting to identify a treatment that has independent effects on sleep disturbances alone, rather than depression, given the close relationship between these two conditions in Parkinson’s disease. In addition to the drugs already highlighted, venlafaxine also seems to have a role in this clinical setting [95,96].
An off phase during the night can manifest itself as insomnia and modulation of dopaminergic therapy can be the best treatment. It is therefore essential to frame the sleep disorder presented by the patient with a correct medical history also detailed in the history of pharmacological therapy. There are numerous studies in the literature that have tried to endorse this therapeutic attitude. The use of a dose of levodopa upon awakening during the night as the main therapeutic action in insomnia linked to Parkinson’s disease is an approach that has yet to be validated in the literature, though it is certainly supported by numerous indirect evidence, which indicates that more constant dopaminergic stimulation is effective in this regard. Treatment with a levodopa-carbidopa gastrointestinal gel that achieves a constant therapeutic drug plasma concentration was shown in one study to improve sleep disturbances together with other symptoms in Parkinson’s disease [97].
Dopaminergic agonists are known to have a longer half-life and are less subject to change in pharmacokinetics than levodopa. A transdermal system for the release of rotigotine in patients complaining of sleep disturbances has shown how this treatment could improve the quality of sleep by reducing nocturnal awakenings and improving motor performance upon morning awakening [98]. In patients with advanced Parkinson’s disease, both the immediate-release and prolonged-release pharmaceutical formulations of pramipexole have been shown to be effective in improving the subjective quality of sleep [99]. Ropinirole as an add on to levodopa therapy has also been shown to improve subjective symptoms in patients with Parkinson’s disease, both with immediate-release pharmaceutical formulations and with prolonged-release pharmaceutical formulations in different disease stages [100,101]. Cabergoline therapy as an add on to levodopa monotherapy has also been shown to be effective in improving both polysomnographic parameters and the subjective quality of sleep in patients with idiopathic Parkinson’s disease [102].
Dopaminergic stimulation has also been shown to be effective in treating sleep disorders in Parkinson’s disease other than insomnia. In a randomized controlled study of over 300 patients, the efficacy of levodopa and cabergoline in the treatment of RLS was compared; the study showed a greater efficacy on symptoms for the cabergoline treatment, while patients in the levodopa treatment group reported better tolerability [103]. Pramipexole, rotigotine, and ropinirole have also shown good efficacy in controlling RLS symptoms [104,105,106]. Finally, both immediate-release and extended-release ropinirole have been shown to have a significant effect in mitigating daytime sleepiness episodes in EDS [107].
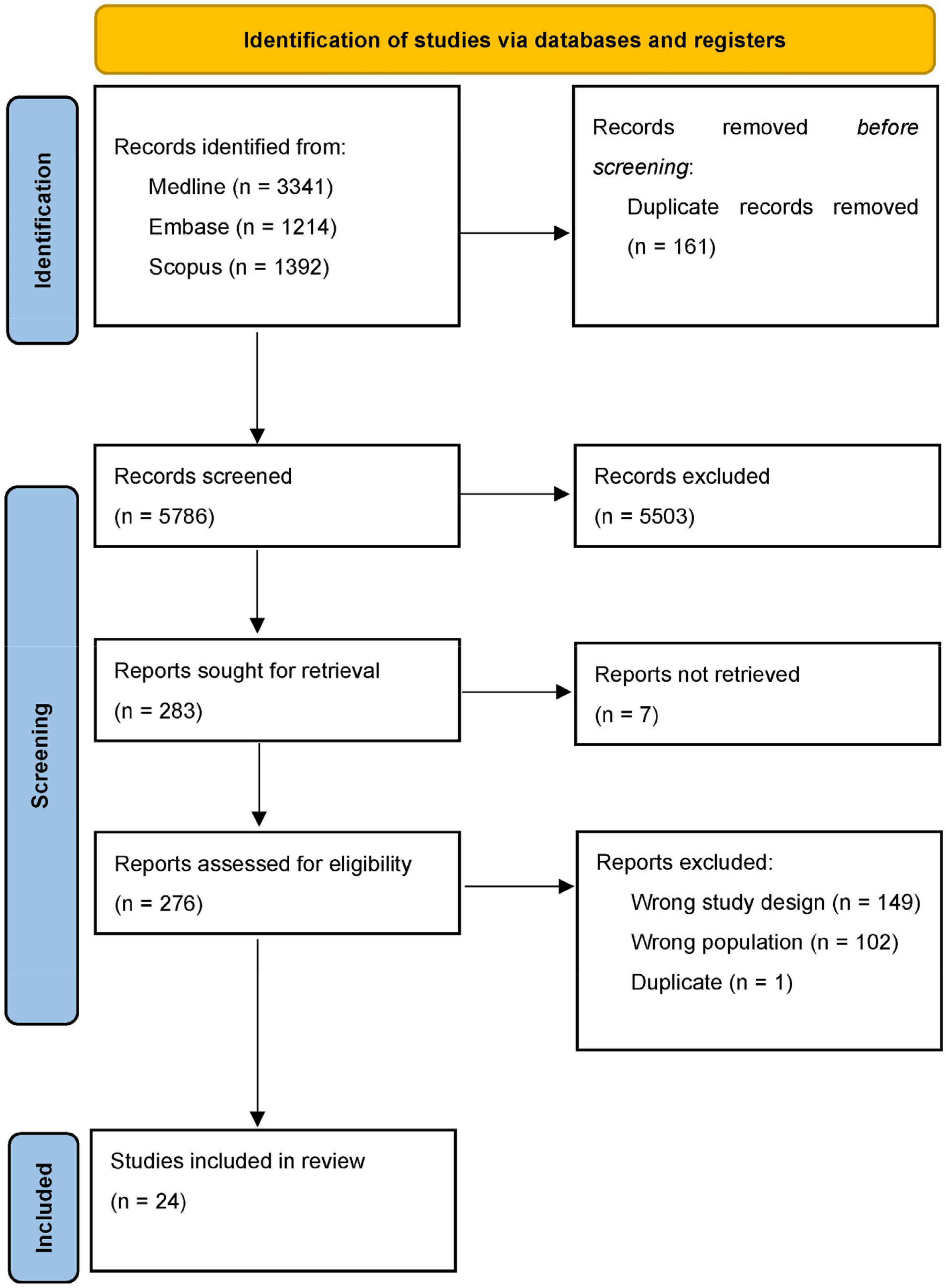
To support our hypothesis postulating the undertreatment of sleep disorder in Parkinson’s disease, two authors (FL and CT) separately screened major medical databases in search of clinical randomized controlled trials conducted in the setting of our interest. The keywords “Parkinson’s disease” and “sleep disorder” and all possible combinations were used to screen the Medline, EMBASE, and Scopus databases, and 5786 articles were screened. The article selection process is summarized in Figure 1 according to a PRISMA diagram. In Table 1 are summarized all the randomized controlled trial regard the setting of our interest.

Figure 1.
PRISMA diagram of selection process.

Table 1.
Summary of the evidence currently available in the literature.
6. Right Medication at Right Disease State
Figure 2 is the mainstay of our therapeutic proposal. It is essential to associate the correct treatment for sleep disturbance with a certain disease state. In the prodromal phases, there are medications such as melatonin or antidepressants such as trazodone or mirtazapine. In the symptomatic phases for motor disturbances, treatment with additional doses of levodopa in the night or with prolonged release formulations of levodopa or dopamine agonists may be useful, supporting the idea that the sleep disorder could be a “non-motor off state”. Finally, in the final stages of the disease, in which modest cognitive impairment with behavior disorders can be present, the use of antipsychotics finds space. In this advanced phase of the disease, normally, many drugs affecting cognition, depression, anxiety, behavior symptoms, and mobility are prescribed with a tailored therapy that could be specific for each patient. This topic was recently emphasized in the context of psychosis, where authors underlined different sleep disorders throughout the course of the disease and different psychosis stages showed distinct abnormalities in sleep quality, architecture, and spindles [128]. These findings altogether suggest that sleep disorders could become a core treatment in different neurodegenerative diseases, such as psychosis, Parkinson’s disease, and dementia [129,130]. In this clinical context, a correct pharmacological treatment can only take place after the comprehensive evaluation of the patient accompanied by a correct pharmacological history. Especially in elderly patient, drug treatment can itself be a cause of clinical worsening and hospitalizations that negatively impact the patient’s prognosis.
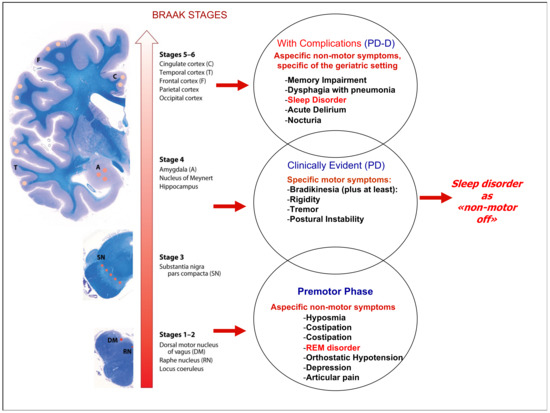
Figure 2.
Correlation between the Braak scale in Parkinson’s disease and symptom presentation. In sleep disorders, the indications for pharmacological therapy depend on the multidimensional evaluation of the patient.
In the advanced stage of neurodegenerative diseases, sleep disorders probably represent a challenge for physicians [131,132], especially geriatricians, where the balance between deprescribing or drug appropriateness could become the key element for maintaining a patient at home.
7. Conclusions
In the world of the geriatric population, polypharmacotherapy frequently occurs. Epidemiological trends indicate that more and more elderly patients are exposed to the risk of being overtreated without a real clinical benefit and to a greater risk of adverse clinical consequences. We have already described how the improper use of sedative drugs in Parkinson’s disease such as benzodiazepines can expose patients to a greater risk of inhalation pneumonia, as reported for other drug classes such as antipsychotics and antidepressants. The treatment of sleep disorders in Parkinson’s disease cannot benefit from dichotomous indications. This clinical problem is strictly dependent on factors such as the stage of the disease and the patient’s insight into the problem. The scenario totally changes in the advanced stages of Parkinson’s dementia. In light of the considerations made, and the available evidence, it is possible to make an indication of a therapeutic attitude rather than a therapeutic indication. The sleep disorders in a patient with Parkinson’s disease must be viewed from a multidimensional perspective. It is essential to indicate therapeutic treatments that are biologically consistent with the stage of the disease. Especially in an elderly patient, the therapeutic indications must be balanced with other pharmacological treatments and the patient’s comorbidities, avoiding the exposure of the patient to sedation and other relevant harmful side effects. In this setting, it will be important in the future to design randomized controlled trials that take into account the heterogeneity of the elderly population with Parkinson’s disease and the different types and modalities of presentation of sleep disorders.
Author Contributions
Conceptualization, F.L., M.M. and C.T.; methodology, C.T. and F.G.; software, M.S.; validation, M.M., F.L. and I.Z.; investigation, F.L.; resources, F.G.; data curation, C.T.; writing—original draft preparation, F.L. and C.T.; writing—review and editing, M.M. and F.G.; visualization, I.Z. and M.S.; supervision, M.M. and F.L.; project administration, F.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, S.; Smailagic, N.; Hyde, C.; Noel-Storr, A.H.; Takwoingi, Y.; McShane, R.; Feng, J. (11)C-PIB-PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2014, 7, CD010386. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Testa, C.; DILorenzo, A.; Parlato, A.; D’Ambrosio, G.; Merolla, A.; Pacileo, M.; Iannuzzo, G.; Gentile, M.; Nugara, C.; Sarullo, F.M.; et al. Exercise for slowing the progression of atherosclerotic process: Effects on inflammatory markers. Panminerva Med. 2021, 63, 122–132. [Google Scholar] [CrossRef]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. Neurochir. Pol. 2022, 56, 148–155. [Google Scholar] [CrossRef]
- Dumbhare, O.; Gaurkar, S.S. A Review of Genetic and Gene Therapy for Parkinson’s Disease. Cureus 2023, 15, e34657. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White Iii, C.L.; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef]
- Dickson, D.W.; Braak, H.; Duda, J.E.; Duyckaerts, C.; Gasser, T.; Halliday, G.M.; Hardy, J.; Leverenz, J.B.; Del Tredici, K.; Wszolek, Z.K.; et al. Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 2009, 8, 1150–1157. [Google Scholar] [CrossRef]
- Lang, A.E.; Obeso, J.A. Challenges in Parkinson’s disease: Restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004, 3, 309–316. [Google Scholar] [CrossRef]
- Langston, J.W. The Parkinson’s complex: Parkinsonism is just the tip of the iceberg. Ann. Neurol. 2006, 59, 591–596. [Google Scholar] [CrossRef]
- Ahlskog, J.E. Beating a dead horse: Dopamine and Parkinson disease. Neurology 2007, 69, 1701–1711. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov. Disord. 2016, 31, 1114–1119. [Google Scholar] [CrossRef]
- Halliday, G.; Lees, A.; Stern, M. Milestones in Parkinson’s disease—Clinical and pathologic features. Mov. Disord. 2011, 26, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Mosley, P.E.; Moodie, R.; Dissanayaka, N. Caregiver Burden in Parkinson Disease: A Critical Review of Recent Literature. J. Geriatr. Psychiatry Neurol. 2017, 30, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Ruffini, L.; Testa, C.; Salvi, M.; Scarlattei, M.; Baldari, G.; Zucchini, I.; Lorenzi, B.; Cattabiani, C.; Maggio, M. Cognitive and Behavior Deficits in Parkinson’s Disease with Alteration of FDG-PET Irrespective of Age. Geriatrics 2021, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Schapira, A.H.; Stocchi, F.; Sethi, K.; Odin, P.; MacPhee, G.; Brown, R.G.; Naidu, Y.; Clayton, L.; Abe, K.; et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov. Disord. 2007, 22, 1623–1629. [Google Scholar] [CrossRef]
- Karlsen, K.H.; Larsen, J.P.; Tandberg, E.; Maeland, J.G. Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1999, 66, 431–435. [Google Scholar] [CrossRef]
- Tholfsen, L.K.; Larsen, J.P.; Schulz, J.; Tysnes, O.B.; Gjerstad, M.D. Changes in insomnia subtypes in early Parkinson disease. Neurology 2017, 88, 352–358. [Google Scholar] [CrossRef]
- Kadastik-Eerme, L.; Rosenthal, M.; Paju, T.; Muldmaa, M.; Taba, P. Health-related quality of life in Parkinson’s disease: A cross-sectional study focusing on non-motor symptoms. Health Qual. Life Outcomes 2015, 13, 83. [Google Scholar] [CrossRef]
- Loddo, G.; Calandra-Buonaura, G.; Sambati, L.; Giannini, G.; Cecere, A.; Cortelli, P.; Provini, F. The Treatment of Sleep Disorders in Parkinson’s Disease: From Research to Clinical Practice. Front. Neurol. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; van Hilten, J.J.; Marinus, J. The course of insomnia in Parkinson’s disease. Park. Relat. Disord. 2016, 33, 51–57. [Google Scholar] [CrossRef]
- Diederich, N.J.; Vaillant, M.; Mancuso, G.; Lyen, P.; Tiete, J. Progressive sleep ‘destructuring’ in Parkinson’s disease. A polysomnographic study in 46 patients. Sleep Med. 2005, 6, 313–318. [Google Scholar] [CrossRef]
- Louter, M.; van Sloun, R.J.; Pevernagie, D.A.; Arends, J.B.; Cluitmans, P.J.; Bloem, B.R.; Overeem, S. Subjectively impaired bed mobility in Parkinson disease affects sleep efficiency. Sleep Med. 2013, 14, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Esteban, J.C.; Zarranz, J.J.; Lezcano, E.; Velasco, F.; Ciordia, R.; Rouco, I.; Losada, J.; Bilbao, I. Sleep complaints and their relation with drug treatment in patients suffering from Parkinson’s disease. Mov. Disord. 2006, 21, 983. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Högl, B.; Arnulf, I.; Comella, C.; Ferreira, J.; Iranzo, A.; Tilley, B.; Trenkwalder, C.; Poewe, W.; Rascol, O.; Sampaio, C.; et al. Scales to assess sleep impairment in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2010, 25, 2704–2716. [Google Scholar] [CrossRef]
- Yang, X.; Liu, B.; Shen, H.; Li, S.; Zhao, Q.; An, R.; Hu, F.; Ren, H.; Xu, Y.; Xu, Z. Prevalence of restless legs syndrome in Parkinson’s disease: A systematic review and meta-analysis of observational studies. Sleep Med. 2018, 43, 40–46. [Google Scholar] [CrossRef]
- Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G.; Jiménez-Jiménez, F.J. Association between restless legs syndrome and other movement disorders. Neurology 2019, 92, 948–964. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Carli, G.; Casoni, F.; Galbiati, A. Restless Legs Syndrome and Parkinson Disease: A Causal Relationship Between the Two Disorders? Front. Neurol. 2018, 9, 551. [Google Scholar] [CrossRef]
- Medicine AAoS. The International Classification of Sleep Disorders: Diagnostic and Coding Manual American Academy of Sleep Medicine, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Zhang, J.; Xu, C.Y.; Liu, J. Meta-analysis on the prevalence of REM sleep behavior disorder symptoms in Parkinson’s disease. BMC Neurol. 2017, 17, 23. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Trautmann, E.; Sixel-Döring, F.; Wicke, T.; Ebentheuer, J.; Schaumburg, M.; Lang, E.; Focke, N.K.; Kumar, K.R.; Lohmann, K.; et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013, 81, 1226–1234. [Google Scholar] [CrossRef]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D. Advances in markers of prodromal Parkinson disease. Nat. Rev. Neurol. 2016, 12, 622–634. [Google Scholar] [CrossRef]
- Boeve, B.F.; Silber, M.H.; Saper, C.B.; Ferman, T.J.; Dickson, D.W.; Parisi, J.E.; Benarroch, E.E.; Ahlskog, J.E.; Smith, G.E.; Caselli, R.C.; et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007, 130 Pt 11, 2770–2788. [Google Scholar] [CrossRef]
- Stiasny-Kolster, K.; Mayer, G.; Schäfer, S.; Möller, J.C.; Heinzel-Gutenbrunner, M.; Oertel, W.H. The REM sleep behavior disorder screening questionnaire—A new diagnostic instrument. Mov. Disord. 2007, 22, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- O’Suilleabhain, P.E.; Dewey, R.B., Jr. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson disease. Arch. Neurol. 2002, 59, 986–989. [Google Scholar] [CrossRef]
- Suzuki, K.; Okuma, Y.; Uchiyama, T.; Miyamoto, M.; Sakakibara, R.; Shimo, Y.; Hattori, N.; Kuwabara, S.; Yamamoto, T.; Kaji, Y.; et al. Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: A multicentre cross-sectional study. J. Neurol. Neurosurg. Psychiatry 2017, 88, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.W.; Chahine, L.M.; Caspell-Garcia, C.; Long, J.D.; Coffey, C.; Högl, B.; Videnovic, A.; Iranzo, A.; Mayer, G.; Foldvary-Schaefer, N.; et al. Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Valko, P.O.; Hauser, S.; Sommerauer, M.; Werth, E.; Baumann, C.R. Observations on sleep-disordered breathing in idiopathic Parkinson’s disease. PLoS ONE 2014, 9, e100828. [Google Scholar] [CrossRef] [PubMed]
- Béland, S.G.; Postuma, R.B.; Latreille, V.; Bertrand, J.A.; Panisset, M.; Chouinard, S.; Wolfson, C.; Gagnon, J.F. Observational Study of the Relation between Parkinson’s Disease and Sleep Apnea. J. Park. Dis. 2015, 5, 805–811. [Google Scholar] [CrossRef]
- Bahia, C.M.C.S.; Pereira, J.S.; Lopes, A.J. Laryngopharyngeal motor dysfunction and obstructive sleep apnea in Parkinson’s disease. Sleep Breath. 2019, 23, 543–550. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Gros, P.; Mery, V.P.; Lafontaine, A.L.; Robinson, A.; Benedetti, A.; Kimoff, R.J.; Kaminska, M. Diagnosis of Obstructive Sleep Apnea in Parkinson’s Disease Patients: Is Unattended Portable Monitoring a Suitable Tool? Park. Dis. 2015, 2015, 258418. [Google Scholar] [CrossRef]
- Kay, D.B.; Tanner, J.J.; Bowers, D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 2018, 8, e00967. [Google Scholar] [CrossRef]
- Lauretani, F.; Testa, C.; Salvi, M.; Zucchini, I.; Lorenzi, B.; Tagliaferri, S.; Cattabiani, C.; Maggio, M. Reward System Dysfunction and the Motoric-Cognitive Risk Syndrome in Older Persons. Biomedicines 2022, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Pitton Rissardo, J.; Fornari Caprara, A.L. Parkinson’s disease rating scales: A literature review. Ann. Mov. Disord. 2020, 3, 3–22. [Google Scholar] [CrossRef]
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C.; The Collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef]
- Ma, J.F.; Wan, Q.; Hu, X.Y.; Sun, S.G.; Wang, W.Z.; Zhao, Z.X.; Wang, Y.J.; Liu, C.F.; Li, J.M.; Jiang, Y.P.; et al. Efficacy and safety of pramipexole in chinese patients with restless legs syndrome: Results from a multi-center, randomized, double-blind, placebo-controlled trial. Sleep Med. 2012, 13, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Bohnen, N.I.; Albin, R.L.; Frey, K.A.; Müller, M.L.; Chervin, R.D. Insomnia and sleepiness in Parkinson disease: Associations with symptoms and comorbidities. J. Clin. Sleep Med. 2013, 9, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.H.; Benes, H.; Garcia-Borreguero, D.; Högl, B.; Poewe, W.; Montagna, P.; Ferini-Strambi, L.; Sixel-Döring, F.; Trenkwalder, C.; Partinen, M.; et al. Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: A randomized, placebo-controlled polysomnographic study. Sleep Med. 2010, 11, 848–856. [Google Scholar] [CrossRef]
- Walters, A.S.; Ondo, W.G.; Dreykluft, T.; Grunstein, R.; Lee, D.; Sethi, K.; TREAT RLS 2 (Therapy with Ropinirole: Efficacy and Tolerability in RLS 2) Study Group. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: A 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov. Disord. 2004, 19, 1414–1423. [Google Scholar] [CrossRef]
- Garcia-Borreguero, D.; Silber, M.H.; Winkelman, J.W.; Högl, B.; Bainbridge, J.; Buchfuhrer, M.; Hadjigeorgiou, G.; Inoue, Y.; Manconi, M.; Oertel, W.; et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: A combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016, 21, 1–11. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Armstrong, M.J.; Allen, R.P.; Chaudhuri, K.R.; Ondo, W.; Trenkwalder, C.; Zee, P.C.; Gronseth, G.S.; Gloss, D.; Zesiewicz, T. Practice guideline summary: Treatment of restless legs syndrome in adults: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2016, 87, 2585–2593. [Google Scholar] [CrossRef]
- Walters, A.S.; Ondo, W.G.; Kushida, C.A.; Becker, P.M.; Ellenbogen, A.L.; Canafax, D.M.; Barrett, R.W.; XP045 Study Group. Gabapentin enacarbil in restless legs syndrome: A phase 2b, 2-week, randomized, double-blind, placebo-controlled trial. Clin. Neuropharmacol. 2009, 32, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Chen, C.; Garcia-Borreguero, D.; Polo, O.; DuBrava, S.; Miceli, J.; Knapp, L.; Winkelman, J.W. Comparison of pregabalin with pramipexole for restless legs syndrome. N. Engl. J. Med. 2014, 370, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.E.; Park, E.O.; Shin, C.W.; Kim, H.J.; Jeon, B. REM sleep behavior disorder portends poor prognosis in Parkinson’s disease: A systematic review. J. Clin. Neurosci. 2018, 47, 6–13. [Google Scholar] [CrossRef]
- St Louis, E.K.; Boeve, B.F. REM Sleep Behavior Disorder: Diagnosis, Clinical Implications, and Future Directions. Mayo Clin. Proc. 2017, 92, 1723–1736. [Google Scholar] [CrossRef]
- Aurora, R.N.; Zak, R.S.; Maganti, R.K.; Auerbach, S.H.; Casey, K.R.; Chowdhuri, S.; Karippot, A.; Ramar, K.; Kristo, D.A.; Morgenthaler, T.I.; et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J. Clin. Sleep Med. 2010, 6, 85–95, Erratum in J. Clin. Sleep Med. 2010, 6, table of contents. [Google Scholar] [PubMed]
- McCarter, S.J.; Boswell, C.L.; St Louis, E.K.; Dueffert, L.G.; Slocumb, N.; Boeve, B.F.; Silber, M.H.; Olson, E.J.; Tippmann-Peikert, M. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013, 14, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gros, P.; Mery, V.P.; Lafontaine, A.L.; Robinson, A.; Benedetti, A.; Kimoff, R.J.; Kaminska, M. Obstructive sleep apnea in Parkinson’s disease patients: Effect of Sinemet CR taken at bedtime. Sleep Breath. 2016, 20, 205–212. [Google Scholar] [CrossRef]
- Medeiros, C.A.; Carvalhedo de Bruin, P.F.; Lopes, L.A.; Magalhães, M.C.; de Lourdes Seabra, M.; de Bruin, V.M. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. A randomized, double blind, placebo-controlled study. J. Neurol. 2007, 254, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.A.; Mastick, J.; Colling, E.; Carter, J.H.; Singer, C.M.; Aminoff, M.J. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005, 6, 459–466. [Google Scholar] [CrossRef]
- Batla, A.; Simeoni, S.; Uchiyama, T.; deMin, L.; Baldwin, J.; Melbourne, C.; Islam, S.; Bhatia, K.P.; Pakzad, M.; Eriksson, S.; et al. Exploratory pilot study of exogenous sustained-release melatonin on nocturia in Parkinson’s disease. Eur. J. Neurol. 2021, 28, 1884–1892. [Google Scholar] [CrossRef]
- De Berardis, D.; Fornaro, M.; Serroni, N.; Olivieri, L.; Marini, S.; Moschetta, F.S.; Srinivasan, V.; Assetta, M.; Valchera, A.; Salone, A.; et al. Agomelatine treatment of major depressive disorder in Parkinson’s disease: A case series. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 343–345. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, M.; Park, S.; Jang, W.; Park, J.; Oh, E.; Cho, J.W.; Kim, J.S.; Youn, J. Prolonged-release melatonin in Parkinson’s disease patients with a poor sleep quality: A randomized trial. Park. Relat. Disord. 2020, 75, 50–54. [Google Scholar] [CrossRef]
- Kashihara, K.; Nomura, T.; Maeda, T.; Tsuboi, Y.; Mishima, T.; Takigawa, H.; Nakashima, K. Beneficial Effects of Ramelteon on Rapid Eye Movement Sleep Behavior Disorder Associated with Parkinson’s Disease—Results of a Multicenter Open Trial. Intern. Med. 2016, 55, 231–236. [Google Scholar] [CrossRef]
- Huang, K.H.; Tai, C.J.; Kuan, Y.H.; Chang, Y.C.; Tsai, T.H.; Lee, C.Y. Pneumonia Risk Associated with the Use of Individual Benzodiazepines and Benzodiazepine Related Drugs among the Elderly with Parkinson’s Disease. Int. J. Environ. Res. Public Health 2021, 18, 9410. [Google Scholar] [CrossRef]
- De Almeida, C.M.O.; Pachito, D.V.; Sobreira-Neto, M.A.; Tumas, V.; Eckeli, A.L. Pharmacological treatment for REM sleep behavior disorder in Parkinson disease and related conditions: A scoping review. J. Neurol. Sci. 2018, 393, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gilat, M.; Marshall, N.S.; Testelmans, D.; Buyse, B.; Lewis, S.J.G. A critical review of the pharmacological treatment of REM sleep behavior disorder in adults: Time for more and larger randomized placebo-controlled trials. J. Neurol. 2022, 269, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Park, H.; Lee, W.W.; Kim, H.J.; Kim, H.J.; Jeon, B. Clonazepam for probable REM sleep behavior disorder in Parkinson’s disease: A randomized placebo-controlled trial. J. Neurol. Sci. 2019, 401, 81–86. [Google Scholar] [CrossRef]
- Werneck, A.L.; Rosso, A.L.; Vincent, M.B. The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’ disease. Arq. Neuropsiquiatr. 2009, 67, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Agah, E.; Tavanbakhsh, S.; Mirsepassi, Z.; Mousavi, S.V.; Talachi, N.; Tafakhori, A.; Aghamollaii, V. Safety and efficacy of melatonin, clonazepam, and trazodone in patients with Parkinson’s disease and sleep disorders: A randomized, double-blind trial. Neurol. Sci. 2022, 43, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.; Gély-Nargeot, M.C.; Rossignol, A.; Geny, C.; Bayard, S. Efficacy of cognitive behavioral therapy for insomnia comorbid to Parkinson’s disease: A focus on psychological and daytime functioning with a single-case design with multiple baselines. J. Clin. Psychol. 2020, 76, 356–376. [Google Scholar] [CrossRef]
- Menza, M.; Dobkin, R.D.; Marin, H.; Gara, M.; Bienfait, K.; Dicke, A.; Comella, C.L.; Cantor, C.; Hyer, L. Treatment of insomnia in Parkinson’s disease: A controlled trial of eszopiclone and placebo. Mov. Disord. 2010, 25, 1708–1714. [Google Scholar] [CrossRef]
- Cochen De Cock, V. Therapies for Restless Legs in Parkinson’s Disease. Curr. Treat. Options Neurol. 2019, 21, 56. [Google Scholar] [CrossRef]
- Silber, M.H.; Becker, P.M.; Earley, C.; Garcia-Borreguero, D.; Ondo, W.G.; Medical Advisory Board of the Willis-Ekbom Disease Foundation. Willis-Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin. Proc. 2013, 88, 977–986. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Allen, R.; Högl, B.; Clemens, S.; Patton, S.; Schormair, B.; Winkelmann, J. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 2018, 17, 994–1005. [Google Scholar] [CrossRef]
- Anderson, S.L.; Vande Griend, J.P. Quetiapine for insomnia: A review of the literature. Am. J. Health Syst. Pharm. 2014, 71, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Juri, C.; Chaná, P.; Tapia, J.; Kunstmann, C.; Parrao, T. Quetiapine for insomnia in Parkinson disease: Results from an open-label trial. Clin. Neuropharmacol. 2005, 28, 185–187. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Okun, M.S.; Rodriguez, R.L.; Malaty, I.A.; Romrell, J.; Sun, A.; Wu, S.S.; Pillarisetty, S.; Nyathappa, A.; Eisenschenk, S. Quetiapine improves visual hallucinations in Parkinson disease but not through normalization of sleep architecture: Results from a double-blind clinical-polysomnography study. Int. J. Neurosci. 2009, 119, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Hinze-Selch, D.; Mullington, J.; Orth, A.; Lauer, C.J.; Pollmächer, T. Effects of clozapine on sleep: A longitudinal study. Biol. Psychiatry 1997, 42, 260–266. [Google Scholar] [CrossRef]
- Neufeld, M.Y.; Rabey, J.M.; Orlov, E.; Korczyn, A.D. Electroencephalographic findings with low-dose clozapine treatment in psychotic Parkinsonian patients. Clin. Neuropharmacol. 1996, 19, 81–86. [Google Scholar] [CrossRef]
- Pham Nguyen, T.P.; Abraham, D.S.; Thibault, D.; Weintraub, D.; Willis, A.W. Low continuation of antipsychotic therapy in Parkinson disease—Intolerance, ineffectiveness, or inertia? BMC Neurol. 2021, 21, 240. [Google Scholar] [CrossRef]
- Patel, R.S.; Bhela, J.; Tahir, M.; Pisati, S.R.; Hossain, S. Pimavanserin in Parkinson’s Disease-induced Psychosis: A Literature Review. Cureus 2019, 11, e5257. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Vanover, K.E.; Weiner, D.M.; Davis, R.E.; van Kammen, D.P. Pimavanserin tartrate, a 5-HT(2A) receptor inverse agonist, increases slow wave sleep as measured by polysomnography in healthy adult volunteers. Sleep Med. 2011, 12, 134–141. [Google Scholar] [CrossRef]
- Jha, M.K.; Fava, M.; Freeman, M.P.; Thase, M.E.; Papakostas, G.I.; Shelton, R.C.; Trivedi, M.H.; Dirks, B.; Liu, K.; Stankovic, S. Effect of Adjunctive Pimavanserin on Sleep/Wakefulness in Patients with Major Depressive Disorder: Secondary Analysis from CLARITY. J. Clin. Psychiatry 2020, 82, 20m13425. [Google Scholar] [CrossRef]
- Patel, N.; LeWitt, P.; Neikrug, A.B.; Kesslak, P.; Coate, B.; Ancoli-Israel, S. Nighttime Sleep and Daytime Sleepiness Improved With Pimavanserin during Treatment of Parkinson’s Disease Psychosis. Clin. Neuropharmacol. 2018, 41, 210–215. [Google Scholar] [CrossRef]
- Rios Romenets, S.; Creti, L.; Fichten, C.; Bailes, S.; Libman, E.; Pelletier, A.; Postuma, R.B. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson’s disease—A randomized study. Park. Relat. Disord. 2013, 19, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cui, Y.; Shen, J.; Wang, P. Suvorexant for the prevention of delirium: A meta-analysis. Medicine 2020, 99, e21043. [Google Scholar] [CrossRef]
- Prange, S.; Klinger, H.; Laurencin, C.; Danaila, T.; Thobois, S. Depression in Patients with Parkinson’s Disease: Current Understanding of its Neurobiology and Implications for Treatment. Drugs Aging 2022, 39, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Agüera-Ortiz, L.; García-Ramos, R.; Grandas Pérez, F.J.; López-Álvarez, J.; Montes Rodríguez, J.M.; Olazarán Rodríguez, F.J.; Olivera Pueyo, J.; Pelegrín Valero, C.; Porta-Etessam, J. Focus on Depression in Parkinson’s Disease: A Delphi Consensus of Experts in Psychiatry, Neurology, and Geriatrics. Park. Dis. 2021, 2021, 6621991. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Poewe, W.; Chaudhuri, K.R.; Jech, R.; Pickut, B.; Pirtošek, Z.; Szasz, J.; Valldeoriola, F.; Winkler, C.; Bergmann, L.; et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Park. Relat. Disord. 2017, 45, 13–20. [Google Scholar] [CrossRef]
- Giladi, N.; Fichtner, A.; Poewe, W.; Boroojerdi, B. Rotigotine transdermal system for control of early morning motor impairment and sleep disturbances in patients with Parkinson’s disease. J. Neural Transm. 2010, 117, 1395–1399. [Google Scholar] [CrossRef]
- Xiang, W.; Sun, Y.Q.; Teoh, H.C. Comparison of nocturnal symptoms in advanced Parkinson’s disease patients with sleep disturbances: Pramipexole sustained release versus immediate release formulations. Drug Des. Dev. Ther. 2018, 12, 2017–2024. [Google Scholar] [CrossRef]
- Mizuno, Y.; Nomoto, M.; Hasegawa, K.; Hattori, N.; Kondo, T.; Murata, M.; Takeuchi, M.; Takahashi, M.; Tomida, T.; Rotigotine Trial Group. Rotigotine vs ropinirole in advanced stage Parkinson’s disease: A double-blind study. Park. Relat. Disord. 2014, 20, 1388–1393. [Google Scholar] [CrossRef]
- Pahwa, R.; Stacy, M.A.; Factor, S.A.; Lyons, K.E.; Stocchi, F.; Hersh, B.P.; Elmer, L.W.; Truong, D.D.; Earl, N.L.; EASE-PD Adjunct Study Investigators. Ropinirole 24-hour prolonged release: Randomized, controlled study in advanced Parkinson disease. Neurology 2007, 68, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Romigi, A.; Stanzione, P.; Marciani, M.G.; Izzi, F.; Placidi, F.; Cervellino, A.; Giacomini, P.; Brusa, L.; Grossi, K.; Pierantozzi, M. Effect of cabergoline added to levodopa treatment on sleep-wake cycle in idiopathic Parkinson’s disease: An open label 24-hour polysomnographic study. J. Neural Transm. 2006, 113, 1909–1913. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Benes, H.; Grote, L.; Happe, S.; Högl, B.; Mathis, J.; Saletu-Zyhlarz, G.M.; Kohnen, R.; CALDIR Study Group. Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: Results from a multi-center, randomized, active controlled trial. Mov. Disord. 2007, 22, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Wurst, M.G.; Whatley, M.F.; Daniels, R.N. Classics in Chemical Neuroscience: Pramipexole. ACS Chem. Neurosci. 2020, 11, 2506–2512. [Google Scholar] [CrossRef]
- Kakar, R.S.; Kushida, C.A. Ropinirole in the treatment of restless legs syndrome. Expert Rev. Neurother. 2005, 5, 35–42. [Google Scholar] [CrossRef]
- Kesayan, T.; Shaw, J.D.; Jones, T.M.; Staffetti, J.S.; Zesiewicz, T.A. Critical appraisal of rotigotine transdermal system in management of Parkinson’s disease and restless legs syndrome—Patient considerations. Degener. Neurol. Neuromuscul. Dis. 2015, 5, 63–72, Erratum in: Degener. Neurol. Neuromuscul. Dis. 2016, 6, 13–15. [Google Scholar]
- Dusek, P.; Busková, J.; Růzicka, E.; Majerová, V.; Srp, A.; Jech, R.; Roth, J.; Sonka, K. Effects of ropinirole prolonged-release on sleep disturbances and daytime sleepiness in Parkinson disease. Clin. Neuropharmacol. 2010, 33, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Hoel, R.W.; Giddings Connolly, R.M.; Takahashi, P.Y. Polypharmacy Management in Older Patients. Mayo Clin. Proc. 2021, 96, 242–256. [Google Scholar] [CrossRef]
- Gilat, M.; Coeytaux Jackson, A.; Marshall, N.S.; Hammond, D.; Mullins, A.E.; Hall, J.M.; Fang, B.A.M.; Yee, B.J.; Wong, K.K.H.; Grunstein, R.R.; et al. Melatonin for rapid eye movement sleep behavior disorder in Parkinson’s disease: A randomised controlled trial. Mov. Disord. 2020, 35, 344–349. [Google Scholar] [CrossRef]
- Amara, A.W.; Wood, K.H.; Joop, A.; Memon, R.A.; Pilkington, J.; Tuggle, S.C.; Reams, J.; Barrett, M.J.; Edwards, D.A.; Weltman, A.L.; et al. Randomized, Controlled Trial of Exercise on Objective and Subjective Sleep in Parkinson’s Disease. Mov. Disord. 2020, 35, 947–958. [Google Scholar] [CrossRef]
- Meloni, M.; Figorilli, M.; Carta, M.; Tamburrino, L.; Cannas, A.; Sanna, F.; Defazio, G.; Puligheddu, M. Preliminary finding of a randomized, double-blind, placebo-controlled, crossover study to evaluate the safety and efficacy of 5-hydroxytryptophan on REM sleep behavior disorder in Parkinson’s disease. Sleep Breath. 2022, 26, 1023–1031. [Google Scholar] [CrossRef]
- Peball, M.; Krismer, F.; Knaus, H.G.; Djamshidian, A.; Werkmann, M.; Carbone, F.; Ellmerer, P.; Heim, B.; Marini, K.; Valent, D.; et al. Non-Motor Symptoms in Parkinson’s Disease are Reduced by Nabilone. Ann. Neurol. 2020, 88, 712–722. [Google Scholar] [CrossRef]
- Stefani, A.; Santamaria, J.; Iranzo, A.; Hackner, H.; Schenck, C.H.; Högl, B. Nelotanserin as symptomatic treatment for rapid eye movement sleep behavior disorder: A double-blind randomized study using video analysis in patients with dementia with Lewy bodies or Parkinson’s disease dementia. Sleep Med. 2021, 81, 180–187. [Google Scholar] [CrossRef]
- Garcia-Borreguero, D.; Garcia-Malo, C.; Granizo, J.J.; Ferré, S. A Randomized, Placebo-Controlled Crossover Study with Dipyridamole for Restless Legs Syndrome. Mov. Disord. 2021, 36, 2387–2392. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Placidi, F.; Liguori, C.; Albanese, M.; Imbriani, P.; Marciani, M.G.; Mercuri, N.B.; Stanzione, P.; Stefani, A. Rotigotine may improve sleep architecture in Parkinson’s disease: A double-blind, randomized, placebo-controlled polysomnographic study. Sleep Med. 2016, 21, 140–144. [Google Scholar] [CrossRef]
- Schrempf, W.; Fauser, M.; Wienecke, M.; Brown, S.; Maaß, A.; Ossig, C.; Otto, K.; Brandt, M.D.; Löhle, M.; Schwanebeck, U.; et al. Rasagiline improves polysomnographic sleep parameters in patients with Parkinson’s disease: A double-blind, baseline-controlled trial. Eur. J. Neurol. 2018, 25, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Kies, B.; Rudzinska, M.; Fine, J.; Nikl, J.; Honczarenko, K.; Dioszeghy, P.; Hill, D.; Anderson, T.; Myllyla, V.; et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: A double-blind, randomized, placebo-controlled study (RECOVER). Mov. Disord. 2011, 26, 90–99. [Google Scholar] [CrossRef]
- Silva-Batista, C.; de Brito, L.C.; Corcos, D.M.; Roschel, H.; de Mello, M.T.; Piemonte, M.E.P.; Tricoli, V.; Ugrinowitsch, C. Resistance Training Improves Sleep Quality in Subjects with Moderate Parkinson’s Disease. J. Strength Cond. Res. 2017, 31, 2270–2277. [Google Scholar] [CrossRef] [PubMed]
- Larsson, V.; Aarsland, D.; Ballard, C.; Minthon, L.; Londos, E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson’s disease dementia. Int. J. Geriatr. Psychiatry 2010, 25, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Di Giacopo, R.; Fasano, A.; Quaranta, D.; Della Marca, G.; Bove, F.; Bentivoglio, A.R. Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson’s disease. Mov. Disord. 2012, 27, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Büchele, F.; Hackius, M.; Schreglmann, S.R.; Omlor, W.; Werth, E.; Maric, A.; Imbach, L.L.; Hägele-Link, S.; Waldvogel, D.; Baumann, C.R. Sodium Oxybate for Excessive Daytime Sleepiness and Sleep Disturbance in Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 114–118. [Google Scholar] [CrossRef]
- Ray Chaudhuri, K.; Martinez-Martin, P.; Rolfe, K.A.; Cooper, J.; Rockett, C.B.; Giorgi, L.; Ondo, W.G. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson’s disease. Eur. J. Neurol. 2012, 19, 105–113. [Google Scholar] [CrossRef]
- Adler, C.H.; Hauser, R.A.; Sethi, K.; Caviness, J.N.; Marlor, L.; Anderson, W.M.; Hentz, J.G. Ropinirole for restless legs syndrome: A placebo-controlled crossover trial. Neurology 2004, 62, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Caviness, J.N.; Hentz, J.G.; Lind, M.; Tiede, J. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov. Disord. 2003, 18, 287–293. [Google Scholar] [CrossRef]
- De Almeida, C.M.O.; Brito, M.M.C.; Bosaipo, N.B.; Pimentel, A.V.; Tumas, V.; Zuardi, A.W.; Crippa, J.A.S.; Hallak, J.E.C.; Eckeli, A.L. Cannabidiol for Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2021, 36, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Plastino, M.; Gorgone, G.; Fava, A.; Ettore, M.; Iannacchero, R.; Scarfone, R.; Vaccaro, A.; De Bartolo, M.; Bosco, D. Effects of safinamide on REM sleep behavior disorder in Parkinson disease: A randomized, longitudinal, cross-over pilot study. J. Clin. Neurosci. 2021, 91, 306–312. [Google Scholar] [CrossRef]
- De Cock, V.C.; Dodet, P.; Leu-Semenescu, S.; Aerts, C.; Castelnovo, G.; Abril, B.; Drapier, S.; Olivet, H.; Corbillé, A.G.; Leclair-Visonneau, L.; et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson’s disease (APOMORPHEE): A multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022, 21, 428–437. [Google Scholar] [CrossRef]
- Wailke, S.; Herzog, J.; Witt, K.; Deuschl, G.; Volkmann, J. Effect of controlled-release levodopa on the microstructure of sleep in Parkinson’s disease. Eur. J. Neurol. 2011, 18, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bagautdinova, J.; Mayeli, A.; Wilson, J.D.; Donati, F.L.; Colacot, R.M.; Meyer, N.; Fusar-Poli, P.; Ferrarelli, F. Sleep Abnormalities in Different Clinical Stages of Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2023, 80, 202–210. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).