A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PCI Model of TBI
2.3. Neurobehavioral Severity Scoring
2.4. Tissue Collection
2.5. Immunofluorescence
2.6. qPCR
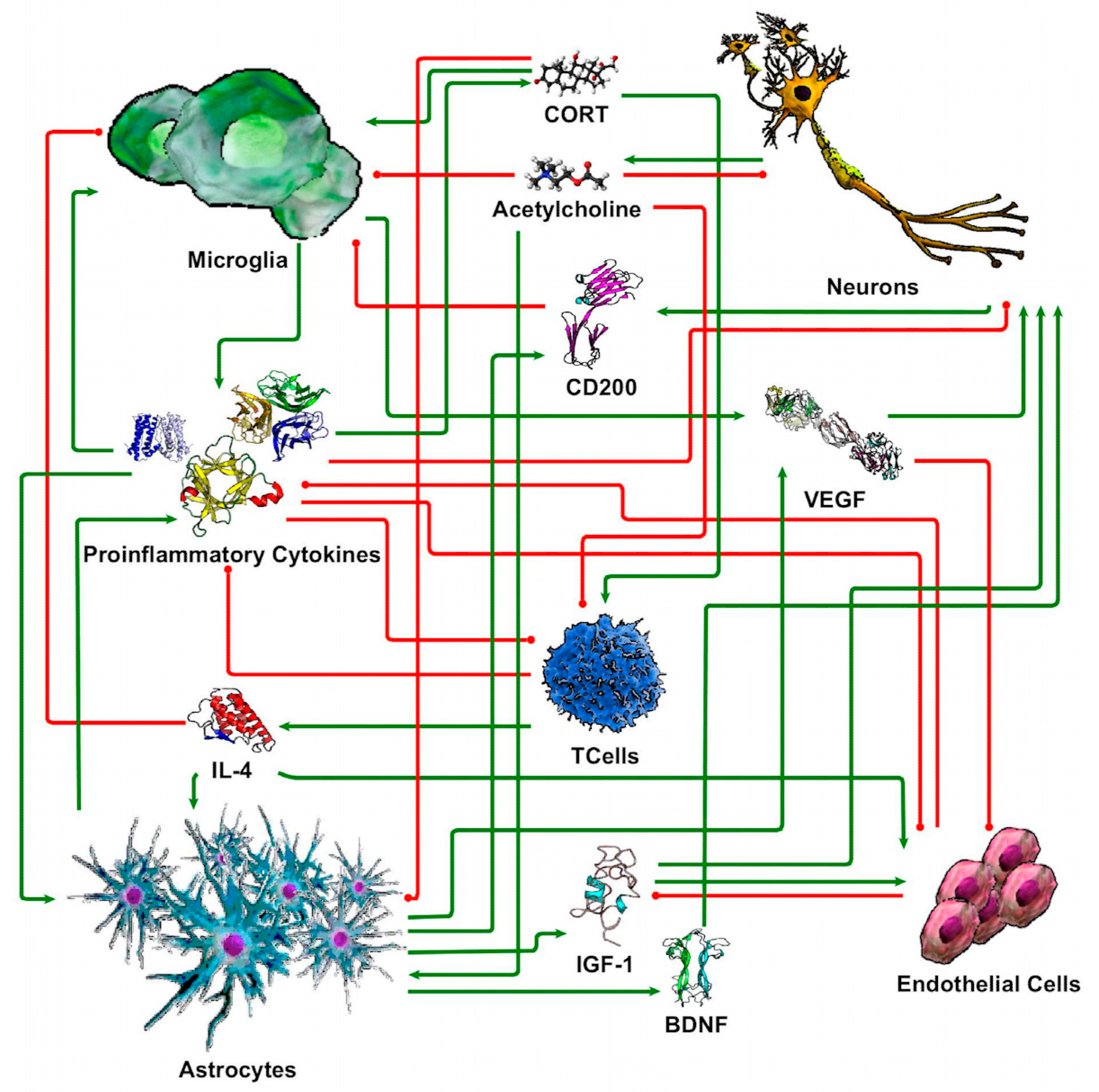
2.7. Discrete Logic Modeling of Neuron-Glia Interaction
2.8. Statistics
3. Results
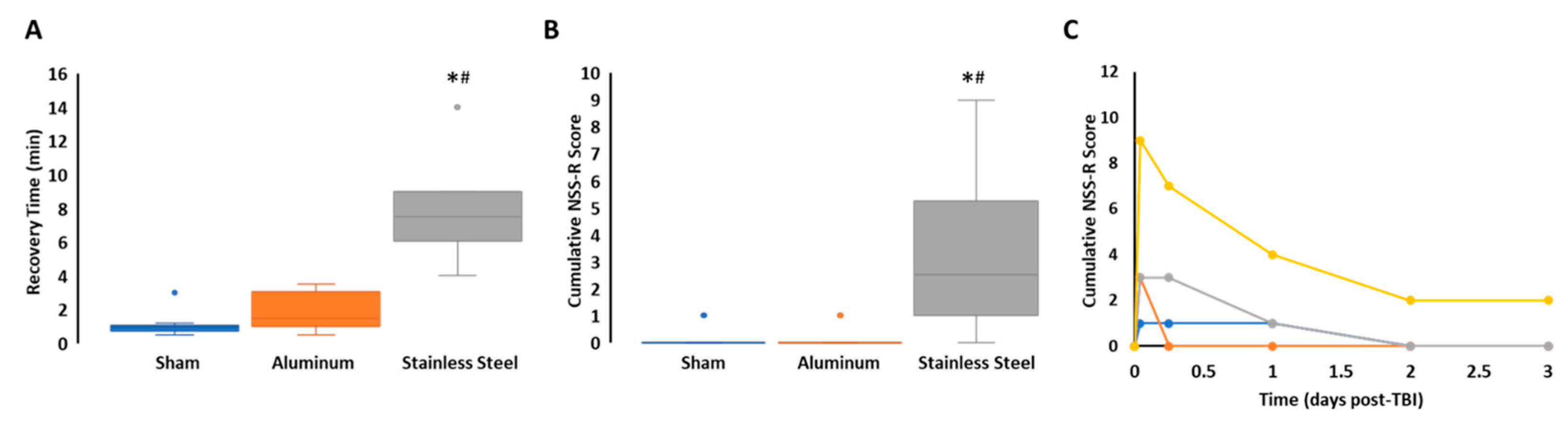
3.1. Impact with a Stainless-Steel Projectile Induced Significantly Prolonged Periods of Unconsciousness and Neurobehavioral Impairment Compared to an Aluminum Projectile
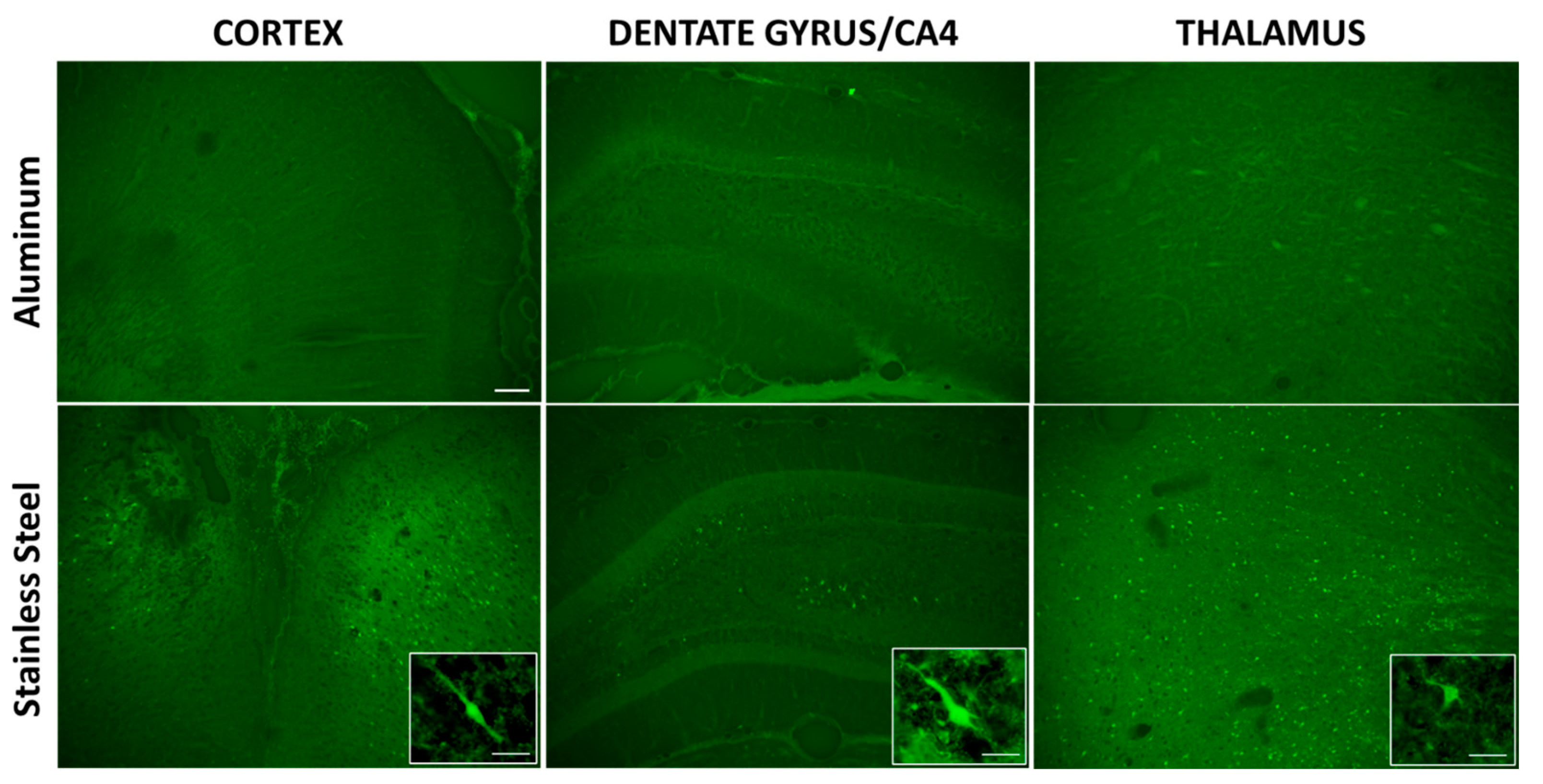
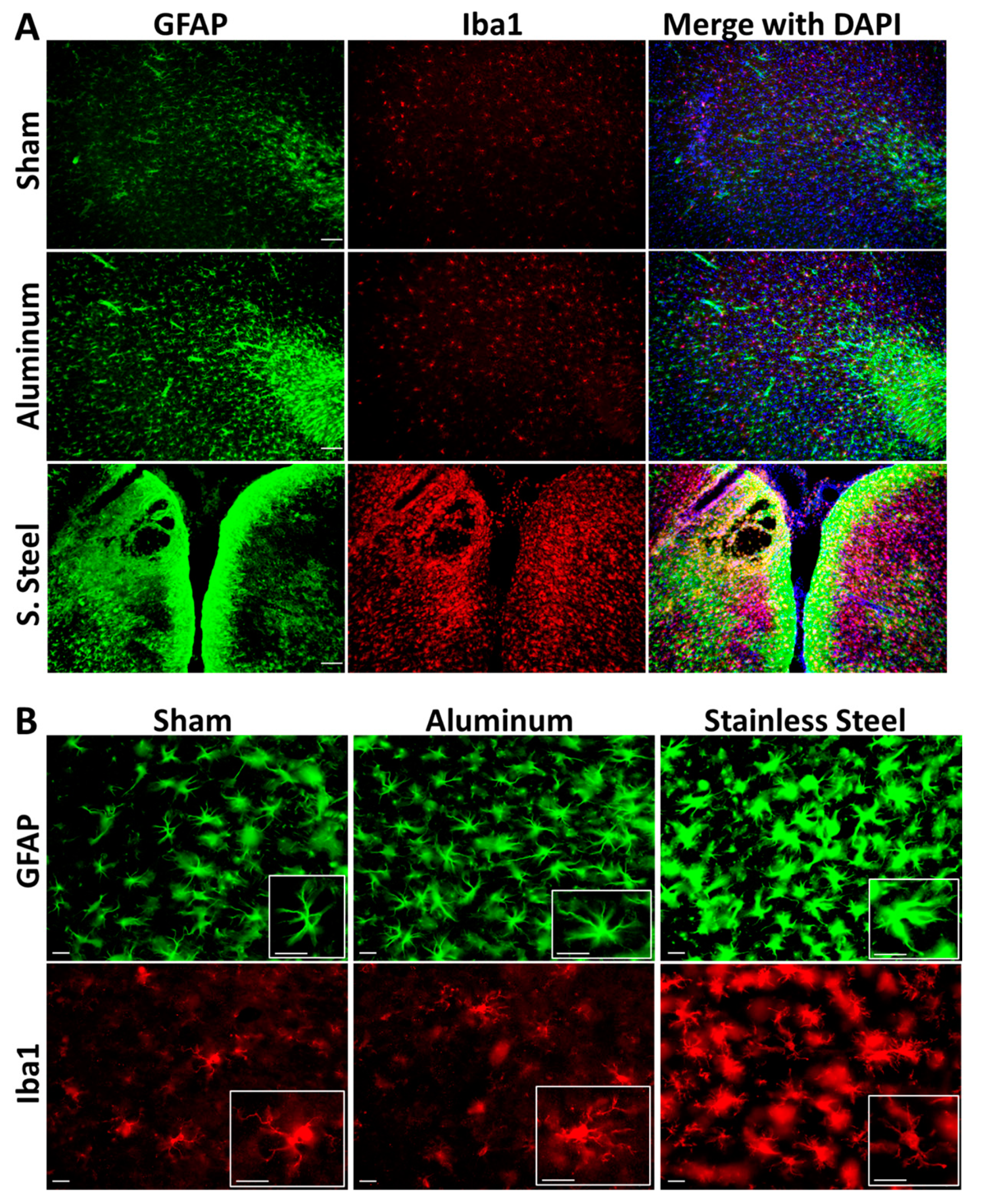
3.2. Impact with a Stainless-Steel Projectile Produced Significant Neuronal Damage and Gliosis Compared to an Aluminum Projectile
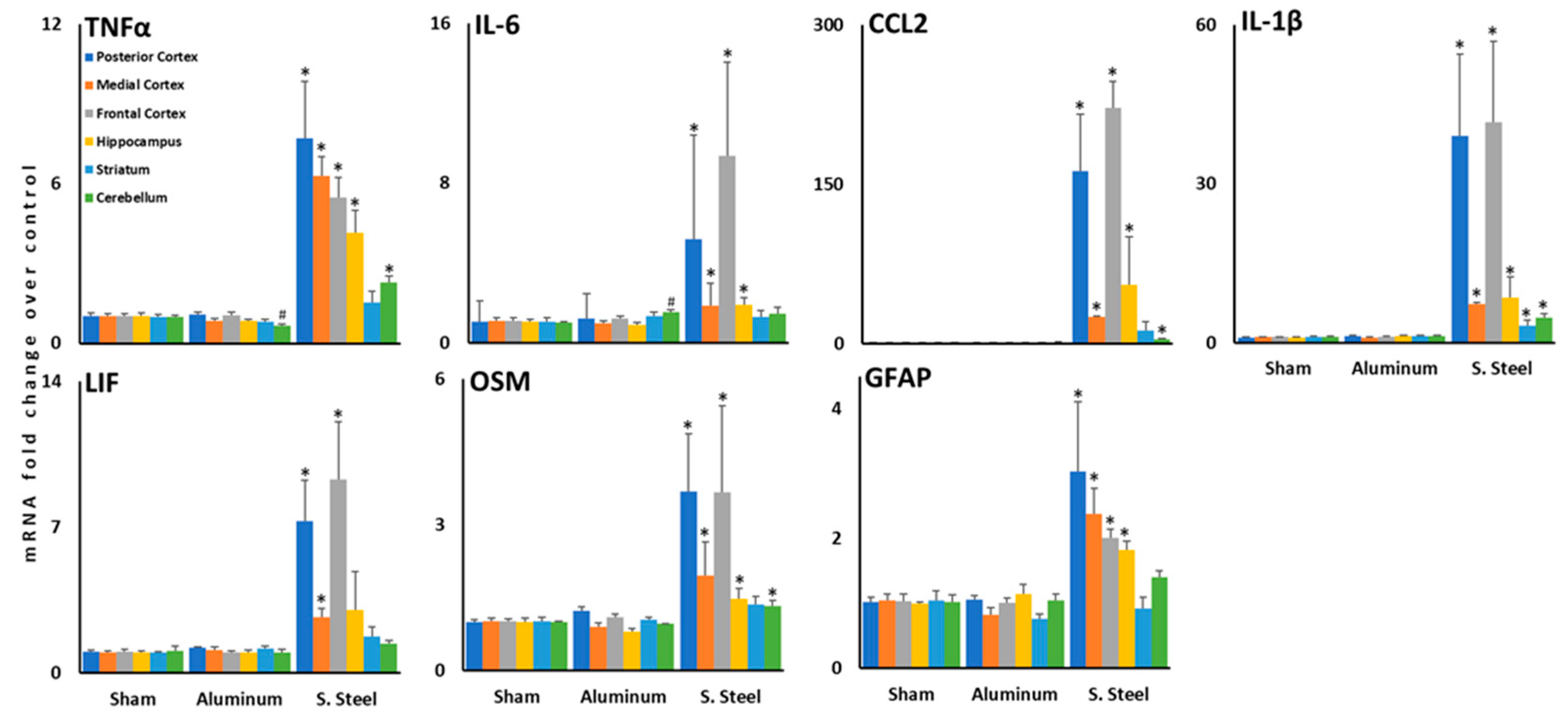
3.3. Impact with a Stainless-Steel Projectile Induced Significant Increases in the Expression of Inflammatory Cytokine mRNA in Several Brain Areas Compared to an Aluminum Projectile
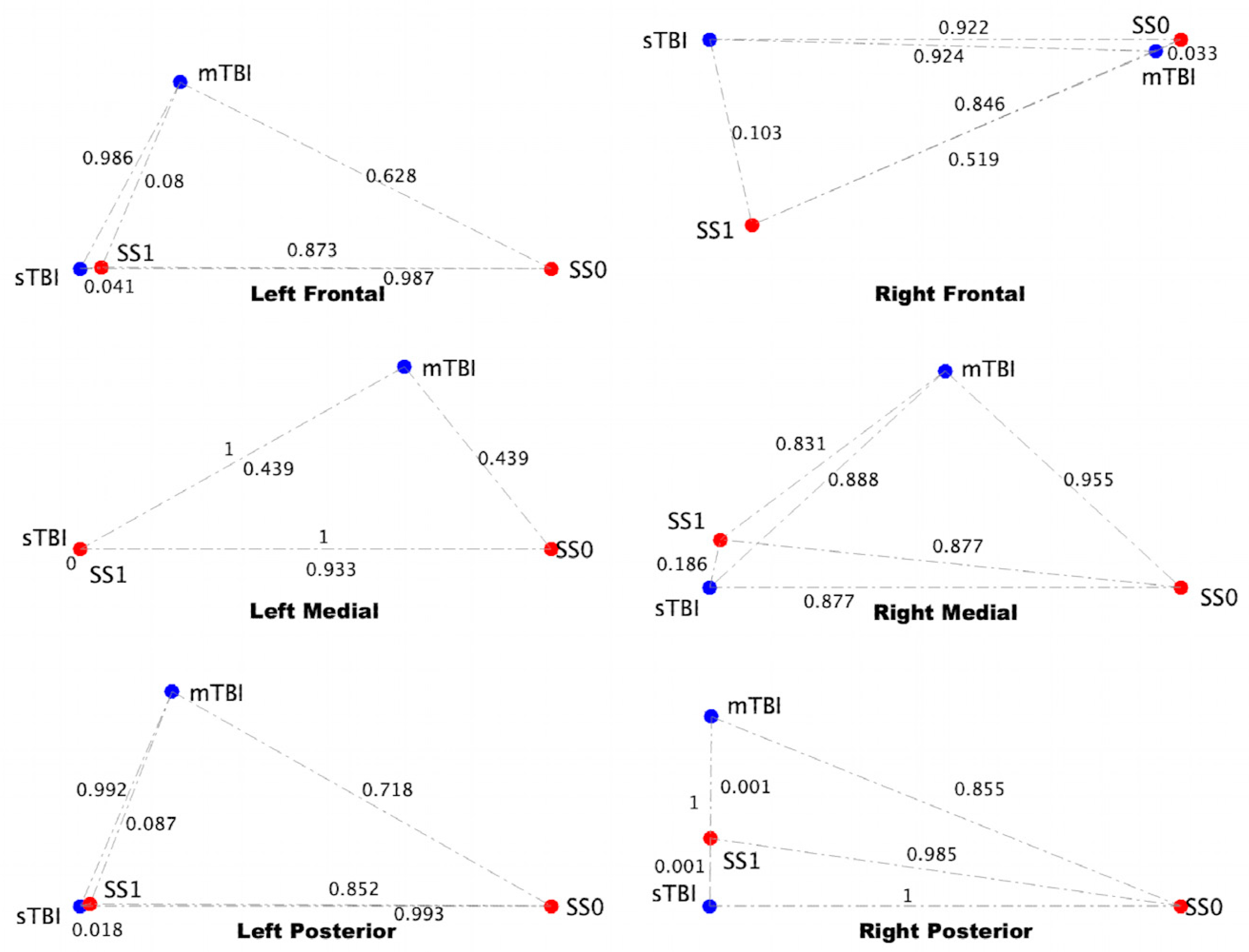
3.4. sTBI Closely Aligns with the Neuroinflammatory State in a Computational Neuron-Glia Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurman, D.J.; Alverson, C.; Dunn, K.A.; Guerrero, J.; Sniezek, J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999, 14, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Liao, Y.E.; Yang, L.Y.; Wang, J.Y.; Tweedie, D.; Karnati, H.K.; Greig, N.H.; Wang, J.Y. Neuroinflammation in animal models of traumatic brain injury. J. Neurosci. Methods 2016, 272, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sussman, E.S.; Pendharkar, A.V.; Ho, A.L.; Ghajar, J. Mild traumatic brain injury and concussion: Terminology and classification. Handb. Clin. Neurol. 2018, 158, 21–24. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014; Centers for Disease Control and Prevention, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Centers for Disease Control and Prevention. “Get the Facts About TBI.” Traumatic Brain Injury & Concussion, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2022. Available online: https://www.cdc.gov/traumaticbraininjury/get_the_facts.html (accessed on 21 March 2022).
- Leo, P.; McCrea, M. Epidemiology. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 1. [Google Scholar]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Sulhan, S.; Lyon, K.A.; Shapiro, L.A.; Huang, J.H. Neuroinflammation and blood-brain barrier disruption following traumatic brain injury: Pathophysiology and potential therapeutic targets. J. Neurosci. Res. 2020, 98, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Bell, B.D.; Primeau, M.; Sweet, J.J.; Lofland, K.R. Neuropsychological functioning in migraine headache, nonheadache chronic pain, and mild traumatic brain injury patients. Arch. Clin. Neuropsychol. 1999, 14, 389–399. [Google Scholar] [CrossRef]
- Smith, D.H.; Johnson, V.E.; Stewart, W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat. Rev. Neurol. 2013, 9, 211–221. [Google Scholar]
- McKee, A.C.; Robinson, M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimer’s Dement. 2014, 10, S242–S253. [Google Scholar] [CrossRef] [Green Version]
- Stein, T.D.; Alvarez, V.E.; McKee, A.C. Chronic traumatic encephalopathy: A spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimer’s Res. Ther. 2014, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Gardner, R.C.; Yaffe, K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell. Neurosci. 2015, 66, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef] [Green Version]
- Merritt, V.C.; Clark, A.L.; Crocker, L.D.; Sorg, S.F.; Werhane, M.L.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. Repetitive mild traumatic brain injury in military veterans is associated with increased neuropsychological intra-individual variability. Neuropsychologia 2018, 119, 340–348. [Google Scholar] [CrossRef]
- Pattinson, C.L.; Shahim, P.; Taylor, P.; Dunbar, K.; Guedes, V.A.; Motamedi, V.; Lai, C.; Devoto, C.; Peyer, J.; Roy, M.J.; et al. Elevated Tau in Military Personnel Relates to Chronic Symptoms Following Traumatic Brain Injury. J. Head Trauma Rehabil. 2020, 35, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kalish, B.T.; Finander, B.; Cao, T.; Jin, G.; Yahya, T.; Levy, E.S.; Kukreja, B.; LaRovere, E.S.; Chung, J.Y.; et al. Repetitive mild closed head injury in adolescent mice is associated with impaired proteostasis, neuroinflammation, and tauopathy. J. Neurosci. 2022, 42, 2418–2432. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.Y.; Larimore, Z.; Holmes, L.; Cartagena, C.; Mountney, A.; Deng-Bryant, Y.; Schmid, K.; Shear, D.; Tortella, F. The WRAIR Projectile Concussive Impact Model of Mild Traumatic Brain Injury: Re-design, Testing and Preclinical Validation. Ann. Biomed. Eng. 2014, 42, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Mouzon, B.C.; Bachmeier, C.; Ferro, A.; Ojo, J.O.; Crynen, G.; Acker, C.M.; Davies, P.; Mullan, M.; Stewart, W.; Crawford, F. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 2014, 75, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Mountney, A.; Boutté, A.M.; Cartagena, C.M.; Flerlage, W.F.; Johnson, W.D.; Rho, C.; Lu, X.-C.; Yarnell, A.; Marcsisin, S.; Sousa, J.; et al. Functional and molecular correlates after single and repeated rat closed-head concussion: Indices of vulnerability after brain injury. J. Neurotrauma 2017, 34, 2768–2789. [Google Scholar] [CrossRef] [PubMed]
- Mouzon, B.C.; Bachmeier, C.; Ojo, J.O.; Acker, C.M.; Ferguson, S.; Paris, D.; Ait-Ghezala, G.; Crynen, G.; Davies, P.; Mullan, M.; et al. Lifelong behavioral and neuropathological consequences of repetitive mild traumatic brain injury. Ann. Clin. Transl. Neurol. 2017, 5, 64–80. [Google Scholar] [CrossRef] [Green Version]
- Simon, D.; McGeachy, M.; Bayır, H.; Clark, R.S.B.; Loane, D.J. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madathil, S.K.; Wilfred, B.S.; Urankar, S.E.; Yang, W.; Leung, L.Y.; Gilsdorf, J.S.; Shear, D.A. Early microglial activation following closed-head concussive injury is dominated by pro-inflammatory M-1 type. Front. Neurol. 2018, 9, 964. [Google Scholar] [CrossRef] [Green Version]
- Izzy, S.; Brown-Whalen, A.; Yahya, T.; Sarro-Schwartz, A.; Jin, G.; Chung, J.Y.; Lule, S.; Morsett, L.M.; Alquraini, A.; Wu, L.; et al. Repetitive Traumatic Brain Injury Causes Neuroinflammation before Tau Pathology in Adolescent P301S Mice. Int. J. Mol. Sci. 2021, 22, 907. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [PubMed] [Green Version]
- Chen, Z.; Leung, L.Y.; Mountney, A.; Liao, Z.; Yang, W.; Lu, X.-C.M.; Dave, J.; Deng-Bryant, Y.; Wei, G.; Schmid, K.; et al. A novel animal model of closed-head concussive-induced mild traumatic brain injury: Development, implementation, and characterization. J. Neurotrauma 2012, 29, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Deng-Bryant, Y.; Leung, L.Y.; Madathil, S.; Flerlage, J.; Yang, F.; Yang, W.; Gilsdorf, J.; Shear, D. Chronic cognitive deficits and associated histopathology following closed-head concussive injury in rats. Front. Neurol. 2019, 10, 699. [Google Scholar] [CrossRef]
- Craddock, T.J.A.; Michalovicz, L.T.; Kelly, K.A.; Rice, M.A.; Miller, D.B., Jr.; Klimas, N.G.; Morris, M.; O’Callaghan, J.P.; Broderick, G. A Logic Model of Neuronal-Glial Interaction Suggests Altered Homeostatic Regulation in the Perpetuation of Neuroinflammation. Front. Cell. Neurosci. 2018, 12, 336. [Google Scholar] [CrossRef]
- Yarnell, A.M.; Barry, E.S.; Mountney, A.; Shear, D.; Tortella, F.; Grunberg, N.E. The revised neurobehavioral severity scale (NSS-R) for rodents. Curr. Protoc. Neurosci. 2016, 75, 9–52. [Google Scholar] [CrossRef] [PubMed]
- Michalovicz, L.T.; Kelly, K.A.; Miller, D.B.; Sullivan, K.; O’Callaghan, J.P. The β-adrenergic receptor blocker and anti-inflammatory drug propranolol mitigates brain cytokine expression in a long-term model of Gulf War Illness. Life Sci. 2021, 285, 119962. [Google Scholar] [CrossRef] [PubMed]
- Locker, A.R.; Michalovicz, L.T.; Kelly, K.A.; Miller, J.V.; Miller, D.B.; O’Callaghan, J.P. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 2017, 142, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.A.; Michalovicz, L.T.; Miller, J.V.; Castranova, V.; Miller, D.B.; O’Callaghan, J.P. Prior exposure to corticosterone markedly enhances and prolongs the neuroinflammatory response to systemic challenge with LPS. PLoS ONE 2018, 13, e0190546. [Google Scholar] [CrossRef] [Green Version]
- Craddock, T.J.; Fritsch, P.; Rice, M.A., Jr.; del Rosario, R.M.; Miller, D.B.; Fletcher, M.A.; Klimas, N.G.; Broderick, G. A role for homeostatic drive in the perpetuation of complex chronic illness: Gulf War Illness and chronic fatigue syndrome. PLoS ONE 2014, 9, e84839. [Google Scholar] [CrossRef]
- Brown, M.B. 400: A method for combining non-independent, one-sided tests of significance. Biometrics 1975, 2, 987–992. [Google Scholar] [CrossRef]
- Craddock, T.J.; Del Rosario, R.R.; Rice, M.; Zysman, J.P.; Fletcher, M.A.; Klimas, N.G.; Broderick, G. Achieving remission in gulf war illness: A simulation-based approach to treatment design. PLoS ONE 2015, 10, e0132774. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, P.; Craddock, T.J.; del Rosario, R.M.; Rice, M.A.; Smylie, A.; Folcik, V.A.; Klimas, N.G.; Broderick, G. Succumbing to the laws of attraction: Exploring the sometimes pathogenic versatility of discrete immune logic. Syst. Biomed. 2013, 1, 179–194. [Google Scholar] [CrossRef]
- Rice, M.A., Jr.; Craddock, T.J.; Folcik, V.A.; del Rosario, R.M.; Barnes, Z.M.; Klimas, N.G.; Fletcher, M.A.; Zysman, J.P.; Broderick, G. Gulf War Illness: Is there lasting damage to the endocrine-immune circuitry? Syst. Biomed. 2014, 2, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Carrera Arias, F.J.; Aenlle, K.; Abreu, M.; Holschbach, M.A.; Michalovicz, L.T.; Kelly, K.A.; Klimas, N.; Craddock, T.J. Modeling neuroimmune interactions in human subjects and animal models to predict subtype-specific multidrug treatments for Gulf War Illness. Int. J. Mol. Sci. 2021, 22, 8546. [Google Scholar] [CrossRef] [PubMed]
- Sammon, J.W. A nonlinear mapping for data structure analysis. IEEE Trans. Comput. 1969, 100, 401–409. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Omelchenko, A.; Shrirao, A.B.; Bhattiprolu, A.K.; Zahn, J.D.; Schloss, R.S.; Dickson, S.; Meaney, D.F.; Boustany, N.N.; Yarmush, M.L.; Firestein, B.L. Dynamin and reverse-mode sodium calcium exchanger blockade confers neuroprotection from diffuse axonal injury. Cell Death Dis. 2019, 10, 727. [Google Scholar] [CrossRef] [Green Version]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.C.; Voorhees, J.R.; Genova, R.M.; Davis, K.C.; Madison, A.M.; Britt, J.K.; Cintrón-Pérez, C.J.; McDaniel, L.; Harper, M.M.; Pieper, A.A. Acute Axonal Degeneration Drives Development of Cognitive, Motor, and Visual Deficits after Blast-Mediated Traumatic Brain Injury in Mice. eNeuro 2016, 3, ENEURO.0220-16.2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, E.J.; Inglese, M. The Role of Thalamic Damage in Mild Traumatic Brain Injury. J. Neurotrauma 2016, 33, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolenec, P.; Pilipović, K.; Janković, T.; Župan, G. Pattern of Neuronal and Axonal Damage, Glial Response, and Synaptic Changes in Rat Cerebellum within the First Week following Traumatic Brain Injury. J. Neuropathol. Exp. Neurol. 2020, 79, 1163–1182. [Google Scholar] [CrossRef]
- Matser, J.T.; Kessels, A.G.; Jordan, B.D.; Lezak, M.D.; Troost, J. Chronic traumatic brain injury in professional soccer players. Neurology 1998, 51, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.K.; Janulewicz, P.A.; Seichepine, D.R.; Sullivan, K.A.; Proctor, S.P.; Krengel, M.H. Multiple Mild Traumatic Brain Injuries Are Associated with Increased Rates of Health Symptoms and Gulf War Illness in a Cohort of 1990-1991 Gulf War Veterans. Brain Sci. 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]


| Group | Total N | Mortality 1 | Acute Porphyrin 2 | Hematoma | Fracture |
|---|---|---|---|---|---|
| Sham | 10 | 0 | 0 | 0 | 0 |
| Aluminum | 10 | 0 | 1 | 0 | 0 |
| Stainless Steel | 10 | 2 | 2 | 5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalovicz, L.T.; Kelly, K.A.; Craddock, T.J.A.; O’Callaghan, J.P. A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury. Brain Sci. 2023, 13, 623. https://doi.org/10.3390/brainsci13040623
Michalovicz LT, Kelly KA, Craddock TJA, O’Callaghan JP. A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury. Brain Sciences. 2023; 13(4):623. https://doi.org/10.3390/brainsci13040623
Chicago/Turabian StyleMichalovicz, Lindsay T., Kimberly A. Kelly, Travis J. A. Craddock, and James P. O’Callaghan. 2023. "A Projectile Concussive Impact Model Produces Neuroinflammation in Both Mild and Moderate-Severe Traumatic Brain Injury" Brain Sciences 13, no. 4: 623. https://doi.org/10.3390/brainsci13040623





