Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson’s Disease
Abstract
1. Introduction
Role of Inflammation in PD
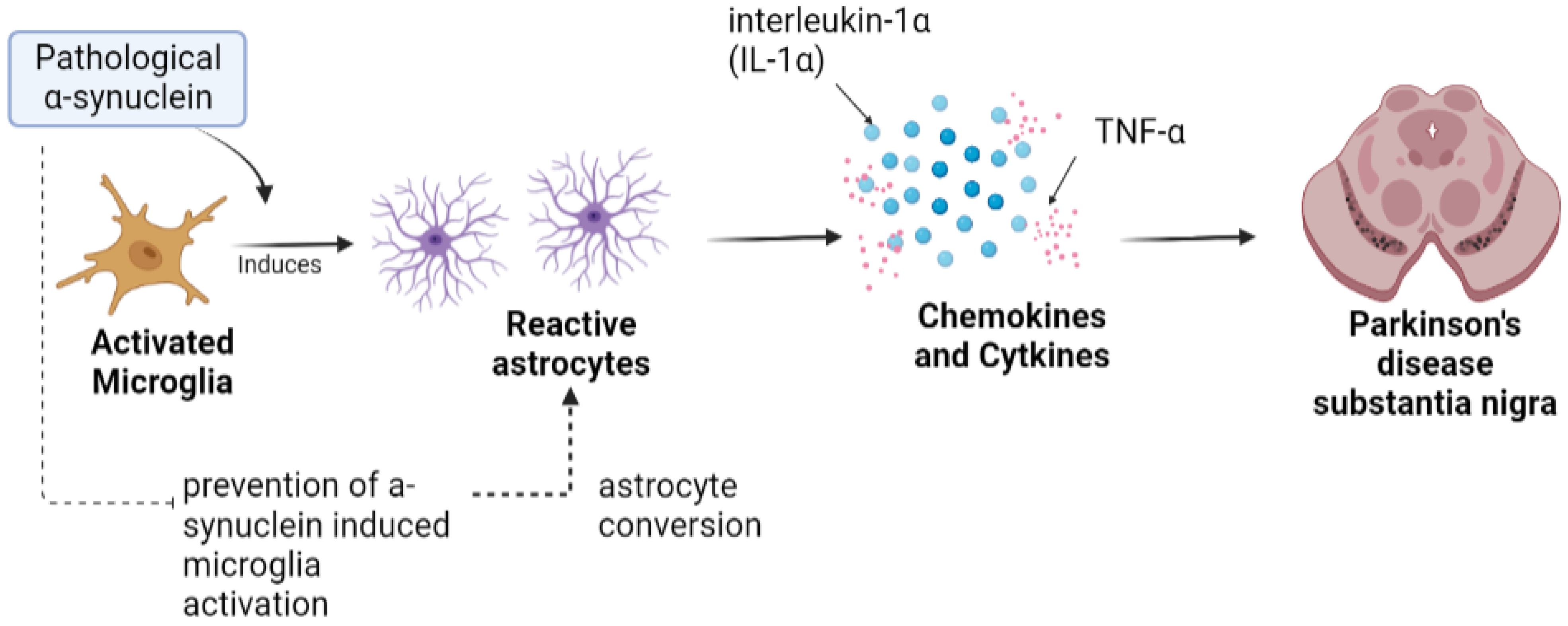
2. Microglia in PD
3. Astrocytes in PD
4. Neuroinflammation-Based Biomarkers in PD
| Inflammatory Biomarker | Sample Source | Summary | Effect in PD Patients | Reference | |
|---|---|---|---|---|---|
| Cytokines | IL-1β | Peripheral blood/CSF | Sustained IL-1β expression in the striatum leads to DAergic neuronal death resulting in motor disabilities | IL-1β levels are elevated in the serum Probability of REM sleep behavior disorder (PRBD) | [69] |
| IL-2 | Blood serum | Blood IL-2 levels are decreased, which reduces the number and function of treg cells, affecting autoimmunity and causing lymphoproliferation | Higher serum IL-2 levels than control participants and occurrence of symptoms of depression or anxiety | [78] | |
| IL-6 | CSF | Neuronal death in neurodegenerative diseases | Higher level of levels of plasma serum IL-6 that are correlated with an intense level of depression | [79] | |
| IL-10 | CSF/serum | Develops neuroprotective effects against LPS-induced cell death | Higher peripheral level of IL-10 | [80] | |
| TNF-α/sTNFRs | CSF | Activates microglia, which further induces the progressive loss of DAergic neurons | Positively associated with symptoms such as cognitive impairment, depression, and disability | [81] | |
| NLRP3 (inflammasome protein) | Biofluids | Progression biomarker in PD | Motor severity | [82] | |
| Chemokines | RANTES | Blood serum | Proinflammatory chemokine involved in the regulation of immunoreactions and the addition of immune cells such as monocytes, granulocytes, and T cells to sites of inflammation | Elevated level in serum of PD patient, with serum RANTES levels positively correlated with H&Y stages and disease duration | [83] |
| Protein | High-sensitivity C-reactive Protein (hsCRP) | Plasma | Indicative marker of ongoing inflammation tissue damage | Serum hsCRP levels are elevated in patients with PD as compared to control participants | [84] |
| Genetic mutation | Leucine-rich repeat kinase 2 (LRRK2) | Familial and sporadic PD patients | Reduces the secretion of TNF-α, as its expression and kinase activity are upregulated in lipopolysaccharide (LPS)-activated microglia | Higher expression of IL-6 and TNFα | [85] |
| PTEN-induced putative Kinase 1 (PINK1) | Familial PD patients | Detects mitochondrial dysfunction of phosphorylates in the PARKIN that degrade damaged mitochondria through a process called mitophagy | The upregulation of TNF-α, IL-1β, and IL-6 levels and mitophagy reduces inflammation by removing the damaged mitochondria | [72] | |
| Parkin (PRKN) | Autosomal-recessive early PD patients | Involved in mitophagy to degrade damaged mitochondria | Chronic inflammation | [86,87] | |
| DJ-1 | Familial-recessive PD patients | Interrupts the role of protein in the regulation of membrane receptor tracing DJ-1, which connects to the p65 subunit of NFκB, and the knockdown of DJ-1, which directs p65 nuclear translocation | - | [74] | |
| Radiotracers | Translocater protein TSPO-1 ([11C]PK11195) | PD patients | Elevated expression of mitochondrial translocator protein in activated microglia | Positively associated with a high level of motor dysfunction | [88,89] |
| TSPO-2 [18F]-FEPPA | PD patients | Detects neuroinflammation that is specific to the striatum | - | [90,91] | |
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmood, A.; Shah, A.A.; Umair, M.; Wu, Y.; Khan, A. Recalling the pathology of Parkinson’s disease; lacking exact figure of prevalence and genetic evidence in Asia with an alarming outcome: A time to step-up. Clin. Genet. 2021, 100, 659–677. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.; Coetzee, G.A. Parkinson’s disease-associated genetic variation is linked to quantitative expression of inflammatory genes. PLoS ONE 2017, 12, e0175882. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A. Inflammation and neurodegeneration: Chronicity matters. Aging 2019, 11, 3. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, Y.; Zhu, K.; Xie, A. The association between TLR3 rs3775290 polymorphism and sporadic Parkinson’s disease in Chinese Han population. Neurosci. Lett. 2020, 728, 135005. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Garcia, C.P. Inflammation-related gene polymorphisms associated with Parkinson’s disease: An updated meta-analysis. Egypt. J. Med. Hum. Genet. 2020, 21, 14. [Google Scholar] [CrossRef]
- Theodore, S.; Cao, S.; McLean, P.J.; Standaert, D.G. Targeted overexpression of human α-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2008, 67, 1149–1158. [Google Scholar] [CrossRef]
- Graeber, M.B.; Streit, W.J. Microglia: Biology and pathology. Acta Neuropathol. 2010, 119, 89–105. [Google Scholar] [CrossRef]
- Graeber, M.B. Changing face of microglia. Science 2010, 330, 783–788. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Pannese, E. Neurocytology: Fine Structure of Neurons, Nerve Processes, and Neuroglial Cells; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Holtbernd, F.; Ma, Y.; Peng, S.; Schwartz, F.; Timmermann, L.; Kracht, L.; Fink, G.R.; Tang, C.C.; Eidelberg, D.; Eggers, C. Dopaminergic correlates of metabolic network activity in Parkinson’s disease. Hum. Brain Mapp. 2015, 36, 3575–3585. [Google Scholar] [CrossRef]
- Escartin, C.; Guillemaud, O.; Carrillo-de Sauvage, M. Questions and (some) answers on reactive astrocytes. Glia 2019, 67, 2221–2247. [Google Scholar] [CrossRef]
- Sher, A.A.; Gao, A.; Coombs, K.M. Autophagy Modulators Profoundly Alter the Astrocyte Cellular Proteome. Cells 2020, 9, 805. [Google Scholar] [CrossRef]
- Pang, S.Y.-Y.; Ho, P.W.-L.; Liu, H.-F.; Leung, C.-T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Cunningham, C.; Lopez-Rodriguez, A.B. Astrocytes: Heterogeneous and Dynamic Phenotypes in Neurodegeneration and Innate Immunity. Neuroscientist 2018, 25, 455–474. [Google Scholar] [CrossRef]
- Ho, D.H.; Lee, H.; Son, I.; Seol, W. G2019s LRRK2 promotes mitochondrial fission and increases TNFalpha-mediated neuroinflammation responses. Anim. Cells Syst. 2019, 23, 106–111. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Zhuang, Q.-Q.; Zhu, L.-B.; Zhu, H.; Li, T.; Li, R.; Chen, S.-F.; Huang, C.-P.; Zhang, X.; Zhu, J.-H. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci. Rep. 2016, 6, 36669. [Google Scholar] [CrossRef]
- Meara, J.; Bhowmick, B.K.; Hobson, P. Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age Ageing 1999, 28, 99–102. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.W.; Schuh, A.F.S.; Saute, J.; Townsend, R.; Fricke, D.; Leke, R.; Souza, D.O.; Portela, L.V.; Chaves, M.L.F.; Rieder, C.R.M. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res. 2009, 34, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. S erum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef]
- Ezcurra, A.L.D.L.; Chertoff, M.; Ferrari, C.; Graciarena, M.; Pitossi, F. Chronic expression of low levels of tumor necrosis factor-α in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol. Dis. 2010, 37, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.P.; Schonhoff, A.M.; Sette, A.; Lindestam Arlehamn, C.S. Central and peripheral inflammation: Connecting the immune responses of Parkinson’s disease. J. Park. Dis. 2022, 12, S129–S136. [Google Scholar] [CrossRef]
- Loane, C.; Politis, M. Positron emission tomography neuroimaging in Parkinson’s disease. Am. J. Transl. Res. 2011, 3, 323. [Google Scholar]
- Latz, E.; Sam Xiao, T.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Mittelbronn, M.; Dietz, K.; Schluesener, H.J.; Meyermann, R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001, 101, 249–255. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; Ragozzino, D.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Askew, K.; Olmos-Alonso, A.; Garcia-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.A.; Riecken, K.; Beccari, S.; Sierra, A.; et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017, 18, 391–405. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Lev, N.; Barhum, Y.; Ben-Zur, T.; Melamed, E.; Steiner, I.; Offen, D. Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 2013, 50, 542–550. [Google Scholar] [CrossRef]
- Spangenberg, E.E.; Lee, R.J.; Najafi, A.R.; Rice, R.A.; Elmore, M.R.P.; Blurton-Jones, M.; West, B.L.; Green, K.N. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 2016, 139, 1265–1281. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Zhang, H.; Wang, T.; Zheng, Q.; Li, Z. Controlled activation of TRPV1 channels on microglia to boost their autophagy for clearance of alpha-Synuclein and enhance therapy of Parkinson’s disease. Adv. Mater. 2022, 34, e2108435. [Google Scholar] [CrossRef]
- Hu, Y.; Reggiori, F. Molecular regulation of autophagosome formation. Biochem. Soc. Trans. 2022, 50, 55–69. [Google Scholar] [CrossRef]
- Ronan, J.L.; Wu, W.; Crabtree, G.R. From neural development to cognition: Unexpected roles for chromatin. Nat. Rev. Genet. 2013, 14, 347–359. [Google Scholar] [CrossRef]
- Huang, W.; Lv, Q.; Xiao, Y.; Zhong, Z.; Hu, B.; Yan, S.; Yan, Y.; Zhang, J.; Shi, T.; Jiang, L.; et al. Triggering Receptor Expressed on Myeloid Cells 2 Protects Dopaminergic Neurons by Promoting Autophagy in the Inflammatory Pathogenesis of Parkinson’s Disease. Front. Neurosci. 2021, 15, 745815. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Bartels, A.; Willemsen, A.T.M.; Doorduin, J.; de Vries, E.F.J.; Dierckx, R.A.; Leenders, K.L. [11C]-PK11195 PET: Quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Park. Relat. Disord. 2010, 16, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.; Nakamura, M.C.; Hsieh, C.L. Brain trauma elicits non-canonical macrophage activation states. J. Neuroinflamm. 2016, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.-K. Functional diversity of microglia–how heterogeneous are they to begin with? Front. Cell. Neurosci. 2013, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, C.; Gustin, A.; Birck, C.; Kirchmeyer, M.; Beaume, N.; Felten, P.; Grandbarbe, L.; Heuschling, P.; Heurtaux, T. Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: Stronger effects of the A53T mutant. PLoS ONE 2016, 11, e0162717. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Standaert, D.G.; Harms, A.S. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J. Neuroinflamm. 2012, 9, 259. [Google Scholar] [CrossRef]
- Lee, E.-J.; Woo, M.-S.; Moon, P.-G.; Baek, M.-C.; Choi, I.-Y.; Kim, W.-K.; Junn, E.; Kim, H.-S. α-Synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J. Immunol. 2010, 185, 615–623. [Google Scholar] [CrossRef]
- Li, S.; Bi, G.; Han, S.; Huang, R. MicroRNAs Play a Role in Parkinson’s Disease by Regulating Microglia Function: From Pathogenetic Involvement to Therapeutic Potential. Front. Mol. Neurosci. 2022, 14, 744942. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Astrocyte–neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef]
- Cabezas, R.; Ãvila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Coronel, J.C.J.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Solano, S.M.; Miller, D.W.; Augood, S.J.; Young, A.B.; Penney, J.B. Expression of α-synuclein, parkin, and ubiquitin carboxy-terminal hydrolase L1 mRNA in human brain: Genes associated with familial Parkinson’s disease. Ann. Neurol. 2000, 47, 201–210. [Google Scholar] [CrossRef]
- Braak, H.; Sastre, M.; Del Tredici, K. Development of α-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007, 114, 231–241. [Google Scholar] [CrossRef]
- Hansen, C.; Angot, E.; Bergström, A.-L.; Steiner, J.A.; Pieri, L.; Paul, G.; Outeiro, T.F.; Melki, R.; Kallunki, P.; Fog, K.; et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Investig. 2011, 121, 715–725. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, C.; Lee, S.-J. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxidative Med. Cell. Longev. 2010, 3, 283–287. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015, 16, 57. [Google Scholar] [CrossRef]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef]
- Klegeris, A.; Giasson, B.I.; Zhang, H.; Maguire, J.; Pelech, S.; McGeer, P.L. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006, 20, 2000–2008. [Google Scholar] [CrossRef]
- Gu, X.-L.; Long, C.-X.; Sun, L.; Xie, C.; Lin, X.; Cai, H. Astrocytic expression of Parkinson’s disease-related A53T α-synuclein causes neurodegeneration in mice. Mol. Brain 2010, 3, 12. [Google Scholar] [CrossRef]
- Kim, J.-M.; Cha, S.-H.; Choi, Y.R.; Jou, I.; Joe, E.-H.; Park, S.M. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci. Rep. 2016, 6, 28823. [Google Scholar] [CrossRef]
- Mullett, S.J.; Hinkle, D.A. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009, 33, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Santaella, A.; Kuiperij, H.B.; van Rumund, A.; Esselink, R.A.J.; van Gool, A.J.; Bloem, B.R.; Verbeek, M.M. Inflammation biomarker discovery in Parkinson’s disease and atypical parkinsonisms. BMC Neurol. 2020, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko, N.; Friedman, A. Neutrophil-to-lymphocyte ratio (NLR) at boundaries of Progressive Supranuclear Palsy Syndrome (PSPS) and Corticobasal Syndrome (CBS). Neurol. Neurochir. Pol. 2021, 55, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D. Friedman, Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. Neurochir. Pol. 2022, 56, 148–155. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Park. Dis. 2023, 9, 18. [Google Scholar] [CrossRef]
- Eusebi, P.; Giannandrea, D.; Biscetti, L.; Abraha, I.; Chiasserini, D.; Orso, M.; Calabresi, P.; Parnetti, L. Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2017, 32, 1389–1400. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Caspell-Garcia, C.J.; Coffey, C.S.; Taylor, P.; Shaw, L.M.; Trojanowski, J.Q.; Singleton, A.; Frasier, M.; Marek, K.; Galasko, D. Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. Neurology 2017, 89, 1959–1969. [Google Scholar] [CrossRef]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef]
- Liu, T.-W.; Chen, C.-M.; Chang, K.-H. Biomarker of neuroinflammation in Parkinson’s disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef]
- Paisán-Ruíz, C.; Jain, S.; Evans, E.W.; Gilks, W.P.; Simón, J.; van der Brug, M.; de Munain, A.L.; Aparicio, S.; Gil, A.M.; Khan, N.; et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 2004, 44, 595–600. [Google Scholar] [CrossRef]
- Cookson, M.R. Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb. Perspect. Med. 2012, 2, a009415. [Google Scholar] [CrossRef]
- Shimura, H.; Hattori, N.; Kubo, S.; Mizuno, Y.; Asakawa, S.; Minoshima, S.; Shimizu, N.; Iwai, K.; Chiba, T.; Tanaka, K.; et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000, 25, 302–305. [Google Scholar] [CrossRef]
- Bonifati, V. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Banati, R.B. Visualising microglial activation in vivo. Glia 2002, 40, 206–217. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Zimmermann, J.; Sixel-Döring, F.; Focke, N.K.; Wicke, T.; Ebentheuer, J.; Schaumburg, M.; Lang, E.; Trautmann, E.; Zetterberg, H.; et al. Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 2016, 87, 168–177. [Google Scholar] [CrossRef]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J.M. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: A cohort study. Lancet Neurol. 2017, 16, 66–75. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Nagatsu, T. Interleukin-2 but not basic fibroblast growth factor is elevated in parkinsonian brain. J. Neural Transm. 1996, 103, 1077–1081. [Google Scholar] [CrossRef]
- Bu, X.-L.; Wang, X.; Xiang, Y.; Shen, L.-L.; Wang, Q.-H.; Liu, Y.-H.; Jiao, S.-S.; Wang, Y.-R.; Cao, H.-Y.; Yi, X.; et al. The association between infectious burden and Parkinson’s disease: A case-control study. Park. Relat. Disord. 2015, 21, 877–881. [Google Scholar] [CrossRef]
- Brodacki, B.; Staszewski, J.; Toczyłowska, B.; Kozłowska, E.; Drela, N.; Chalimoniuk, M.; Stępien, A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFα, and INFγ concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 2008, 441, 158–162. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Riederer, P.; Narabayashi, H.; Fujita, K.; Nagatsu, T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994, 165, 208–210. [Google Scholar] [CrossRef]
- Fan, Z.; Pan, Y.-T.; Zhang, Z.-Y.; Yang, H.; Yu, S.-Y.; Zheng, Y.; Ma, J.-H.; Wang, X.-M. Systemic activation of NLRP3 inflammasome and plasma α-synuclein levels are correlated with motor severity and progression in Parkinson’s disease. J. Neuroinflamm. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, P.; de Miranda, A.S.; Guerra Amaral, D.C.; de Carvalho Vilela, M.; Cardoso, F.; Teixeira, A.L. Serum levels of chemokines in Parkinson’s disease. Neuroimmunomodulation 2011, 18, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.-J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.-R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Kwon, E.H.; Tennagels, S.; Gold, R.; Gerwert, K.; Beyer, L.; Tönges, L. Update on CSF biomarkers in Parkinson’s disease. Biomolecules 2022, 12, 329. [Google Scholar] [CrossRef]
- Koshimori, Y.; Ko, J.-H.; Mizrahi, R.; Rusjan, P.; Mabrouk, R.; Jacobs, M.F.; Christopher, L.; Hamani, C.; Lang, A.E.; Wilson, A.A.; et al. Imaging striatal microglial activation in patients with Parkinson’s disease. PLoS ONE 2015, 10, e0138721. [Google Scholar] [CrossRef]
- Xue, X.; Duan, R.; Zheng, G.; Chen, H.; Zhang, W.; Shi, L. Translocator protein (18 kDa) regulates the microglial phenotype in Parkinson’s disease through P47. Bioengineered 2022, 13, 11062–11072. [Google Scholar] [CrossRef]
- Booth, H.D.; Hirst, W.D.; Wade-Martins, R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Corsi, F.; Baglini, E.; Barresi, E.; Salerno, S.; Cerri, C.; Martini, C.; Da Settimo Passetti, F.; Taliani, S.; Gargini, C.; Piano, I. Targeting TSPO Reduces Inflammation and Apoptosis in an In Vitro Photoreceptor-Like Model of Retinal Degeneration. ACS Chem. Neurosci. 2022, 13, 3188–3197. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, H.; Ilyas, I.; Mahmood, A.; Hou, L. Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson’s Disease. Brain Sci. 2023, 13, 634. https://doi.org/10.3390/brainsci13040634
Chen K, Wang H, Ilyas I, Mahmood A, Hou L. Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson’s Disease. Brain Sciences. 2023; 13(4):634. https://doi.org/10.3390/brainsci13040634
Chicago/Turabian StyleChen, Kun, Haoyang Wang, Iqra Ilyas, Arif Mahmood, and Lijun Hou. 2023. "Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson’s Disease" Brain Sciences 13, no. 4: 634. https://doi.org/10.3390/brainsci13040634
APA StyleChen, K., Wang, H., Ilyas, I., Mahmood, A., & Hou, L. (2023). Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson’s Disease. Brain Sciences, 13(4), 634. https://doi.org/10.3390/brainsci13040634







