Effect of Post-COVID-19 on Brain Volume and Glucose Metabolism: Influence of Time Since Infection and Fatigue Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Protocol
2.3. Fatigue and Fatigability Assessments
2.4. Imaging Acquisition
2.5. MRI Analysis
2.6. PET Analysis
2.7. Normative Database Comparison
2.8. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Structural Analysis
3.2.1. Relative Regional Volume Comparison: <6 Months Post-Infection vs. >6 Months Post-Infection
3.2.2. Relative Regional Volume Comparison: Fatigued vs. Non-Fatigued in >6 Months Post-Infection Group
3.2.3. Relative Regional Volume Comparison: Fatigued vs. Non-Fatigued in <6 Months Post-Infection
3.2.4. Relative Regional Volume and FSS Correlations
3.2.5. Relative Regional Volumes and FAS Correlations
3.3. Metabolic Analysis
3.3.1. SUV and Relative Regional Metabolism
3.3.2. Normative Database Comparison
4. Discussion
Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Zhao, W.; Huang, S.; Huang, Y.; Chen, Y.; Zhang, H.; Guo, H.; Liu, J. Two-year follow-up of brain structural changes in patients who recovered from COVID-19: A prospective study. Psychiatry Res. 2023, 319, 114969. [Google Scholar] [CrossRef]
- Tian, T.; Wu, J.; Chen, T.; Li, J.; Yan, S.; Zhou, Y.; Peng, X.; Li, Y.; Zheng, N.; Cai, A.; et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight 2022, 7, e155827. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, I.; Chatys-Bogacka, Z.; Slowik, J.; Klich-Raczka, A.; Fedyk-Lukasik, M.; Slowik, A.; Wnuk, M.; Drabik, L. Fatigue after COVID-19 in non-hospitalized patients according to sex. Brain Behav. 2023, 13, e2849. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Fietsam, A.C.; Deters, J.R.; Bryant, A.D.; Kamholz, J. Post-COVID-19 Fatigue: Potential Contributing Factors. Brain Sci. 2020, 10, 1012. [Google Scholar] [CrossRef]
- Fietsam, A.C.; Bryant, A.D.; Rudroff, T. Fatigue and perceived fatigability, not objective fatigability, are prevalent in people with post-COVID-19. Exp. Brain Res. 2022, 241, 211–219. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Fumagalli, C.; Zocchi, C.; Tassetti, L.; Silverii, M.V.; Amato, C.; Livi, L.; Giovannoni, L.; Verrillo, F.; Bartoloni, A.; Marcucci, R.; et al. Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur. J. Intern. Med. 2022, 97, 36–41. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Martini, A.L.; Carli, G.; Kiferle, L.; Piersanti, P.; Palumbo, P.; Morbelli, S.; Calcagni, M.L.; Perani, D.; Sestini, S. Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 90–102. [Google Scholar] [CrossRef]
- Valizadeh, N.; Rudmann, E.A.; Solomon, I.H.; Mukerji, S.S. Mechanisms of Entry Into the Central Nervous System by Neuroinvasive Pathogens. J. Neuroophthalmol. 2022, 42, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Besteher, B.; Machnik, M.; Troll, M.; Toepffer, A.; Zerekidze, A.; Rocktäschel, T.; Heller, C.; Kikinis, Z.; Brodoehl, S.; Finke, K.; et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res. 2022, 317, 114836. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Ponto, L.L.B. (18)F-FDG-PET Imaging for Post-COVID-19 Brain and Skeletal Muscle Alterations. Viruses 2021, 13, 2283. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Barthel, H.; Tolboom, N.; Fraioli, F.; Cecchin, D.; Albert, N.L.; Van Berckel, B.; Boellaard, R.; Brendel, M.; Ekmekcioglu, O.; et al. 2-[18F]-FDG PET for imaging brain involvement in patients with long COVID: Perspective of the EANM Neuroimaging Committee. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3599–3606. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.T.; Hellwig, S.; Blazhenets, G.; Hosp, J.A. Molecular Imaging Findings on Acute and Long-Term Effects of COVID-19 on the Brain: A Systematic Review. J. Nucl. Med. 2022, 63, 971–980. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef]
- Sollini, M.; Morbelli, S.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Chiola, S.; Gelardi, F.; Chiti, A. Long COVID hallmarks on [18F]FDG-PET/CT: A case-control study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3187–3197. [Google Scholar] [CrossRef]
- Morand, A.; Campion, J.-Y.; Lepine, A.; Bosdure, E.; Luciani, L.; Cammilleri, S.; Chabrol, B.; Guedj, E. Similar patterns of [18F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: A paediatric case series. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 913–920. [Google Scholar] [CrossRef]
- Dressing, A.; Bormann, T.; Blazhenets, G.; Schroeter, N.; Walter, L.I.; Thurow, J.; August, D.; Hilger, H.; Stete, K.; Gerstacker, K.; et al. Neuropsychological profiles and cerebral glucose metabolism in neurocognitive Long COVID-syndrome. J. Nucl. Med. 2021, 63, 1058–1063. [Google Scholar] [CrossRef]
- Okrzeja, J.; Garkowski, A.; Kubas, B.; Moniuszko-Malinowska, A. Imaging and neuropathological findings in patients with Post COVID-19 Neurological Syndrome-A review. Front. Neurol. 2023, 14, 1136348. [Google Scholar] [CrossRef]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Michielsen, H.J.; De Vries, J.; Van Heck, G.L. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J. Psychosom. Res. 2003, 54, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; McFadyen, D.A.; Wood, D.L.; Moffat, D.F.; Paul, P.L. Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 35, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bernstein, M.A.; Fox, N.C.; Thompson, P.; Alexander, G.; Harvey, D.; Borowski, B.; Britson, P.J.; Whitwell, J.L.; Ward, C.; et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Hammers, A.; Allom, R.; Koepp, M.J.; Free, S.L.; Myers, R.; Lemieux, L.; Mitchell, T.N.; Brooks, D.J.; Duncan, J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003, 19, 224–247. [Google Scholar] [CrossRef]
- Bispo, D.D.C.; Brandão, P.R.P.; Pereira, D.A.; Maluf, F.B.; Dias, B.A.; Paranhos, H.R.; von Glehn, F.; de Oliveira, A.C.P.; Regattieri, N.A.T.; Silva, L.S.; et al. Brain microstructural changes and fatigue after COVID-19. Front. Neurol. 2022, 13, 1029302. [Google Scholar] [CrossRef]
- Capone, F.; Collorone, S.; Cortese, R.; Di Lazzaro, V.; Moccia, M. Fatigue in multiple sclerosis: The role of thalamus. Mult. Scler. J. 2020, 26, 6–16. [Google Scholar] [CrossRef]
- Sepulcre, J.; Masdeu, J.C.; Goñi, J.; Arrondo, G.; Vélez de Mendizábal, N.; Bejarano, B.; Villoslada, P. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult. Scler. 2009, 15, 337–344. [Google Scholar] [CrossRef]
- Miller, A.H.; Jones, J.F.; Drake, D.F.; Tian, H.; Unger, E.R.; Pagnoni, G. Decreased basal ganglia activation in subjects with chronic fatigue syndrome: Association with symptoms of fatigue. PLoS ONE 2014, 9, e98156. [Google Scholar] [CrossRef]
- Han, P.; Zang, Y.; Akshita, J.; Hummel, T. Magnetic Resonance Imaging of Human Olfactory Dysfunction. Brain Topogr. 2019, 32, 987–997. [Google Scholar] [CrossRef]
- Yus, M.; Matias-Guiu, J.A.; Gil-Martínez, L.; Gómez-Ruiz, N.; Polidura, C.; Jorquera, M.; Delgado-Alonso, C.; Díez-Cirarda, M.; Matías-Guiu, J.; Arrazola, J. Persistent olfactory dysfunction after COVID-19 is associated with reduced perfusion in the frontal lobe. Acta. Neurol. Scand 2022, 146, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, J.G.; Heckmann, S.M.; Lang, C.J.G.; Hummel, T. Neurological Aspects of Taste Disorders. Arch. Neurol. 2003, 60, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C.; Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. The D614G Virus Mutation Enhances Anosmia in COVID-19 Patients: Evidence from a Systematic Review and Meta-analysis of Studies from South Asia. ACS Chem. Neurosci. 2021, 12, 3535–3549. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Goñi, M.; Basu, N.; Murray, A.; Waiter, G. Neural Indicators of Fatigue in Chronic Diseases: A Systematic Review of MRI Studies. Diagnostics 2018, 8, 42. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000, 179, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Penner, I.-K.; Paul, F. Fatigue as a symptom or comorbidity of neurological diseases. Nat. Rev. Neurol. 2017, 13, 662–675. [Google Scholar] [CrossRef]
- Clark, A.L.; Sorg, S.F.; Holiday, K.; Bigler, E.D.; Bangen, K.J.; Evangelista, N.D.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. Fatigue Is Associated With Global and Regional Thalamic Morphometry in Veterans With a History of Mild Traumatic Brain Injury. J. Head Trauma. Rehabil. 2018, 33, 382–392. [Google Scholar] [CrossRef]
- Angioni, D.; Virecoulon Giudici, K.; Montoya Martinez, M.; Rolland, Y.; Vellas, B.; de Souto Barreto, P. Neuroimaging markers of chronic fatigue in older people: A narrative review. Aging Clin. Exp. Res. 2021, 33, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.B.; Brann, D.H.; Abi Hachem, R.; Jang, D.W.; Oliva, A.D.; Ko, T.; Gupta, R.; Wellford, S.A.; Moseman, E.A.; Jang, S.S.; et al. Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci. Transl. Med. 2022, 14, eadd0484. [Google Scholar] [CrossRef]
- Han, B.H.; Moore, A.A. Prevention and Screening of Unhealthy Substance Use by Older Adults. Clin. Geriatr. Med. 2018, 34, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Díez-Cirarda, M.; Yus, M.; Gómez-Ruiz, N.; Polidura, C.; Gil-Martínez, L.; Delgado-Alonso, C.; Jorquera, M.; Gómez-Pinedo, U.; Matias-Guiu, J.; Arrazola, J.; et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 2022. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.-Y.; Huang, C.-C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients–An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef]
- Tu, Y.; Zhang, Y.; Li, Y.; Zhao, Q.; Bi, Y.; Lu, X.; Kong, Y.; Wang, L.; Lu, Z.; Hu, L. Post-traumatic stress symptoms in COVID-19 survivors: A self-report and brain imaging follow-up study. Mol. Psychiatry 2021, 26, 7475–7480. [Google Scholar] [CrossRef]
- Conner, A.K.; Briggs, R.G.; Sali, G.; Rahimi, M.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 13: Tractographic Description of the Inferior Fronto-Occipital Fasciculus. Oper. Neurosurg. 2018, 15, S436–S443. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, D.; Wang, Y.; Wang, Y.; Ou, S. Segmentation of the Cingulum Bundle in the Human Brain: A New Perspective Based on DSI Tractography and Fiber Dissection Study. Front. Neuroanat. 2016, 10, 84. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Maestri, R.; Dezi, S.; Spampinato, D.; Saltuari, L.; Alibardi, A.; Engl, M.; Kofler, M.; et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19. Eur. J. Neurol. 2022, 29, 1652–1662. [Google Scholar] [CrossRef]
- Bellucci, G.; Rinaldi, V.; Buscarinu, M.C.; Reniè, R.; Bigi, R.; Pellicciari, G.; Morena, E.; Romano, C.; Marrone, A.; Mechelli, R.; et al. Multiple Sclerosis and SARS-CoV-2: Has the Interplay Started? Front. Immunol. 2021, 12, 755333. [Google Scholar] [CrossRef] [PubMed]
- Novo, A.M.; Batista, S.; Alves, C.; d’Almeida, O.C.; Marques, I.B.; Macário, C.; Santana, I.; Sousa, L.; Castelo-Branco, M.; Cunha, L. The neural basis of fatigue in multiple sclerosis: A multimodal MRI approach. Neurol. Clin. Pract. 2018, 8, 492–500. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Chammas, A.; Bund, C.; Lersy, F.; Brisset, J.C.; Ardellier, F.D.; Kremer, S.; Namer, I.J. Collicular Hyperactivation in Patients with COVID-19: A New Finding on Brain MRI and PET/CT. Am. J. Neuroradiol. 2021, 42, 1410–1414. [Google Scholar] [CrossRef]
- Hosp, J.A.; Dressing, A.; Blazhenets, G.; Bormann, T.; Rau, A.; Schwabenland, M.; Thurow, J.; Wagner, D.; Waller, C.; Niesen, W.D.; et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021, 144, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Yousefi-Koma, A.; Haseli, S.; Bakhshayeshkaram, M.; Raad, N.; Karimi-Galougahi, M. Multimodality Imaging With PET/CT and MRI Reveals Hypometabolism in Tertiary Olfactory Cortex in Parosmia of COVID-19. Acad. Radiol. 2021, 28, 749–751. [Google Scholar] [CrossRef]
- Chammas, A.; Namer, I.J.; Lersy, F.; Kremer, S.; Bund, C. Inferior Colliculus’s Hypermetabolism: A New Finding on Brain FDG PET and Perfusion MRI in a Patient With COVID-19. Clin. Nucl. Med. 2021, 46, 413–414. [Google Scholar] [CrossRef]
- Verger, A.; Kas, A.; Dudouet, P.; Goehringer, F.; Salmon-Ceron, D.; Guedj, E. Visual interpretation of brain hypometabolism related to neurological long COVID: A French multicentric experience. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3197–3202. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain 2009, 132, 1355–1365. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Nugent, S.; Castellano, C.A.; Goffaux, P.; Whittingstall, K.; Lepage, M.; Paquet, N.; Bocti, C.; Fulop, T.; Cunnane, S.C. Glucose hypometabolism is highly localized, but lower cortical thickness and brain atrophy are widespread in cognitively normal older adults. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1315–E1321. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Katako, A.; Aljuaid, M.; Goertzen, A.L.; Borys, A.; Hobson, D.E.; Kim, S.M.; Lee, C.S. Distinct brain metabolic patterns separately associated with cognition, motor function, and aging in Parkinson’s disease dementia. Neurobiol. Aging 2017, 60, 81–91. [Google Scholar] [CrossRef]
- Venkataramani, V.; Winkler, F. Cognitive Deficits in Long COVID-19. N. Engl. J. Med. 2022, 387, 1813–1815. [Google Scholar] [CrossRef] [PubMed]
- Guedj, E.; Morbelli, S.; Kaphan, E.; Campion, J.-Y.; Dudouet, P.; Ceccaldi, M.; Cammilleri, S.; Nobili, F.; Eldin, C. From early limbic inflammation to long COVID sequelae. Brain 2021, 144, e65. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.T.; Blazhenets, G.; Prinz, M.; Hosp, J.A. Reply: From early limbic inflammation to long COVID sequelae. Brain 2021, 144, e66. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and older people. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef]

| (a). Subject Characteristic for the <6 Months and >6 Months Groups. | ||||||
|---|---|---|---|---|---|---|
| Group | <6 Months Post-Infection | >6 Months Post-Infection | p-Value | |||
| N (f) | 18 (11) | 15 (6) | 0.49 | |||
| # Fatigued (female) | 9 (6) | 7 (2) | 0.85 | |||
| CFQ-11 Score (F) | 7.80 ± 2.15 | 8.57 ± 2.57 | 0.36 | |||
| CFQ-11 Score (NF) | 1.56 ± 1.58 | 1.13 ± 1.36 | 0.41 | |||
| Age (years) | 36.61 ± 15.66 | 30.40 ± 13.01 | 0.23 | |||
| Weight (kg) | 78.66 ± 21.79 | 80.50 ± 22.42 | 0.81 | |||
| Height (cm) | 167.78 ± 39.81 | 174.75 ± 44.78 | 0.64 | |||
| BMI | 28.09 ± 8.08 | 26.38 ± 7.19 | 0.53 | |||
| Time since Infection (months) | 3.68 ± 1.69 | 12.61 ± 4.79 | <0.01 | |||
| # Vaccinated | 16 | 15 | ||||
| # Vaccinated before infection | 10 | 3 | ||||
| # Vaccinated after infection | 6 | 12 | ||||
| (b). Sub-group characteristics and outcome of the FSS and FAS for the fatigued and non-fatigued groups at each time point. | ||||||
| Group | <6 months F | <6 months NF | p-Value | >6 months F | >6 months NF | p-Value |
| Age | 39.6 ± 16.2 | 36.6 ± 15.7 | 0.70 | 37.1 ± 12.4 (18–54) | 24.5 ± 4.6 (19–32) | 0.02 |
| N (f) | 9 (6) | 9 (5) | 0.65 | 7 (2) | 8 (4) | 0.44 |
| Height (cm) | 169.3 ± 11.98 | 166.2 ± 9.1 | 0.55 | 172.7 ± 7.5 | 176.5 ± 12.3 | 0.49 |
| Weight (kg) | 78.9 ± 11.3 | 78.7 ± 21.8 | 0.98 | 81.8 ± 9.1 | 79.4 ± 11.6 | 0.67 |
| FSS Score | 4.6 ± 1.5 | 2.1 ± 0.5 | <0.001 | 4.4 ± 1.8 | 2.3 ± 0.7 | 0.009 |
| FAS Score | 28.6 ± 7.8 | 16.0 ± 5.1 | <0.001 | 30.0 ± 10.0 | 14.6 ± 3.4 | 0.001 |
| (a). Results of the RRV Comparison between the Two Time Points. | ||||||
|---|---|---|---|---|---|---|
| Region | <6 Months Post-Infection | >6 Months Post-Infection | p-Value | Cohen’s d | ||
| L Putamen | 0.41 ± 0.02 | 0.43 ± 0.02 | 0.01 | 1.00 | ||
| R Putamen | 0.40 ± 0.03 | 0.42 ± 0.02 | 0.03 | 0.77 | ||
| L Pallidum | 0.11 ± 0.008 | 0.12 ± 0.007 | 0.01 | 1.30 | ||
| R Pallidum | 0.11 ± 0.009 | 0.12 ± 0.007 | 0.003 | 1.23 | ||
| L Thalamus | 0.59 ± 0.04 | 0.62 ± 0.03 | 0.03 | 0.84 | ||
| R Thalamus | 0.57 ± 0.04 | 0.60 ± 0.03 | 0.01 | 0.84 | ||
| R Cuneus | 0.59 ± 0.05 | 0.55 ± 0.04 | 0.01 | 0.87 | ||
| (b). Results of the RRV comparisons between fatigued and non-fatigued at each time point. | ||||||
| Region | <6 months F | <6 months NF | >6 months F | >6 months NF | p-value | Cohen’s d |
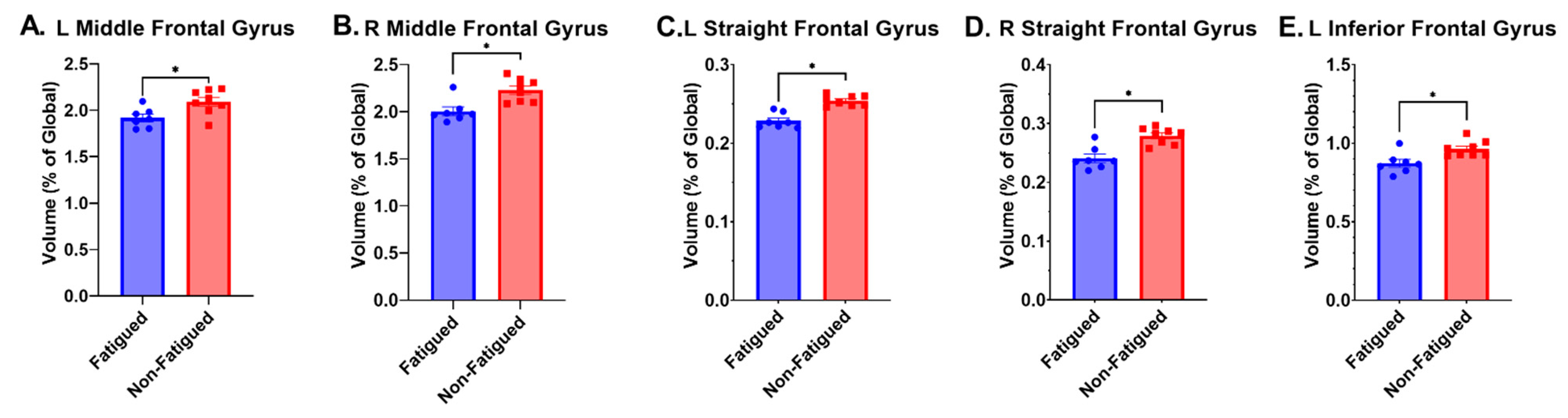
| L Middle Fr. Gyrus | * | * | 1.92 ± 0.11 | 2.09 ± 0.13 | 0.02 | 1.40 |
| R Middle Fr. Gyrus | * | * | 2.01 ± 0.12 | 2.23 ± 0.12 | 0.004 | 1.83 |
| L Straight Fr. Gyrus | * | * | 0.23 ± 0.01 | 0.25 ± 0.01 | <0.001 | 2.00 |
| R Straight Fr. Gyrus | * | * | 0.24 ± 0.02 | 0.28 ± 0.01 | <0.001 | 2.60 |
| L Ant. Orbital Gyrus | * | * | 0.33 ± 0.03 | 0.37 ± 0.01 | 0.02 | 1.85 |
| L Inferior Fr. Gyrus | * | * | 0.87 ± 0.07 | 0.96 ± 0.05 | 0.01 | 1.50 |
| L Mid. Orbital Gyrus | * | * | 0.33 ± 0.02 | 0.37 ± 0.02 | 0.005 | 2.00 |
| R Mid. Orbital Gyrus | * | * | 0.32 ± 0.03 | 0.36 ± 0.03 | 0.03 | 1.33 |
| R Sup. Post. Temp. Gyrus | * | * | 0.65 ± 0.06 | 0.72 ± 0.05 | 0.04 | 1.28 |
| R Ant. Cingulate Gyrus | * | * | 0.41 ± 0.03 | 0.45 ± 0.03 | 0.03 | 1.33 |
| L Caudate Nucleus | 0.32 ± 0.03 | 0.35 ± 0.02 | * | * | 0.05 | 1.19 |
| R Nucleus Accumbens | 0.025 ± 0.002 | 0.027 ± 0.002 | * | * | 0.04 | 1.00 |
| L Substantia Nigra | 0.028 ± 0.002 | 0.030 ± 0.002 | * | * | 0.04 | 1.00 |
| >6 Months Post-Infection | ||||
|---|---|---|---|---|
| Region | FSS r-Value | p-Value | FAS r-Value | p-Value |
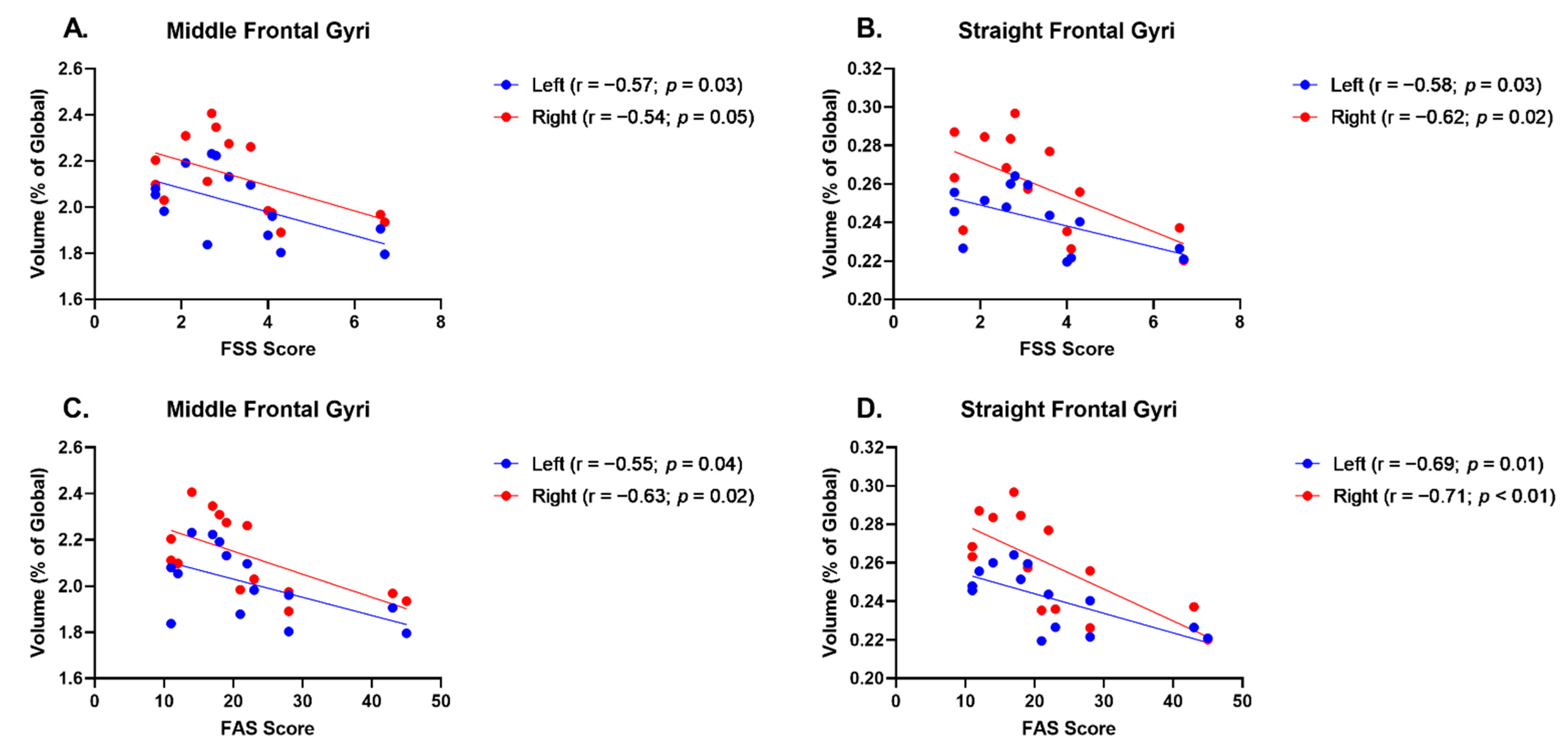
| L Middle Frontal Gyrus | −0.57 | 0.03 | −0.55 | 0.04 |
| R Middle Frontal Gyrus | −0.54 | 0.05 | −0.63 | 0.02 |
| L Straight Frontal Gyrus | −0.58 | 0.03 | −0.69 | 0.01 |
| R Straight Frontal Gyrus | −0.62 | 0.02 | −0.71 | <0.01 |
| L Anterior Orbital Gyrus | −0.43 | 0.13 | −0.48 | 0.09 |
| L Inferior Frontal Gyrus | −0.56 | 0.04 | −0.65 | 0.02 |
| L Middle Orbital Gyrus | −0.46 | 0.10 | −0.55 | 0.04 |
| R Middle Orbital Gyrus | −0.48 | 0.08 | −0.65 | 0.01 |
| R Superior Posterior Temporal Lobe | −0.15 | 0.61 | −0.24 | 0.40 |
| R Anterior Cingulate Gyrus | −0.41 | 0.15 | −0.54 | 0.05 |
| <6 Months Post-Infection | ||||
| Region | FSS r-Value | p-Value | FAS r-Value | p-Value |
| L Caudate Nucleus | −0.22 | 0.40 | −0.22 | 0.40 |
| R Nucleus Accumbens | −0.60 | 0.01 | −0.51 | 0.04 |
| L Substantia Nigra | −0.45 | 0.07 | −0.43 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deters, J.R.; Fietsam, A.C.; Gander, P.E.; Boles Ponto, L.L.; Rudroff, T. Effect of Post-COVID-19 on Brain Volume and Glucose Metabolism: Influence of Time Since Infection and Fatigue Status. Brain Sci. 2023, 13, 675. https://doi.org/10.3390/brainsci13040675
Deters JR, Fietsam AC, Gander PE, Boles Ponto LL, Rudroff T. Effect of Post-COVID-19 on Brain Volume and Glucose Metabolism: Influence of Time Since Infection and Fatigue Status. Brain Sciences. 2023; 13(4):675. https://doi.org/10.3390/brainsci13040675
Chicago/Turabian StyleDeters, Justin R., Alexandra C. Fietsam, Phillip E. Gander, Laura L. Boles Ponto, and Thorsten Rudroff. 2023. "Effect of Post-COVID-19 on Brain Volume and Glucose Metabolism: Influence of Time Since Infection and Fatigue Status" Brain Sciences 13, no. 4: 675. https://doi.org/10.3390/brainsci13040675
APA StyleDeters, J. R., Fietsam, A. C., Gander, P. E., Boles Ponto, L. L., & Rudroff, T. (2023). Effect of Post-COVID-19 on Brain Volume and Glucose Metabolism: Influence of Time Since Infection and Fatigue Status. Brain Sciences, 13(4), 675. https://doi.org/10.3390/brainsci13040675







