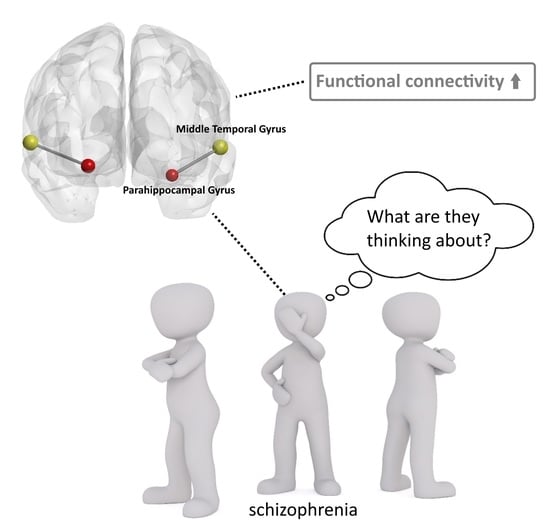
Increased Functional Connectivity Involving the Parahippocampal Gyrus in Patients with Schizophrenia during Theory of Mind Processing: A Psychophysiological Interaction Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
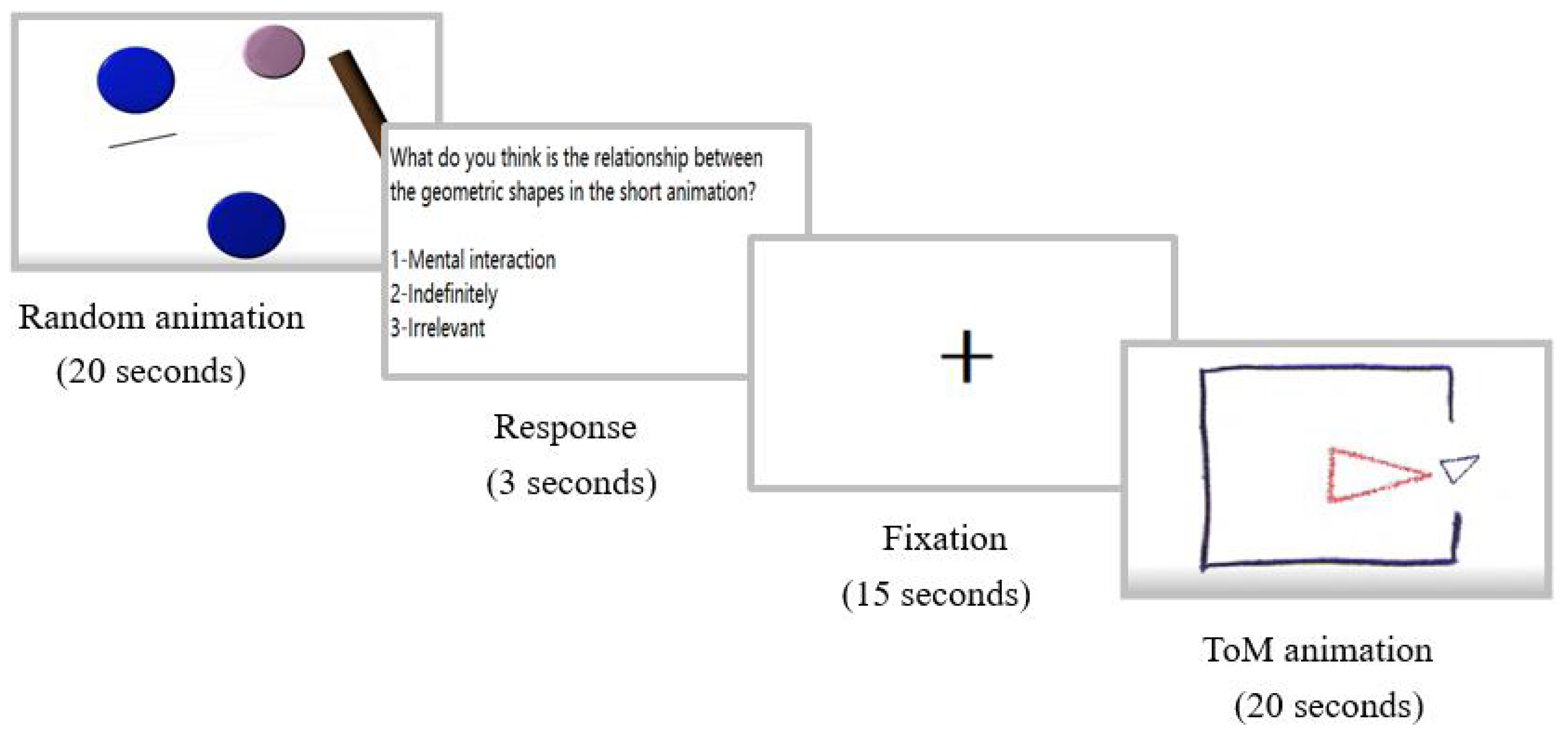
2.2. Stimuli and Task Design
2.3. MRI Data Acquisition
2.4. Data Analyses
2.4.1. Demographics, Clinical, and Behavioral Data Analysis
2.4.2. MRI Data Preprocessing
2.4.3. GLM Analysis
2.4.4. Psychophysiological Interaction Analysis
2.4.5. Correlation Analysis
2.4.6. Exploratory Analysis
3. Results
3.1. Demographic and Clinical Information
3.2. Behavioral Results of fMRI Paradigm
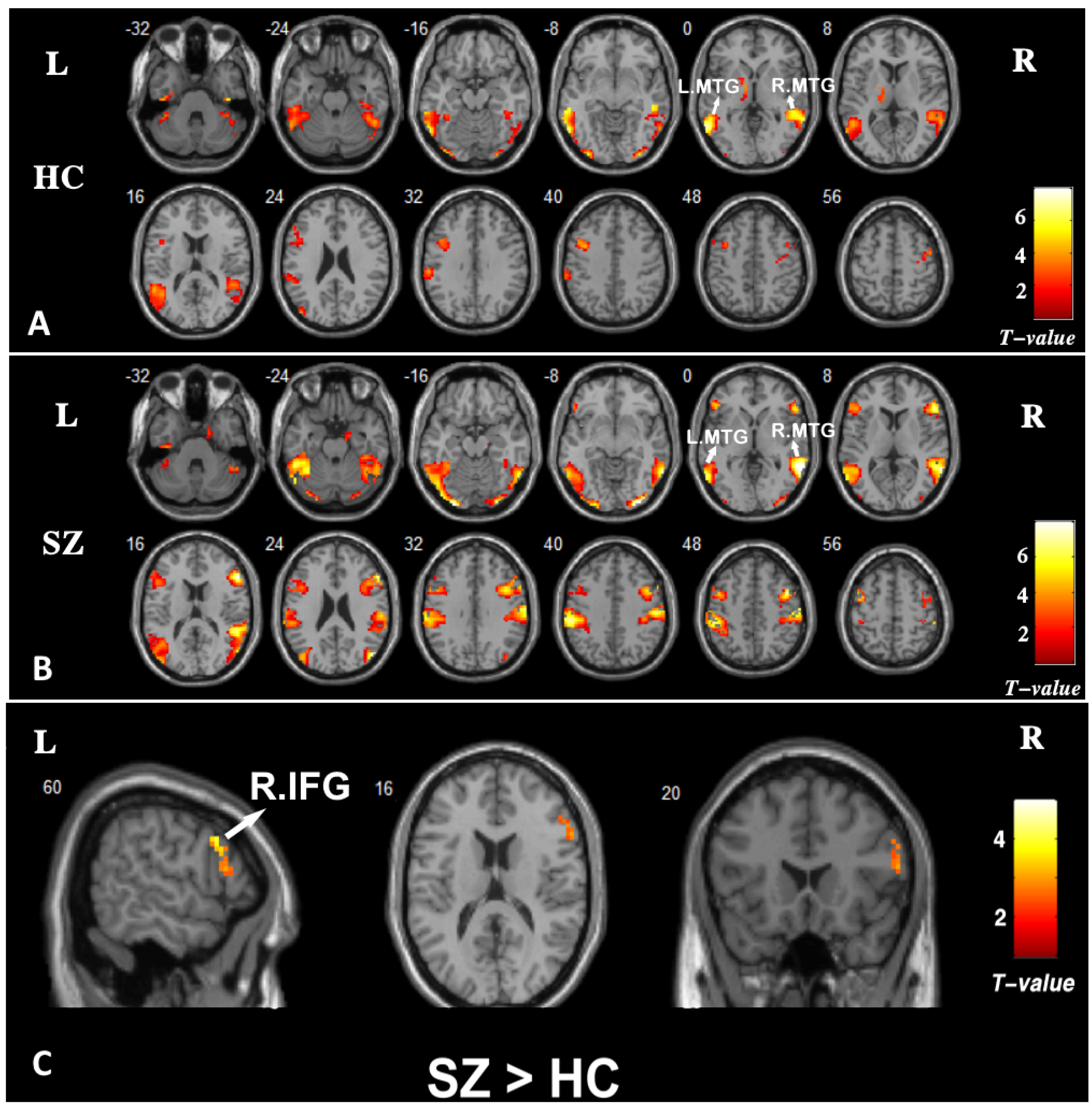
3.3. Brain Activation
3.4. Functional Connectivity: PPI
3.5. Correlation Analysis
3.6. Exploratory Analysis
3.7. Summarize
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III–the final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef]
- Young, J.W.; Geyer, M.A. Developing treatments for cognitive deficits in schizophrenia: The challenge of translation. J. Psychopharmacol. 2015, 29, 178–196. [Google Scholar] [CrossRef]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Erfurth, A.; Barlati, S.; Zanca, F.; Giordano, G.M.; Glenthoj, L.B.; Nordentoft, M.; et al. European Psychiatric Association guidance on assessment of cognitive impairment in schizophrenia. Eur. Psychiatry 2022, 65, e58. [Google Scholar] [CrossRef]
- Green, M.F.; Penn, D.L.; Bentall, R.; Carpenter, W.T.; Gaebel, W.; Gur, R.C.; Kring, A.M.; Park, S.; Silverstein, S.M.; Heinssen, R. Social cognition in schizophrenia: An NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 2008, 34, 1211–1220. [Google Scholar] [CrossRef]
- Kronbichler, L.; Tschernegg, M.; Martin, A.I.; Schurz, M.; Kronbichler, M. Abnormal Brain Activation During Theory of Mind Tasks in Schizophrenia: A Meta-Analysis. Schizophr. Bull. 2017, 43, 1240–1250. [Google Scholar] [CrossRef]
- Kharawala, S.; Hastedt, C.; Podhorna, J.; Shukla, H.; Kappelhoff, B.; Harvey, P.D. The relationship between cognition and functioning in schizophrenia: A semi-systematic review. Schizophr. Res. Cognit. 2022, 27, 100217. [Google Scholar] [CrossRef]
- Sjolie, C.; Meyn, E.K.; Raudeberg, R.; Andreassen, O.A.; Vaskinn, A. Nonsocial cognitive underpinnings of theory of mind in schizophrenia. Psychiatry Res. 2020, 289, 113055. [Google Scholar] [CrossRef]
- Schilbach, L.; Derntl, B.; Aleman, A.; Caspers, S.; Clos, M.; Diederen, K.M.; Gruber, O.; Kogler, L.; Liemburg, E.J.; Sommer, I.E.; et al. Differential Patterns of Dysconnectivity in Mirror Neuron and Mentalizing Networks in Schizophrenia. Schizophr. Bull. 2016, 42, 1135–1148. [Google Scholar] [CrossRef]
- Monticelli, M.; Zeppa, P.; Mammi, M.; Penner, F.; Melcarne, A.; Zenga, F.; Garbossa, D. Where We Mentalize: Main Cortical Areas Involved in Mentalization. Front. Neurol. 2021, 12, 712532. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef]
- Abu-Akel, A.; Shamay-Tsoory, S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 2011, 49, 2971–2984. [Google Scholar] [CrossRef]
- Iacoboni, M.; Dapretto, M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 2006, 7, 942–951. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [CrossRef]
- Van Overwalle, F.; Baetens, K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage 2009, 48, 564–584. [Google Scholar] [CrossRef]
- Arioli, M.; Canessa, N. Neural processing of social interaction: Coordinate-based meta-analytic evidence from human neuroimaging studies. Hum. Brain Mapp. 2019, 40, 3712–3737. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, W.; Yao, L.; Xiao, Y.; Liu, L.; Liu, J.; Li, S.; Tao, B.; Shah, C.; Gong, Q.; et al. Association between structural and functional brain alterations in drug-free patients with schizophrenia: A multimodal meta-analysis. J. Neuropsychiatry Clin. Neurosci. 2018, 43, 131–142. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Song, M.; Lv, L.; Cui, Y.; Liu, Y.; Fan, L.; Zuo, N.; Xu, K.; Du, Y.; et al. Linked 4-Way Multimodal Brain Differences in Schizophrenia in a Large Chinese Han Population. Schizophr. Bull. 2019, 45, 436–449. [Google Scholar] [CrossRef]
- Minzenberg, M.J.; Firl, A.J.; Yoon, J.H.; Gomes, G.C.; Reinking, C.; Carter, C.S. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology 2010, 35, 2590–2599. [Google Scholar] [CrossRef]
- Dabiri, M.; Firouzabadi, F.D.; Yang, K.; Barker, P.B.; Lee, R.R.; Yousem, D.M. Neuroimaging in schizophrenia: A review article. Front. Neurosci. 2022, 16, 1042814. [Google Scholar] [CrossRef]
- Geisler, D.; Walton, E.; Naylor, M.; Roessner, V.; Lim, K.O.; Schulz, S.C.; Gollub, R.L.; Calhoun, V.D.; Sponheim, S.R.; Ehrlich, S. Brain structure and function correlates of cognitive subtypes in schizophrenia. Psychiatry Res. 2015, 234, 74–83. [Google Scholar] [CrossRef]
- Choe, E.; Lee, T.Y.; Kim, M.; Hur, J.W.; Yoon, Y.B.; Cho, K.K.; Kwon, J.S. Aberrant within- and between-network connectivity of the mirror neuron system network and the mentalizing network in first episode psychosis. Schizophr. Res. 2018, 199, 243–249. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.; Ha, M.; Moon, S.Y.; Lho, S.K.; Kim, M.; Kwon, J.S. Intrinsic cerebellar functional connectivity of social cognition and theory of mind in first-episode psychosis patients. NPJ Schizophr. 2021, 7, 59. [Google Scholar] [CrossRef]
- Amft, M.; Bzdok, D.; Laird, A.R.; Fox, P.T.; Schilbach, L.; Eickhoff, S.B. Definition and characterization of an extended social-affective default network. Brain Struct. Funct. 2015, 220, 1031–1049. [Google Scholar] [CrossRef]
- Mothersill, O.; Tangney, N.; Morris, D.W.; McCarthy, H.; Frodl, T.; Gill, M.; Corvin, A.; Donohoe, G. Further evidence of alerted default network connectivity and association with theory of mind ability in schizophrenia. Schizophr. Res. 2017, 184, 52–58. [Google Scholar] [CrossRef]
- Ilzarbe, D.; de la Serna, E.; Baeza, I.; Rosa, M.; Puig, O.; Calvo, A.; Masias, M.; Borras, R.; Pariente, J.C.; Castro-Fornieles, J.; et al. The relationship between performance in a theory of mind task and intrinsic functional connectivity in youth with early onset psychosis. Dev. Cognit. Neurosci. 2019, 40, 100726. [Google Scholar] [CrossRef]
- Martin, A.K.; Dzafic, I.; Robinson, G.A.; Reutens, D.; Mowry, B. Mentalizing in schizophrenia: A multivariate functional MRI study. Neuropsychologia 2016, 93, 158–166. [Google Scholar] [CrossRef]
- Mier, D.; Eisenacher, S.; Rausch, F.; Englisch, S.; Gerchen, M.F.; Zamoscik, V.; Meyer-Lindenberg, A.; Zink, M.; Kirsch, P. Aberrant activity and connectivity of the posterior superior temporal sulcus during social cognition in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 597–610. [Google Scholar] [CrossRef]
- Barch, D.M.; Burgess, G.C.; Harms, M.P.; Petersen, S.E.; Schlaggar, B.L.; Corbetta, M.; Glasser, M.F.; Curtiss, S.; Dixit, S.; Feldt, C.; et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage 2013, 80, 169–189. [Google Scholar] [CrossRef]
- Das, P.; Lagopoulos, J.; Coulston, C.M.; Henderson, A.F.; Malhi, G.S. Mentalizing impairment in schizophrenia: A functional MRI study. Schizophr. Res. 2012, 134, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.; Koelkebeck, K.; Brandt, M.; Wee, M.; Kueppers, K.A.; Kugel, H.; Kohl, W.; Bauer, J.; Ohrmann, P. Theory of mind in patients with schizophrenia: Is mentalizing delayed? Schizophr. Res. 2012, 137, 224–229. [Google Scholar] [CrossRef]
- Bliksted, V.; Frith, C.; Videbech, P.; Fagerlund, B.; Emborg, C.; Simonsen, A.; Roepstorff, A.; Campbell-Meiklejohn, D. Hyper- and Hypomentalizing in Patients with First-Episode Schizophrenia: fMRI and Behavioral Studies. Schizophr. Bull. 2019, 45, 377–385. [Google Scholar] [CrossRef]
- Dietz, M.J.; Zhou, Y.; Veddum, L.; Frith, C.D.; Bliksted, V.F. Aberrant effective connectivity is associated with positive symptoms in first-episode schizophrenia. Neuroimage Clin. 2020, 28, 102444. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Gibbon, M.; Spitzer, R.L.; Williams, J.B.W. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV); American Psychiatric Press: Washington, DC, USA, 1997. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.J.; Friston, K.J. Analysis of fMRI time-series revisited–again. Neuroimage 1995, 2, 173–181. [Google Scholar] [CrossRef]
- Friston, K.J.; Buechel, C.; Fink, G.R.; Morris, J.; Rolls, E.; Dolan, R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997, 6, 218–229. [Google Scholar] [CrossRef]
- Csulak, T.; Hajnal, A.; Kiss, S.; Dembrovszky, F.; Varju-Solymar, M.; Sipos, Z.; Kovacs, M.A.; Herold, M.; Varga, E.; Hegyi, P.; et al. Implicit Mentalizing in Patients With Schizophrenia: A Systematic Review and Meta-Analysis. Front. Psychol. 2022, 13, 790494. [Google Scholar] [CrossRef]
- Bohec, A.L.; Baltazar, M.; Tassin, M.; Rey, R. Theory of mind and schizotypy: A review. Encephale 2021, 47, 254–262. [Google Scholar] [CrossRef]
- Russell, T.A.; Rubia, K.; Bullmore, E.T.; Soni, W.; Suckling, J.; Brammer, M.J.; Simmons, A.; Williams, S.C.; Sharma, T. Exploring the social brain in schizophrenia: Left prefrontal underactivation during mental state attribution. Am. J. Psychiatry 2000, 157, 2040–2042. [Google Scholar] [CrossRef]
- Leube, D.; Straube, B.; Green, A.; Blumel, I.; Prinz, S.; Schlotterbeck, P.; Kircher, T. A possible brain network for representation of cooperative behavior and its implications for the psychopathology of schizophrenia. Neuropsychobiology 2012, 66, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Aoki, Y.; Yahata, N.; Kawakubo, Y.; Inoue, H.; Iwashiro, N.; Natsubori, T.; Koike, S.; Gonoi, W.; Sasaki, H.; et al. Neural basis for inferring false beliefs and social emotions in others among individuals with schizophrenia and those at ultra-high risk for psychosis. Psychiatry Res. Neuroimaging 2017, 259, 34–41. [Google Scholar] [CrossRef]
- Herold, R.; Varga, E.; Hajnal, A.; Hamvas, E.; Berecz, H.; Toth, B.; Tenyi, T. Altered Neural Activity during Irony Comprehension in Unaffected First-Degree Relatives of Schizophrenia Patients—An fMRI Study. Front. Psychol. 2017, 8, 2309. [Google Scholar] [CrossRef]
- Bekkali, S.; Youssef, G.J.; Donaldson, P.H.; Albein-Urios, N.; Hyde, C.; Enticott, P.G. Is the Putative Mirror Neuron System Associated with Empathy? A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2021, 31, 14–57. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Calhoun, V.; Malhi, G.S. Mentalizing in male schizophrenia patients is compromised by virtue of dysfunctional connectivity between task-positive and task-negative networks. Schizophr. Res. 2012, 140, 51–58. [Google Scholar] [CrossRef]
- Castelli, F.; Happe, F.; Frith, U.; Frith, C. Movement and mind: A functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage 2000, 12, 314–325. [Google Scholar] [CrossRef]
- Potkin, S.G.; Turner, J.A.; Brown, G.G.; McCarthy, G.; Greve, D.N.; Glover, G.H.; Manoach, D.S.; Belger, A.; Diaz, M.; Wible, C.G.; et al. Working memory and DLPFC inefficiency in schizophrenia: The FBIRN study. Schizophr. Bull. 2009, 35, 19–31. [Google Scholar] [CrossRef]
- Whitney, C.; Jefferies, E.; Kircher, T. Heterogeneity of the left temporal lobe in semantic representation and control: Priming multiple versus single meanings of ambiguous words. Cereb. Cortex 2011, 21, 831–844. [Google Scholar] [CrossRef]
- Weng, Y.; Lin, J.; Ahorsu, D.K.; Tsang, H.W.H. Neuropathways of Theory of Mind in Schizophrenia: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2022, 137, 104625. [Google Scholar] [CrossRef]
- Aminoff, E.M.; Kveraga, K.; Bar, M. The role of the parahippocampal cortex in cognition. Trends Cognit. Sci. 2013, 17, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Moreau, N.; Viallet, F.; Champagne-Lavau, M. Using memories to understand others: The role of episodic memory in theory of mind impairment in Alzheimer disease. Ageing Res. Rev. 2013, 12, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, O.P.; Coundouris, S.P.; Grainger, S.A.; Henry, J.D. Empathy and Theory of Mind in Alzheimer’s Disease: A Meta-analysis. J. Int. Neuropsychol. Soc. 2020, 26, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, P.; Berrewaerts, J.; Hupet, M. Pragmatic skills in the early stages of Alzheimer’s disease: An analysis by means of a referential communication task. Int. J. Lang. Commun. Disord. 2007, 42, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Antoine, N.; Bahri, M.A.; Bastin, C.; Collette, F.; Phillips, C.; Balteau, E.; Genon, S.; Salmon, E. Anosognosia and default mode subnetwork dysfunction in Alzheimer’s disease. Hum. Brain Mapp. 2019, 40, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, X.; Yang, J.; Qiu, J. The role of the MTG in negative emotional processing in young adults with autistic-like traits: A fMRI task study. J. Affect. Disord. 2020, 276, 890–897. [Google Scholar] [CrossRef]
- Zou, L.; Wu, X.; Tao, S.; Yang, Y.; Zhang, Q.; Hong, X.; Xie, Y.; Li, T.; Zheng, S.; Tao, F. Functional connectivity between the parahippocampal gyrus and the middle temporal gyrus moderates the relationship between problematic mobile phone use and depressive symptoms: Evidence from a longitudinal study. J. Behav. Addict. 2022, 11, 40–48. [Google Scholar] [CrossRef]
- Jouen, A.L.; Ellmore, T.M.; Madden, C.J.; Pallier, C.; Dominey, P.F.; Ventre-Dominey, J. Beyond the word and image: Characteristics of a common meaning system for language and vision revealed by functional and structural imaging. Neuroimage 2015, 106, 72–85. [Google Scholar] [CrossRef]
- Yuan, Q.; Qi, W.; Xue, C.; Ge, H.; Hu, G.; Chen, S.; Xu, W.; Song, Y.; Zhang, X.; Xiao, C.; et al. Convergent Functional Changes of Default Mode Network in Mild Cognitive Impairment Using Activation Likelihood Estimation. Front. Aging Neurosci. 2021, 13, 708687. [Google Scholar] [CrossRef]
- King, S.; Mothersill, D.; Holleran, L.; Patlola, S.; McManus, R.; Kenyon, M.; McDonald, C.; Hallahan, B.; Corvin, A.; Morris, D.W.; et al. Childhood trauma, IL-6 and weaker suppression of the default mode network (DMN) during theory of mind (ToM) performance in schizophrenia. Brain Behav. Immun. Health 2022, 26, 100540. [Google Scholar] [CrossRef]
- Guo, W.; Liu, F.; Chen, J.; Wu, R.; Li, L.; Zhang, Z.; Chen, H.; Zhao, J. Hyperactivity of the default-mode network in first-episode, drug-naive schizophrenia at rest revealed by family-based case-control and traditional case-control designs. Medicine (Baltimore) 2017, 96, e6223. [Google Scholar] [CrossRef]
- Tarcijonas, G.; Sarpal, D.K. Neuroimaging markers of antipsychotic treatment response in schizophrenia: An overview of magnetic resonance imaging studies. Neurobiol. Dis. 2019, 131, 104209. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tang, J.; Liu, N.; Yao, L.; Xu, M.; Sun, H.; Tao, B.; Gong, Q.; Cao, H.; Zhang, W.; et al. The Effects of Antipsychotic Treatment on the Brain of Patients With First-Episode Schizophrenia: A Selective Review of Longitudinal MRI Studies. Front. Psychiatry 2021, 12, 593703. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, F.; Blasi, G.; Fazio, L.; Caforio, G.; Taurisano, P.; Romano, R.; Di Giorgio, A.; Gelao, B.; Lo Bianco, L.; Papazacharias, A.; et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology 2010, 35, 904–912. [Google Scholar] [CrossRef] [PubMed]
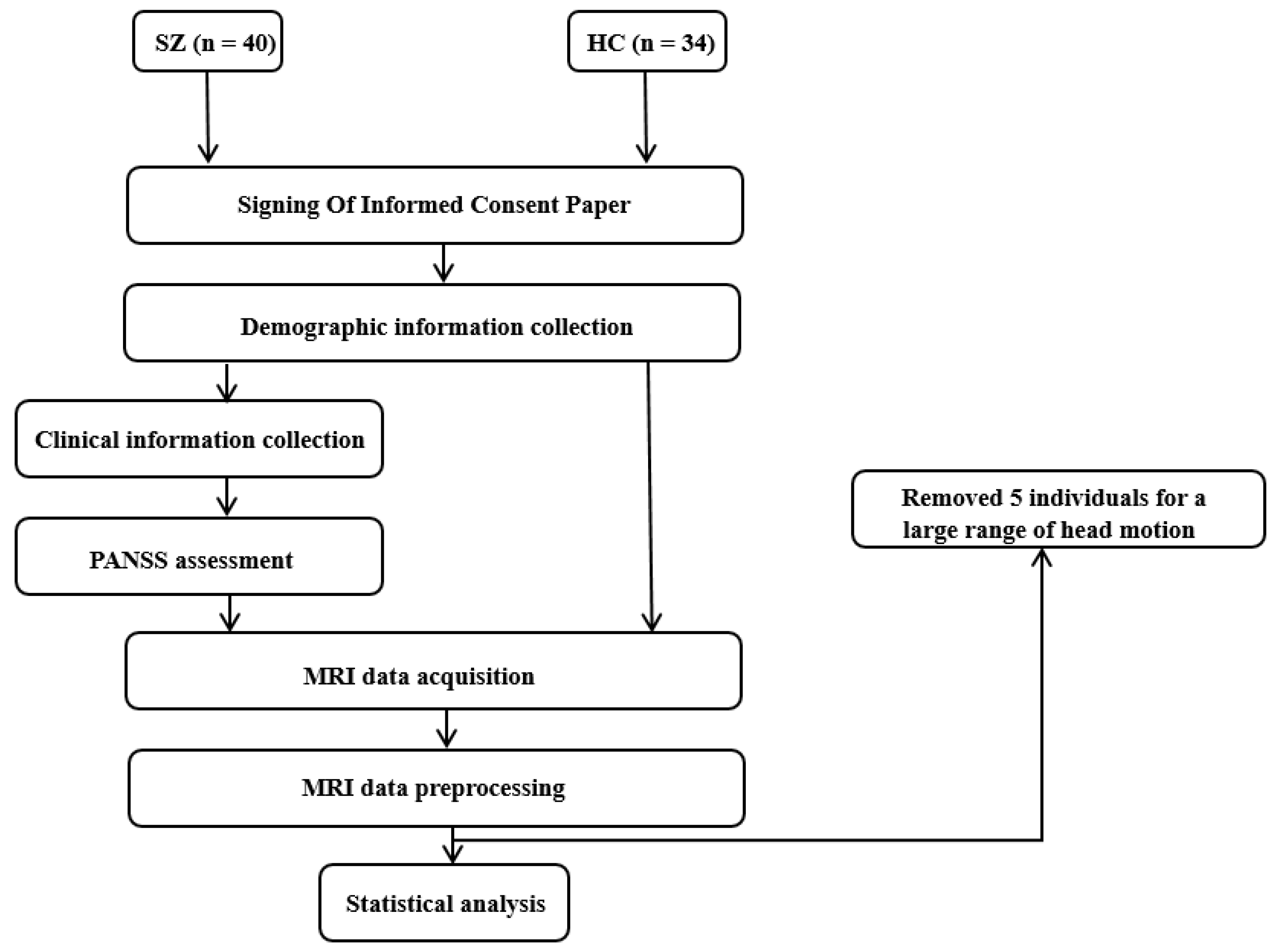
| HC (n = 33) | SZ (n = 36) | T//Z | p Value | |
|---|---|---|---|---|
| Age (Years) | 26.45 ± 4.50 | 24.44 ± 3.96 | 1.98 | 0.052 |
| Male/Female | 14/19 | 18/18 | 0.40 | 0.529 a |
| Education (Years) | 15.70 ± 2.02 | 12.94 ± 2.50 | 5.00 | <0.001 * |
| Age of onset (Years) | 22.19 ± 4.13 | |||
| Duration of illness (Years) | 2.22 ± 2.36 | |||
| CPZ equivalent (mg) | 455.42 ± 236.42 | |||
| PANSS | ||||
| positive | 18.11 ± 4.09 | |||
| negative | 18.34 ± 5.05 | |||
| general | 40.51 ± 4.97 | |||
| total | 78.25 ± 7.03 | |||
| Accuracy scores of tasks | ||||
| Random | 1.73 ± 0.91 | 1.47 ± 1.03 | 1.16 | 0.246 b |
| ToM | 1.70 ± 0.53 | 1.39 ± 0.60 | 2.31 | 0.021 b,* |
| Total | 3.42 ± 1.12 | 2.86 ± 1.13 | 2.17 | 0.030 b,* |
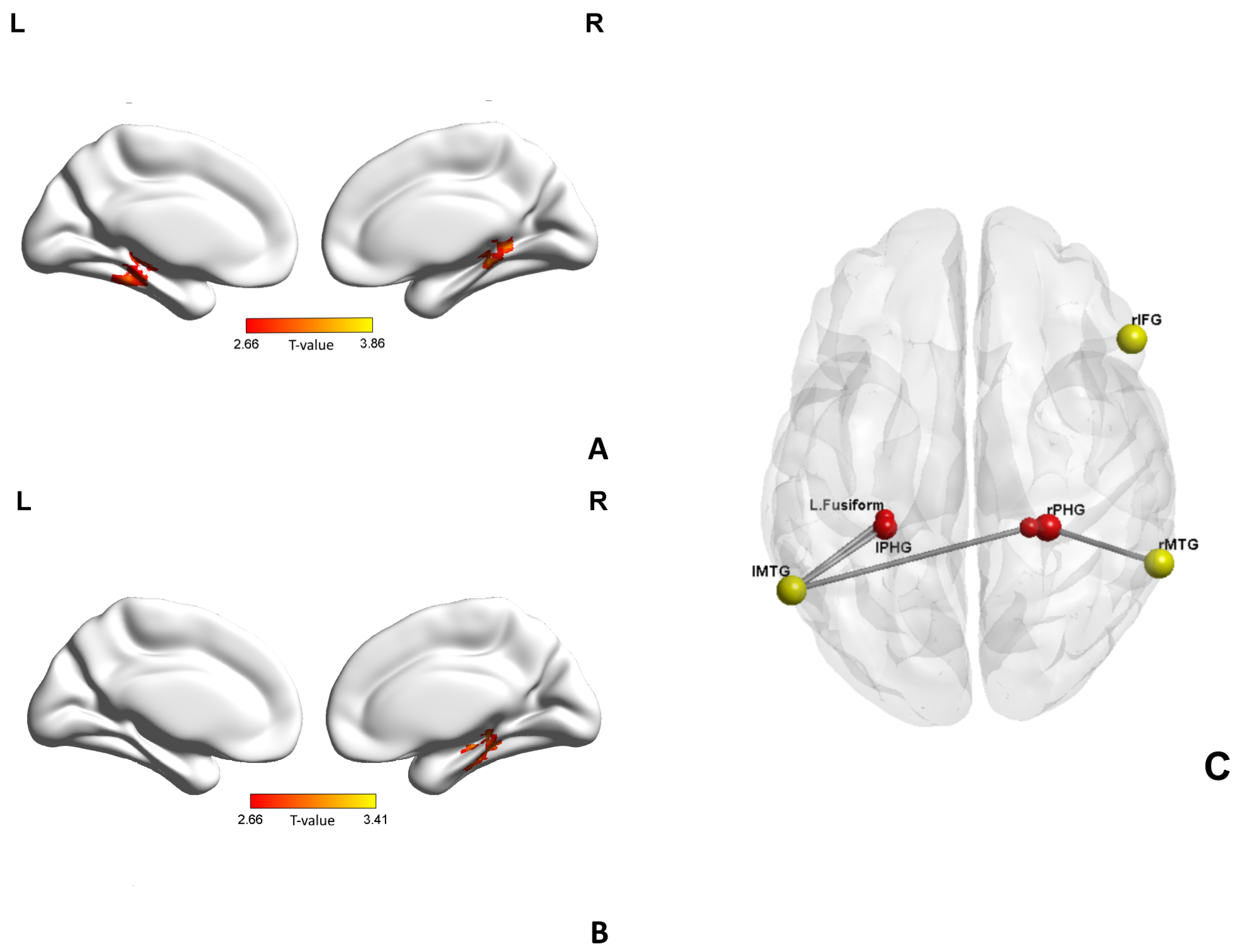
| Conditions | Seed | Structures | Cluster Size | Peak Coordinates x,y,z (mm) | p Value |
|---|---|---|---|---|---|
| SZ > HC | L.MTG | L.Parahippocampal gyrus | 54 | −30 −36 −6 | 3.43 |
| L.Fusiform gyrus | 26 | −33 −42 −18 | 2.92 | ||
| R.Parahippocampal gyrus | 30 | 21 −36 −9 | 3.37 | ||
| R.MTG | R.Parahippocampal gyrus | 79 | 27 −36 −12 | 3.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Huang, H.; Liu, Y.; Zheng, F.; Zhou, Y.; Wang, H. Increased Functional Connectivity Involving the Parahippocampal Gyrus in Patients with Schizophrenia during Theory of Mind Processing: A Psychophysiological Interaction Study. Brain Sci. 2023, 13, 692. https://doi.org/10.3390/brainsci13040692
Qin X, Huang H, Liu Y, Zheng F, Zhou Y, Wang H. Increased Functional Connectivity Involving the Parahippocampal Gyrus in Patients with Schizophrenia during Theory of Mind Processing: A Psychophysiological Interaction Study. Brain Sciences. 2023; 13(4):692. https://doi.org/10.3390/brainsci13040692
Chicago/Turabian StyleQin, Xucong, Huan Huang, Ying Liu, Fanfan Zheng, Yuan Zhou, and Huiling Wang. 2023. "Increased Functional Connectivity Involving the Parahippocampal Gyrus in Patients with Schizophrenia during Theory of Mind Processing: A Psychophysiological Interaction Study" Brain Sciences 13, no. 4: 692. https://doi.org/10.3390/brainsci13040692
APA StyleQin, X., Huang, H., Liu, Y., Zheng, F., Zhou, Y., & Wang, H. (2023). Increased Functional Connectivity Involving the Parahippocampal Gyrus in Patients with Schizophrenia during Theory of Mind Processing: A Psychophysiological Interaction Study. Brain Sciences, 13(4), 692. https://doi.org/10.3390/brainsci13040692