Altered Resting-State Brain Activity and Functional Connectivity in Post-Stroke Apathy: An fMRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Neuropsychological Evaluations
2.3. Imaging Data Acquisition
2.4. Preprocessing
2.5. fALFF Calculation
2.6. Infarct Volume and Surface Thickness
2.7. Statistical Analysis
3. Results
3.1. Demographic Data
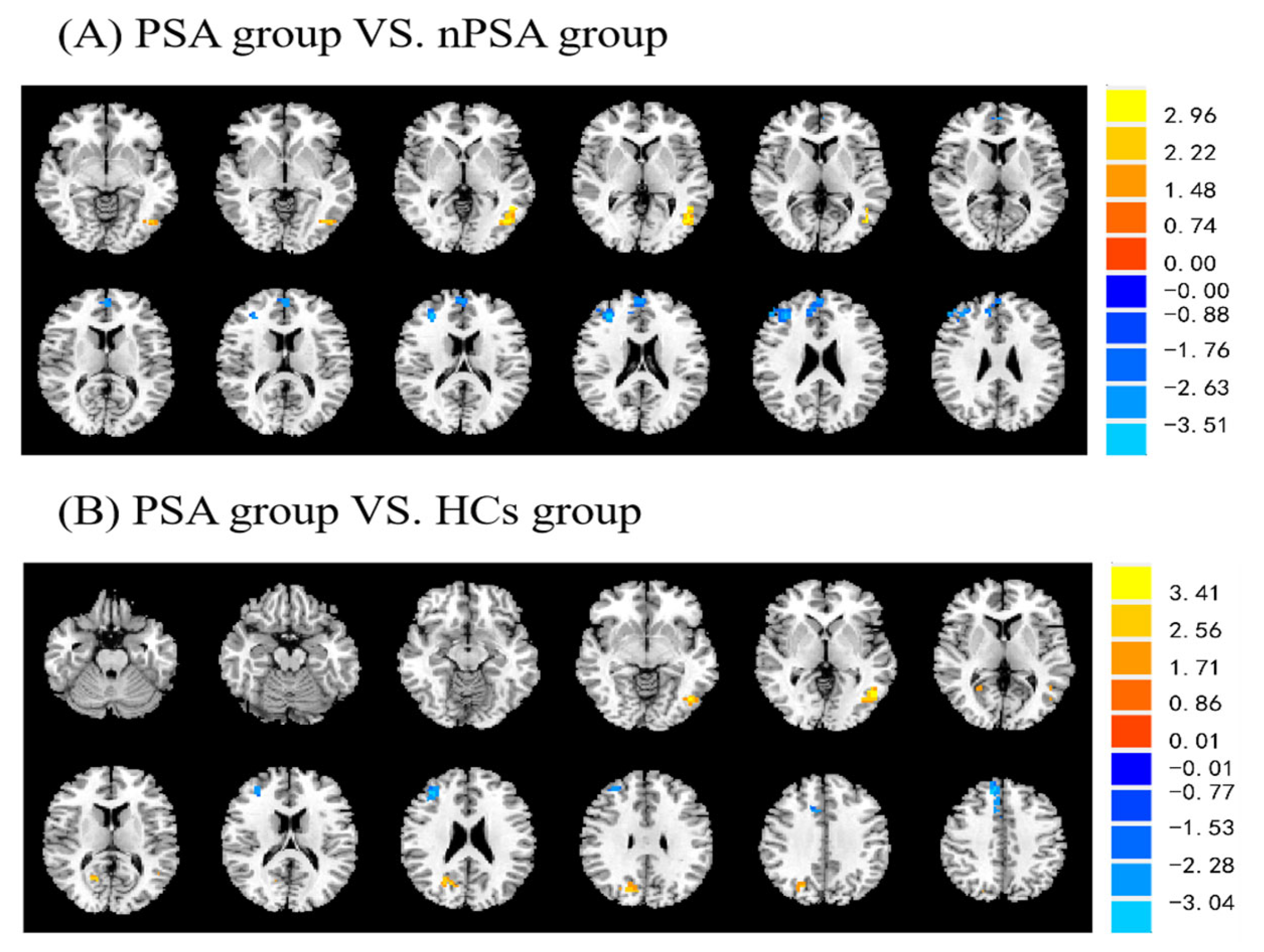
3.2. Post-Stroke Apathy and fALFF
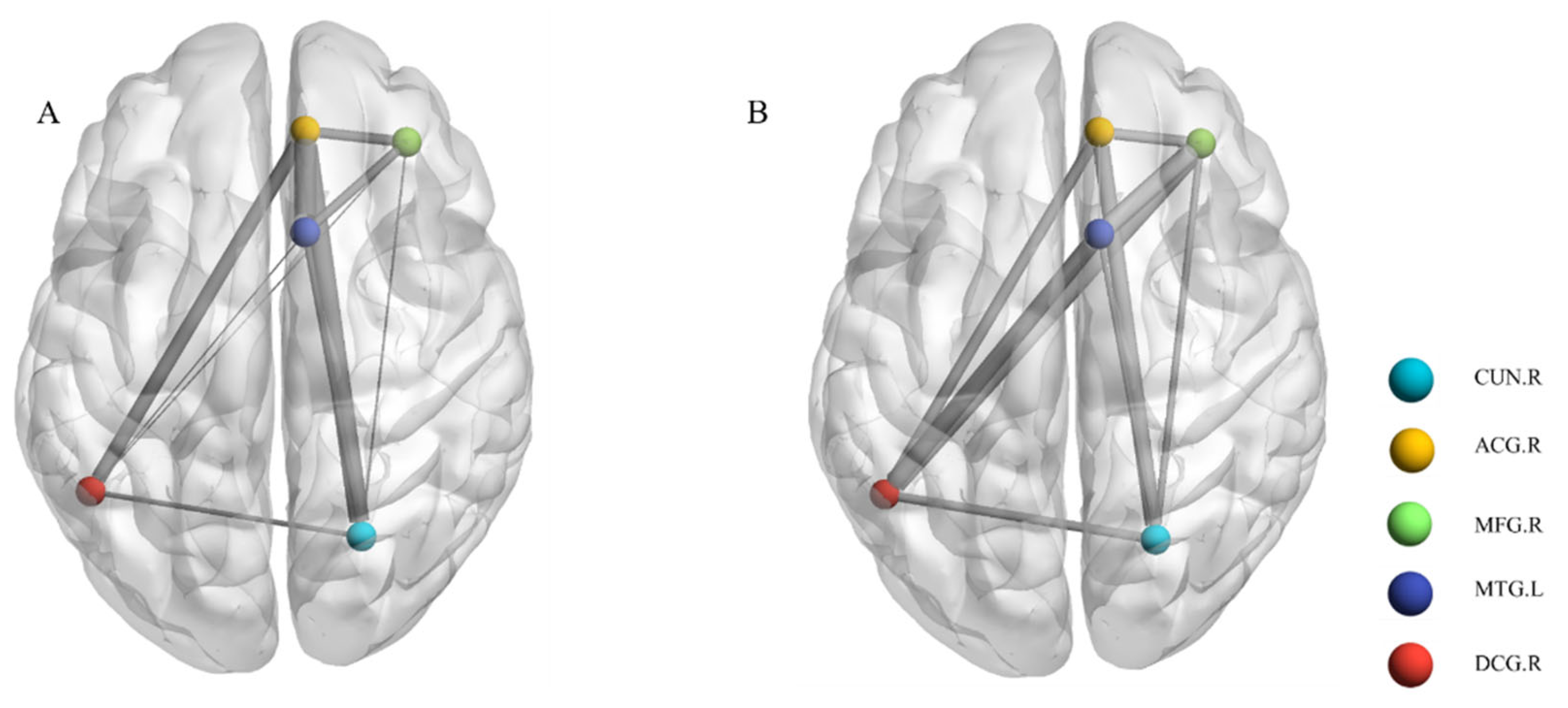
3.3. Post-Stroke Apathy and Local Functional Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferro, J.M.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Lanctôt, K.; Agüera-Ortiz, L.; Aalten, P.; Bremond, F.; Defrancesco, M.; Hanon, C.; David, R.; Dubois, B.; Dujardin, K. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur. Psychiatry 2018, 54, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sagen-Vik, U.; Finset, A.; Moum, T.; Vik, T.G.; Dammen, T. The longitudinal course of anxiety, depression and apathy through two years after stroke. J. Psychosom. Res. 2022, 162, 111016. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-K.; Lau, C.G.; Mok, V.; Ungvari, G.S.; Wong, K.-S. Apathy and health-related quality of life in stroke. Arch. Phys. Med. Rehabil. 2014, 95, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S. Post-stroke cognitive impairment, depression and apathy: Untangling the relationship. Am. J. Geriatr. Psychiatry 2018, 26, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Caeiro, L.; Lau, C.G.; Liang, H.; Mok, V.; Ungvari, G.S.; Wong, K.S. Apathy and suicide-related ideation 3 months after stroke: A cross-sectional study. BMC Neurol. 2015, 15, 60. [Google Scholar] [CrossRef]
- Douven, E.; Staals, J.; Schievink, S.H.; van Oostenbrugge, R.J.; Verhey, F.R.; Wetzels-Meertens, S.; Köhler, S.; Aalten, P. Personality traits and course of symptoms of depression and apathy after stroke: Results of the CASPER study. J. Psychosom. Res. 2018, 111, 69–75. [Google Scholar] [CrossRef]
- Tang, W.; Wong, L.; Mok, V.; Chu, W.; Wang, D. Apathy after stroke: Potential risk factors and magnetic resonance imaging markers. Hong Kong Med. J. 2018, 24, 18–20. [Google Scholar]
- Sutoko, S.; Atsumori, H.; Obata, A.; Funane, T.; Kandori, A.; Shimonaga, K.; Hama, S.; Yamawaki, S.; Tsuji, T. Lesions in the right Rolandic operculum are associated with self-rating affective and apathetic depressive symptoms for post-stroke patients. Sci. Rep. 2020, 10, 20264. [Google Scholar] [CrossRef]
- Le Heron, C.; Holroyd, C.B.; Salamone, J.; Husain, M. Brain mechanisms underlying apathy. J. Neurol. Neurosurg. Psychiatry 2019, 90, 302–312. [Google Scholar] [CrossRef]
- Le Heron, C.; Apps, M.A.J.; Husain, M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia 2018, 118, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Lisiecka-Ford, D.M.; Hollocks, M.J.; Tuladhar, A.M.; Barrick, T.R.; Forster, A.; O’Sullivan, M.J.; Husain, M.; de Leeuw, F.-E.; Morris, R.G. Network neuroscience of apathy in cerebrovascular disease. Prog. Neurobiol. 2020, 188, 101785. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Beckmann, C.F.; De Stefano, N.; Matthews, P.M.; Smith, S.M. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 2006, 29, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-r.; Hua, P.; Shang, X.; Cui, Z.; Zhong, S.; Gong, G.; William Humphreys, G. Deficiency of brain structural sub-network underlying post-ischaemic stroke apathy. Eur. J. Neurol. 2015, 22, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Smitha, K.; Akhil Raja, K.; Arun, K.; Rajesh, P.; Thomas, B.; Kapilamoorthy, T.; Kesavadas, C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef]
- Yang, H.; Long, X.-Y.; Yang, Y.; Yan, H.; Zhu, C.-Z.; Zhou, X.-P.; Zang, Y.-F.; Gong, Q.-Y. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 2007, 36, 144–152. [Google Scholar] [CrossRef]
- Sokoloff, L.; Wechsler, R.L.; Mangold, R.; Balls, K.; Kety, S.S. Cerebral blood flow and oxygen consumption in hyperthyroidism before and after treatment. J. Clin. Investig. 1953, 32, 202–208. [Google Scholar] [CrossRef]
- Egorova, N.; Veldsman, M.; Cumming, T.; Brodtmann, A. Fractional amplitude of low-frequency fluctuations (fALFF) in post-stroke depression. NeuroImage Clin. 2017, 16, 116–124. [Google Scholar] [CrossRef]
- Lee, M.H.; Smyser, C.D.; Shimony, J.S. Resting-state fMRI: A review of methods and clinical applications. Am. J. Neuroradiol. 2013, 34, 1866–1872. [Google Scholar] [CrossRef]
- Zhang, D.; Raichle, M.E. Disease and the brain’s dark energy. Nat. Rev. Neurol. 2010, 6, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, H.; Yu, Q.; Yin, L.; Li, K.; Li, Y.; Fu, J. Cerebral functional manipulation of repetitive transcranial magnetic stimulation in cognitive impairment patients after stroke: An fMRI study. Front. Neurol. 2020, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, L.; Ferro, J.M.; Costa, J. Apathy secondary to stroke: A systematic review and meta-analysis. Cerebrovasc. Dis. 2013, 35, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Sagen, U.; Faerden, A.; Haug, T.; Melle, I.; Finset, A.; Dammen, T. Are there common core features of apathy in different neuropsychiatric samples as assessed by the Apathy Evaluation Scale? Nord. J. Psychiatry 2010, 64, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Fedoroff, J.P.; Price, T.R.; Leiguarda, R.n.; Robinson, R.G. Apathy following cerebrovascular lesions. Stroke 1993, 24, 1625–1630. [Google Scholar] [CrossRef]
- Song, X.-W.; Dong, Z.-Y.; Long, X.-Y.; Li, S.-F.; Zuo, X.-N.; Zhu, C.-Z.; He, Y.; Yan, C.-G.; Zang, Y.-F. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011, 6, e25031. [Google Scholar] [CrossRef]
- Zou, Q.-H.; Zhu, C.-Z.; Yang, Y.; Zuo, X.-N.; Long, X.-Y.; Cao, Q.-J.; Wang, Y.-F.; Zang, Y.-F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef]
- Cotter, G.; Salah Khlif, M.; Bird, L.; Howard, M.E.; Brodtmann, A.; Egorova-Brumley, N. Post-stroke fatigue is associated with resting state posterior hypoactivity and prefrontal hyperactivity. Int. J. Stroke 2021, 75, 1516–1525. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Aviles, M.; Sánchez-Reyes, L.-M.; Fuentes-Aguilar, R.Q.; Toledo-Pérez, D.C.; Rodríguez-Reséndiz, J. A Novel Methodology for Classifying EMG Movements Based on SVM and Genetic Algorithms. Micromachines 2022, 13, 2108. [Google Scholar] [CrossRef]
- Ibrahimi, D.; Rodríguez-Reséndiz, J.; Mendiola-Santibañez, J.D. Differences in the Visual Performances of Patients with Strabismus, Amblyopia, and Healthy Controls. Bioengineering 2022, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Reyes, L.-M.; Rodríguez-Reséndiz, J.; Avecilla-Ramírez, G.N.; García-Gomar, M.-L.; Robles-Ocampo, J.-B. Impact of eeg parameters detecting dementia diseases: A systematic review. IEEE Access. 2021, 9, 78060–78074. [Google Scholar] [CrossRef]
- Gallegos-Duarte, M.; Mendiola-Santibañez, J.D.; Ibrahimi, D.; Paredes-Orta, C.; Rodríguez-Reséndiz, J.; González-Gutiérrez, C.A. A novel method for measuring subtle alterations in pupil size in children with congenital strabismus. IEEE Access 2020, 8, 125331–125344. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Brockman, S. The neuroimaging basis of apathy: Empirical findings and conceptual challenges. Neuropsychologia 2018, 118, 48–53. [Google Scholar] [CrossRef]
- Siegel, J.; Snyder, A.; Metcalf, N.; Fucetola, R.; Hacker, C.; Shimony, J.; Shulman, G.; Corbetta, M. The circuitry of abulia: Insights from functional connectivity MRI. NeuroImage Clin. 2014, 6, 320–326. [Google Scholar] [CrossRef]
- Okada, K.; Kobayashi, S.; Yamagata, S.; Takahashi, K.; Yamaguchi, S. Poststroke apathy and regional cerebral blood flow. Stroke 1997, 28, 2437–2441. [Google Scholar] [CrossRef]
- Guercio, B.J.; Donovan, N.J.; Ward, A.; Schultz, A.; Lorius, N.; Amariglio, R.E.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A.; Marshall, G.A. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e22–e27. [Google Scholar] [CrossRef]
- Robert, G.; Le Jeune, F.; Lozachmeur, C.; Drapier, S.; Dondaine, T.; Péron, J.; Travers, D.; Sauleau, P.; Millet, B.; Vérin, M. Apathy in patients with Parkinson disease without dementia or depression: A PET study. Neurology 2012, 79, 1155–1160. [Google Scholar] [CrossRef]
- Onoda, K.; Yamaguchi, S. Dissociative contributions of the anterior cingulate cortex to apathy and depression: Topological evidence from resting-state functional MRI. Neuropsychologia 2015, 77, 10–18. [Google Scholar] [CrossRef]
- Marshall, G.A.; Fairbanks, L.A.; Tekin, S.; Vinters, H.V.; Cummings, J.L. Neuropathologic correlates of apathy in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2006, 21, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Signori, R. Neural correlates for apathy: Frontal-prefrontal and parietal cortical-subcortical circuits. Front. Aging Neurosci. 2016, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Douven, E.; Köhler, S.; Schievink, S.H.; van Oostenbrugge, R.J.; Staals, J.; Verhey, F.R.; Aalten, P. Temporal associations between fatigue, depression, and apathy after stroke: Results of the cognition and affect after stroke, a prospective evaluation of risks study. Cerebrovasc. Dis. 2017, 44, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Hernandez, L.M.; Bowman, H.C.; Padgaonkar, N.T.; Fuster, E.; Jack, A.; Aylward, E.; Gaab, N.; Van Horn, J.D.; Bernier, R.A. Sex differences in functional connectivity of the salience, default mode, and central executive networks in youth with ASD. Cereb. Cortex 2020, 30, 5107–5120. [Google Scholar] [CrossRef]
- Androulakis, X.M.; Krebs, K.A.; Jenkins, C.; Maleki, N.; Finkel, A.G.; Rorden, C.; Newman, R. Central executive and default mode network intranet work functional connectivity patterns in chronic migraine. J. Neurol. Disord. 2018, 6, 393. [Google Scholar] [CrossRef]
- Greicius, M.D.; Supekar, K.; Menon, V.; Dougherty, R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 2009, 19, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, S.; Liu, Y.; Lin, M.; Wang, J.; Zhong, R.; Zhao, J.; Liu, W.; Zhu, L.; Shang, X. C-reactive protein can be an early predictor of poststroke apathy in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2018, 27, 1861–1869. [Google Scholar] [CrossRef]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage 2014, 99, 180–190. [Google Scholar] [CrossRef]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar]
- Satpute, A.B.; Lindquist, K.A. The default mode network’s role in discrete emotion. Trends Cogn. Sci. 2019, 23, 851–864. [Google Scholar] [CrossRef]
- Cabeza, R.; St Jacques, P. Functional neuroimaging of autobiographical memory. Trends Cogn. Sci. 2007, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Fishman, K.N.; Ashbaugh, A.R.; Lanctôt, K.L.; Cayley, M.L.; Herrmann, N.; Murray, B.J.; Sicard, M.; Lien, K.; Sahlas, D.J.; Swartz, R.H. The role of apathy and depression on verbal learning and memory performance after stroke. Arch. Clin. Neuropsychol. 2019, 34, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, J.; Aragon, D.F.; Rogge-Obando, K.; Campbell, R.A.; Shuttleworth, C.W.; Avila-Rieger, R.E.; Yeo, R.A.; Wilson, J.K.; Fratzke, V.; Brandt, E. Ventromedial prefrontal-anterior cingulate hyperconnectivity and resilience to apathy in traumatic brain injury. J. Neurotrauma 2021, 38, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Hamada, C.; Kawagoe, T.; Takamura, M.; Nagai, A.; Yamaguchi, S.; Onoda, K. Altered resting-state functional connectivity of the frontal-striatal circuit in elderly with apathy. PLoS ONE 2021, 16, e0261334. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.S.; Gunning-Dixon, F.M.; Hoptman, M.J.; AbdelMalak, B.; McGovern, A.R.; Seirup, J.K.; Alexopoulos, G.S. The salience network in the apathy of late-life depression. Int. J. Geriatr. Psychiatry 2014, 29, 1116–1124. [Google Scholar] [CrossRef]
- Van Dalen, J.W.; van Charante, E.P.M.; Nederkoorn, P.J.; van Gool, W.A.; Richard, E. Poststroke apathy. Stroke 2013, 44, 851–860. [Google Scholar] [CrossRef]
- Brodaty, H.; Liu, Z.; Withall, A.; Sachdev, P.S. The longitudinal course of post-stroke apathy over five years. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 283–291. [Google Scholar] [CrossRef]
- Worthington, A.; Wood, R.L. Apathy following traumatic brain injury: A review. Neuropsychologia 2018, 118, 40–47. [Google Scholar] [CrossRef]
- Brodaty, H.; Sachdev, P.S.; Withall, A.; Altendorf, A.; Valenzuela, M.J.; Lorentz, L. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke–the Sydney Stroke Study. Psychol. Med. 2005, 35, 1707–1716. [Google Scholar] [CrossRef]
- Caeiro, L.; Ferro, J.M.; e Melo, T.P.; Canhão, P.; Figueira, M.L. Post-stroke apathy: An exploratory longitudinal study. Cerebrovasc. Dis. 2013, 35, 507–513. [Google Scholar] [CrossRef]
- Dean, A.C.; Victor, T.L.; Boone, K.B.; Philpott, L.M.; Hess, R.A. Dementia and effort test performance. Clin. Neuropsychol. 2009, 23, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.A.; Maoz, O.; Hook, J.N.; Steiner, A.R.; Bieliauskas, L.A. IQ and scores on the Mini-Mental State Examination (MMSE): Controlling for effort and education among geriatric inpatients. Aging Neuropsychol. Cogn. 2007, 14, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Morris, R.G.; Markus, H.S. Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment. Int. J. Stroke 2021, 16, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Eurelings, L.S.; van Dalen, J.W.; Ter Riet, G.; van Charante, E.P.M.; Richard, E.; van Gool, W.A.; Almeida, O.P.; Alexandre, T.S.; Baune, B.T.; Bickel, H. Apathy and depressive symptoms in older people and incident myocardial infarction, stroke, and mortality: A systematic review and meta-analysis of individual participant data. Clin. Epidemiol. 2018, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Büyükgök, D.; Bayraktaroğlu, Z.; Buker, H.S.; Kulaksızoğlu, M.I.B.; Gurvit, İ.H. Resting-state FMRI analysis in apathetic Alzheimer’s disease. Diagn. Interv. Radiol. 2020, 26, 363. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, M.; Wang, Z.; Yang, Y.; Chang, Y.; Liu, L. Abnormal brain activities in multiple frequency bands in Parkinson’s disease with apathy. Front. Neurosci. 2022, 16, 975189. [Google Scholar] [CrossRef]


| PSA (n = 21) | nPSA (n = 38) | HCs (n = 29) | P1 | P2 | |
|---|---|---|---|---|---|
| Age, mean (SD), years | 61.7 (11.8) | 58.4 (12.5) | 56.2 (8.9) | 0.33 | 0.32 |
| Female, n (%) | 9 (42.9) | 12 (31.6) | 12 (41.4) | 0.43 | 0.60 |
| Education < 12, n (%) | 15 (71.4) | 23 (60.5) | 20 (69.0) | 0.40 | 0.64 |
| BMI, mean (SD), kg/m2 | 24.1 (4.04) | 23.4 (3.7) | 24.4 (2.0) | 0.28 | 0.38 |
| Smoking, n (%) | 6 (28.6) | 15 (39.5) | 6 (20.1) | 0.40 | 0.25 |
| MMSE, mean (SD) | 21.9 (3.1) | 23.8 (2.7) | 25.46 (2.1) | 0.02 | <0.001 |
| AES, median (IQR) | 41.2 (38.9, 45.2) | 26.0 (22.8, 31.1) | 23.5 (21.7, 27.4) | <0.001 | <0.001 |
| HAMD, median (IQR) | 4.7 (1.6, 8.0) | 3.0 (1.6, 7.2) | 1.1 (0.2, 7.5) | 0.06 | <0.001 |
| LSNS, median (IQR) | 26.0 (22.3, 28.8) | 29.0 (25.0, 34.0) | 30.0 (23.0, 34.0) | 0.06 | 0.07 |
| Imaging time, mean (SD) | 13.8 (1.1) | 15.4 (2.4) | - | 0.43 | - |
| Infarct volumes, cm3 (SD) | 6.5 (2.3) | 4.6 (1.6) | - | 0.32 | - |
| NIHSS median (IQR) | 6.0 (2.4, 8.4) | 0.6 (2.5, 5.3) | - | 0.02 | - |
| mRS, median (IQR) | 1.2 (1.0, 2.0) | 1.0 (0.8, 1.3) | - | 0.04 | - |
| TIV cm3, mean (SD) | 1450 (146) | 1304 (137) | 1355 (121) | 0.36 | 0.56 |
| Surface thickness mm (SD) | 2.16 (0.60) | 2.26 (0.57) | 2.20 (0.64) | 0.53 | 0.64 |
| Anterior circulation, n (%) | 15 (71.4) | 24 (63.2) | - | 0.45 | - |
| Posterior circulation, n (%) | 6 (28.6) | 8 (21.1) | - | 0.71 | - |
| Peak Location MNI (mm) | T-Score | Cluster Size | |||
|---|---|---|---|---|---|
| X | Y | Z | |||
| PSA versus nPSA | |||||
| Temporal_Mid_L | −48 | −54 | 3 | 3.70 | 38 |
| Cingulum_Ant_R | 9 | 42 | 24 | −3.43 | 13 |
| Frontal_Mid_R | 36 | 39 | 21 | −4.03 | 72 |
| PSA versus HCs | |||||
| Temporal_Mid_L | −48 | −63 | 0 | 4.26 | 28 |
| Cuneus_R | 24 | −66 | 21 | 3.31 | 33 |
| Frontal_Mid_R | 36 | 39 | 24 | −3.50 | 59 |
| Cingulum_Mid_R | 9 | 15 | 39 | −3.51 | 22 |
| Title | Year | Cohort Size | Main Achievements and Results |
|---|---|---|---|
| Altered resting-state brain activity and functional connectivity in post-stroke apathy: an fMRI study | 2023 | PSA (n = 21) nPSA (n = 38) HCs (n = 29) | Abnormalities in brain activity and functional connectivity in the left middle temporal region, right middle frontal region, right cuneate gyrus, right anterior, and middle cingulate region were associated with PSA, revealing a possible neural mechanism and providing new clues for the diagnosis and treatment of PSA. |
| Resting-state fMRI analysis in apathetic Alzheimer’s disease [65] | 2020 | aAD (n = 10) naAD (n = 10) CN (n = 10) | Motivation to start acting seems to be controlled by regions near and inside the pregenual anterior cingulate cortex, and this function seems to weaken when apathy gets worse in AD. |
| Abnormal brain activities in multiple frequency bands in Parkinson’s disease with apathy [66] | 2022 | PD-A (n = 28) nPD-A (n = 19) HCs (n = 32) | PD-A and PD-NA might involve different brain processes. At the same time, ALFF in the slow-5 band and fALFF in the regular band can tell PD-A and PD-NA apart. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Zhang, H.; Fang, Y.; Yin, D.; Dong, Y.; Chao, X.; Gong, X.; Wang, J.; Sun, W. Altered Resting-State Brain Activity and Functional Connectivity in Post-Stroke Apathy: An fMRI Study. Brain Sci. 2023, 13, 730. https://doi.org/10.3390/brainsci13050730
Jiang S, Zhang H, Fang Y, Yin D, Dong Y, Chao X, Gong X, Wang J, Sun W. Altered Resting-State Brain Activity and Functional Connectivity in Post-Stroke Apathy: An fMRI Study. Brain Sciences. 2023; 13(5):730. https://doi.org/10.3390/brainsci13050730
Chicago/Turabian StyleJiang, Shiyi, Hui Zhang, Yirong Fang, Dawei Yin, Yiran Dong, Xian Chao, Xiuqun Gong, Jinjing Wang, and Wen Sun. 2023. "Altered Resting-State Brain Activity and Functional Connectivity in Post-Stroke Apathy: An fMRI Study" Brain Sciences 13, no. 5: 730. https://doi.org/10.3390/brainsci13050730





