Comparison of Analgesic Efficacy between Epidural and Perineural Administration of Autologous Conditioned Serum in the Conservative Treatment of Low Back Pain Due to Lumbar Degenerative Disc Disease: A Randomized, Open-Label, Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Consideration
2.3. Participants
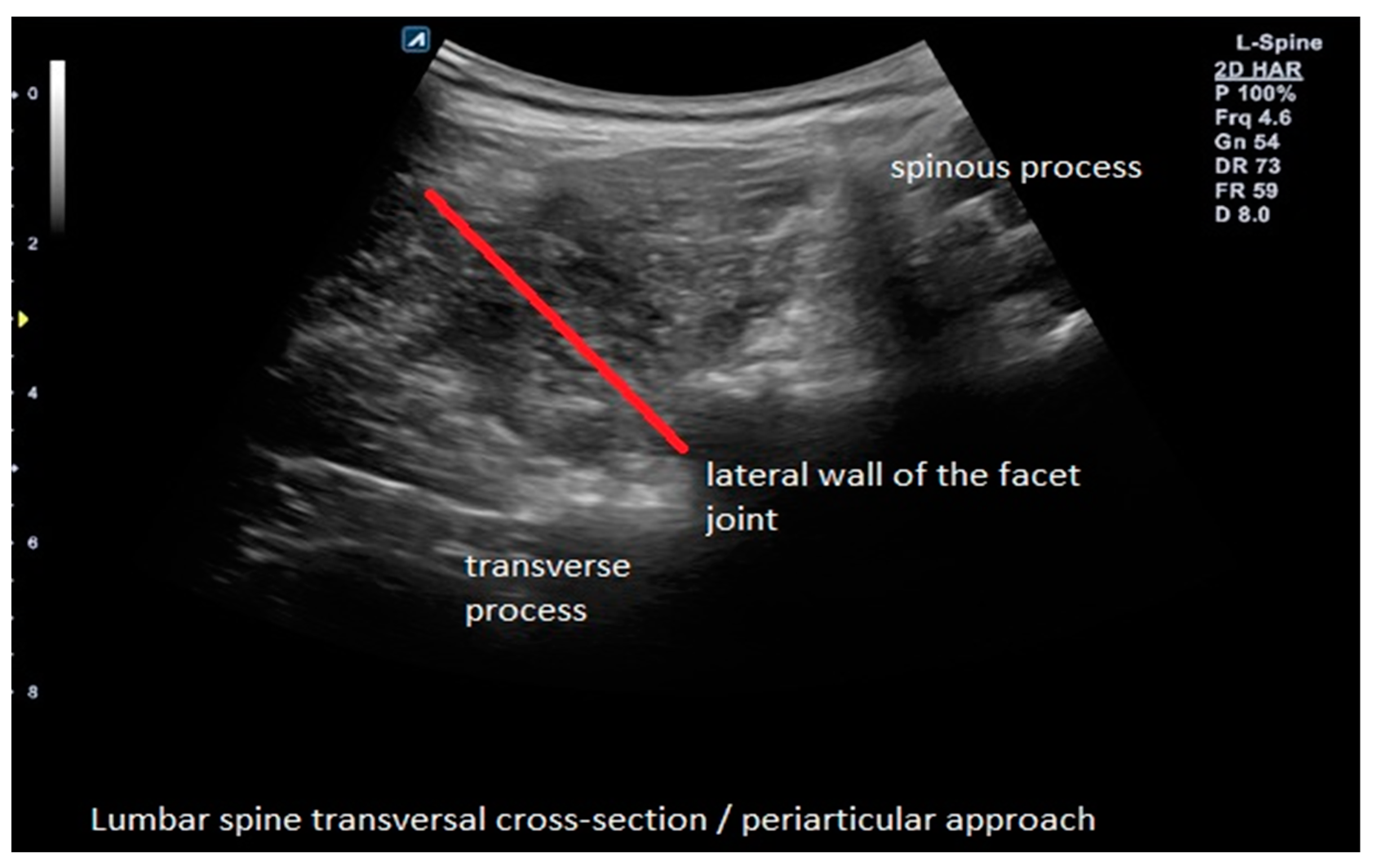
2.4. Interventions
2.5. Outcomes
2.6. Sample Size
2.7. Randomization and Blinding
2.8. Statistical Methods
3. Results
3.1. Participants
3.2. Baseline Data
3.3. Primary Outcome (Change in EQ-5D-5L Index from Baseline to 24 Weeks)
3.4. Primary Outcome (Change in Oswestry Disability Index from Baseline to 24 Weeks)
3.5. Primary Outcome (Change in Roland Morris Questionnaire Score from Baseline to 24 Weeks)
3.6. Outcomes and Estimations (Intra-Group)
3.7. Outcomes and Estimations (Superiority between Groups)
3.8. Outcomes and Estimations (Non-Inferiority)
3.9. Ancillary Analyses
3.10. Adverse Events
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schröder, C.; Nienhaus, A. Intervertebral Disc Disease of the Lumbar Spine in Health Personnel with Occupational Exposure to Patient Handling—A Systematic Literature Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4832. [Google Scholar] [CrossRef] [PubMed]
- Deane, J.A.; McGregor, A.H. Current and Future Perspectives on Lumbar Degenerative Disc Disease: A UK Survey Exploring Specialist Multidisciplinary Clinical Opinion. BMJ Open 2016, 6, e011075. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wen, Z.; Li, D. The Clinical Diagnostic Value of Lumbar Intervertebral Disc Herniation Based on MRI Images. J. Healthc. Eng. 2021, 2021, 5594920. [Google Scholar] [CrossRef]
- Hao, D.-J.; Duan, K.; Liu, T.-J.; Liu, J.-J.; Wang, W.-T. Development and Clinical Application of Grading and Classification Criteria of Lumbar Disc Herniation. Medicine 2017, 96, e8676. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Andrade, N.S.; Neuman, B.J. Lumbar Disc Herniation. Curr. Rev. Musculoskelet. Med. 2017, 10, 507–516. [Google Scholar] [CrossRef]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of Intervertebral Disc Degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef]
- Xin, J.; Wang, Y.; Zheng, Z.; Wang, S.; Na, S.; Zhang, S. Treatment of Intervertebral Disc Degeneration. Orthop. Surg. 2022, 14, 1271–1280. [Google Scholar] [CrossRef]
- Liyew, W.A. Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions. Int. J. Rheumatol. 2020, 2020, 2919625. [Google Scholar] [CrossRef]
- Hasvik, E.; Haugen, A.J.; Grøvle, L. Symptom Descriptors and Patterns in Lumbar Radicular Pain Caused by Disc Herniation: A 1-Year Longitudinal Cohort Study. BMJ Open 2022, 12, e065500. [Google Scholar] [CrossRef]
- Suthar, P.; Patel, R.; Mehta, C.; Patel, N. MRI Evaluation of Lumbar Disc Degenerative Disease. J. Clin. Diagn. Res. 2015, 9, TC04–TC09. [Google Scholar] [CrossRef]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A Systematic Review of the Global Prevalence of Low Back Pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Rodríguez-Salazar, A.; Martín-Alonso, J.; Martín-Velasco, V. Lumbar disc herniation: Natural history, role of physical examination, timing of surgery, treatment options and conflicts of interests. Neurocirugia 2017, 28, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V.; LeQuang, J.A. Rehabilitation for Low Back Pain: A Narrative Review for Managing Pain and Improving Function in Acute and Chronic Conditions. Pain Ther. 2020, 9, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Simson, K.J.; Miller, C.T.; Ford, J.; Hahne, A.; Main, L.; Rantalainen, T.; Teo, W.-P.; Teychenne, M.; Connell, D.; Trudel, G.; et al. Optimising Conservative Management of Chronic Low Back Pain: Study Protocol for a Randomised Controlled Trial. Trials 2017, 18, 184. [Google Scholar] [CrossRef]
- Owen, P.J.; Miller, C.T.; Rantalainen, T.; Simson, K.J.; Connell, D.; Hahne, A.J.; Trudel, G.; Ford, J.J.; Belavy, D.L. Exercise for the Intervertebral Disc: A 6-Month Randomised Controlled Trial in Chronic Low Back Pain. Eur. Spine J. 2020, 29, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory Biomarkers of Low Back Pain and Disc Degeneration: A Review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Tagowski, M.; Lewandowski, Z.; Hodler, J.; Spiegel, T.; Goerres, G.W. Pain Reduction after Lumbar Epidural Injections Using Particulate versus Non-Particulate Steroids: Intensity of the Baseline Pain Matters. Eur. Radiol. 2019, 29, 3379–3389. [Google Scholar] [CrossRef]
- Bensler, S.; Sutter, R.; Pfirrmann, C.W.A.; Peterson, C.K. Is There a Difference in Treatment Outcomes between Epidural Injections with Particulate versus Non-Particulate Steroids? Eur. Radiol. 2017, 27, 1505–1511. [Google Scholar] [CrossRef]
- Mehta, P.; Syrop, I.; Singh, J.R.; Kirschner, J. Systematic Review of the Efficacy of Particulate Versus Nonparticulate Corticosteroids in Epidural Injections. PM R 2017, 9, 502–512. [Google Scholar] [CrossRef]
- Evans, C.H. Novel Biological Approaches to the Intra-Articular Treatment of Osteoarthritis. BioDrugs 2005, 19, 355–362. [Google Scholar] [CrossRef]
- Wehling, P.; Moser, C.; Frisbie, D.; McIlwraith, C.W.; Kawcak, C.E.; Krauspe, R.; Reinecke, J.A. Autologous Conditioned Serum in the Treatment of Orthopedic Diseases: The Orthokine Therapy. BioDrugs 2007, 21, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Camino, J.-C.; Vázquez-Delgado, E.; Gay-Escoda, C. Use of Autologous Conditioned Serum (Orthokine) for the Treatment of the Degenerative Osteoarthritis of the Temporomandibular Joint. Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e433–e438. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sánchez-Margallo, F.M.; Reinecke, J.; Álvarez, V.; López, E.; Marinaro, F.; Casado, J.G. Conditioned Serum Enhances the Chondrogenic and Immunomodulatory Behavior of Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Parisien, M.; Lima, L.V.; Dagostino, C.; El-Hachem, N.; Drury, G.L.; Grant, A.V.; Huising, J.; Verma, V.; Meloto, C.B.; Silva, J.R.; et al. Acute Inflammatory Response via Neutrophil Activation Protects against the Development of Chronic Pain. Sci. Transl. Med. 2022, 14, eabj9954. [Google Scholar] [CrossRef]
- Wright-Carpenter, T.; Opolon, P.; Appell, H.J.; Meijer, H.; Wehling, P.; Mir, L.M. Treatment of Muscle Injuries by Local Administration of Autologous Conditioned Serum: Animal Experiments Using a Muscle Contusion Model. Int. J. Sports Med. 2004, 25, 582–587. [Google Scholar] [CrossRef]
- Shirokova, L.; Noskov, S.; Gorokhova, V.; Reinecke, J.; Shirokova, K. Intra-Articular Injections of a Whole Blood Clot Secretome, Autologous Conditioned Serum, Have Superior Clinical and Biochemical Efficacy Over Platelet-Rich Plasma and Induce Rejuvenation-Associated Changes of Joint Metabolism: A Prospective, Controlled Open-Label Clinical Study in Chronic Knee Osteoarthritis. Rejuvenation Res. 2020, 23, 401–410. [Google Scholar] [CrossRef]
- Merry, T.L.; Chan, A.; Woodhead, J.S.T.; Reynolds, J.C.; Kumagai, H.; Kim, S.-J.; Lee, C. Mitochondrial-Derived Peptides in Energy Metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E659–E666. [Google Scholar] [CrossRef]
- Corp, N.; Mansell, G.; Stynes, S.; Wynne-Jones, G.; Morsø, L.; Hill, J.C.; van der Windt, D.A. Evidence-Based Treatment Recommendations for Neck and Low Back Pain across Europe: A Systematic Review of Guidelines. Eur. J. Pain 2021, 25, 275–295. [Google Scholar] [CrossRef]
- Becker, C.; Heidersdorf, S.; Drewlo, S.; de Rodriguez, S.Z.; Krämer, J.; Willburger, R.E. Efficacy of Epidural Perineural Injections with Autologous Conditioned Serum for Lumbar Radicular Compression: An Investigator-Initiated, Prospective, Double-Blind, Reference-Controlled Study. Spine (Phila Pa 1976) 2007, 32, 1803–1808. [Google Scholar] [CrossRef]
- HS, R.K.; Goni, V.G.; Batra, Y.K. Autologous Conditioned Serum as a Novel Alternative Option in the Treatment of Unilateral Lumbar Radiculopathy: A Prospective Study. Asian Spine J. 2015, 9, 916–922. [Google Scholar] [CrossRef]
- Godek, P. Use of Autologous Serum in Treatment of Lumbar Radiculopathy Pain. Pilot Study. Ortop. Traumatol. Rehabil. 2016, 18, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.; Groenemeyer, D.D.; Becker, J.; Hartmann, J.; Wehling, P. 464 Intradiscal Injections of Orthokine-Derived Autologous Conditioned Serum (ACS) for Lumbar Disc Degeneration. Osteoarthr. Cartil. 2011, 19, S215. [Google Scholar] [CrossRef]
- Godek, P.; Szajkowski, S.; Golicki, D. Evaluation of the Effectiveness of Orthokine Therapy: Retrospective Analysis of 1000 Cases. Ortop. Traumatol. Rehabil. 2020, 22, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, L.; Jiang, C.; Cao, K.; Gao, Z.; Wang, Y. Microglia and Macrophages Contribute to the Development and Maintenance of Sciatica in Lumbar Disc Herniation. Pain 2023, 164, 362–374. [Google Scholar] [CrossRef]





| Character | Epidural Administration | Perineural Administration | p-Value * | ||
|---|---|---|---|---|---|
| N | M ± SD (95% CI of M) | N | M ± SD (95% CI of M) | ||
| Age (in years) | 50 | 47.06 ± 11.86 (43.69 to 50.43) | 50 | 45.52 ± 15.25 (41.19 to 49.85) | 0.5742 |
| Height (in cm) | 50 | 173.74 ± 9.36 (171.08 to 176.40) | 50 | 171.46 ± 9.55 (168.8 to 174.17) | 0.2308 |
| Weight (in kg) | 50 | 81.10 ± 16.01 (76.55 to 85.65) | 50 | 78.00 ± 14.38 (73.91 to 82.09) | 0.3109 |
| Body mass index (BMI) | 50 | 26.85 ± 4.93 (25.45 to 28.25) | 50 | 26.48 ± 4.05 (25.33 to 27.63) | 0.6836 |
| Duration of complaints (in weeks) | 50 | 19.10 ± 19.03 (13.69 to 24.51) | 50 | 19.56 ± 19.27 (14.08 to 25.04) | 0.9047 |
| N (%) | N (%) | p-Value ** | |||
| Total | 50 (100) | 50 (100) | |||
| Gender | 0.3196 | ||||
| Female | 23 (46) | 28 (56) | |||
| Male | 27 (54) | 22 (44) | |||
| Disease phase | 0.4230 | ||||
| Acute (>4 weeks) | 7 (14) | 10 (20) | |||
| Subacute (4–12 weeks) | 21 (42) | 15 (30) | |||
| Chronic (>12 weeks) | 22 (44) | 25 (50) | |||
| Side of discomfort | 0.4244 | ||||
| Left side | 20 (40) | 20 (40) | |||
| Right side | 20 (40) | 15 (30) | |||
| Both sides | 10 (20) | 15 (30) | |||
| Type of hernia | 0.0951 | ||||
| Bulging | 13 (26) | 7 (14) | |||
| Extrusion | 6 (12) | 6 (12) | |||
| Intravertebral | 2 (4) | 2 (4) | |||
| Prolapse | 17 (34) | 28 (56) | |||
| Residual | 12 (24) | 5 (10) | |||
| Sequestration | 0 (0) | 2 (4) | |||
| Predominant level of hernia | 0.5696 | ||||
| Th12/L1 | 1 (2) | 0 (0) | |||
| L2/L3 | 0 (0) | 1 (2) | |||
| L3/L4 | 2 (4) | 1 (2) | |||
| L4/L5 | 16 (32) | 20 (40) | |||
| L5/S1 | 31 (62) | 28 (56) | |||
| Type of discopathy | 0.2821 | ||||
| Single level | 13 (26) | Single level | 13 (26) | ||
| Multilevel | 37 (74) | Multilevel | 37 (74) | ||
| Diabetes | 0.5597 | ||||
| No | 49 (98) | No | 49 (98) | ||
| Yes | 1 (2) | Yes | 1 (2) | ||
| Peripheral vascular disease | 1.0000 | ||||
| No | 48 (96) | No | 48 (96) | ||
| Yes | 2 (4) | Yes | 2 (4) | ||
| Disorders of bone metabolism | 0.5597 | ||||
| No | 49 (98) | No | 49 (98) | ||
| Yes | 1 (2) | Yes | 1 (2) | ||
| Polyneuropathy | 0.3173 | ||||
| No | 50 (100) | No | 50 (100) | ||
| Yes | 0 (0) | Yes | 0 (0) | ||
| Epidural | Perineural | |||
|---|---|---|---|---|
| N | M ± SD (95% CI of M) | N | M ± SD (95% CI of M) | |
| Numeric Rating Scale (NRS) | ||||
| Baseline | 50 | 6.10 ± 1.99 (5.53 to 6.67) | 50 | 5.68 ± 2.17 (5.06 to 6.30) |
| Week 4 | 49 | 3.43 ± 2.17 (2.81 to 4.05) | 50 | 3.26 ± 2.18 (2.64 to 3.88) |
| Week 12 | 46 | 3.44 ± 2.52 (2.69 to 4.18) | 49 | 2.62 ± 2.17 (2.01 to 3.24) |
| Week 24 | 40 | 2.80 ± 2.34 (2.05 to 3.55) | 46 | 2.37 ± 2.12 (1.74 to 3.00) |
| Oswestry Disability Index (ODI) | ||||
| Baseline | 50 | 18.26 ± 6.04 (16.54 to 19.98) | 50 | 19.60 ± 8.60 (17.16 to 22.04) |
| Week 4 | 49 | 12.39 ± 6.87 (10.41 to 14.36) | 50 | 13.78 ± 8.90 (11.25 to 16.31) |
| Week 12 | 46 | 11.83 ± 7.26 (9.67 to 13.98) | 49 | 10.31 ± 7.72 (8.09 to 12.52) |
| Week 24 | 40 | 9.65 ± 6.65 (7.52 to 11.78) | 46 | 9.91 ± 10.09 (6.92 to 12.91) |
| Roland Morris Questionnaire (RMQ) | ||||
| Baseline | 50 | 7.40 ± 4.29 (6.18 to 8.62) | 50 | 8.76 ± 5.55 (7.18 to 10.34) |
| Week 4 | 49 | 4.84 ± 4.32 (3.60 to 6.08) | 50 | 5.42 ± 4.63 (4.10 to 6.74) |
| Week 12 | 46 | 4.41 ± 4.29 (3.14 to 5.69) | 49 | 4.06 ± 4.28 (2.83 to 5.29) |
| Week 24 | 40 | 3.08 ± 3.21 (2.05 to 4.10) | 46 | 4.37 ± 5.14 (2.84 to 5.90) |
| EQ-5D-5L mobility | ||||
| Baseline | 50 | 1.94 ± 0.84 (1.70 to 2.18) | 50 | 2.26 ± 0.96 (1.99 to 2.53) |
| Week 4 | 49 | 1.69 ± 0.74 (1.48 to 1.91) | 50 | 1.88 ± 0.85 (1.64 to 2.12) |
| Week 12 | 46 | 1.74 ± 0.77 (1.51 to 1.97) | 49 | 1.63 ± 0.81 (1.40 to 1.87) |
| Week 24 | 40 | 1.58 ± 0.71 (1.35 to 1.80) | 45 | 1.53 ± 0.87 (1.27 to 1.79) |
| EQ-5D-5L self-care | ||||
| Baseline | 50 | 1.74 ± 0.78 (1.52 to 1.96) | 50 | 1.84 ± 1.00 (1.56 to 2.12) |
| Week 4 | 49 | 1.57 ± 0.71 (1.37 to 1.78) | 50 | 1.64 ± 0.83 (1.41 to 1.88) |
| Week 12 | 46 | 1.50 ± 0.75 (1.28 to 1.72) | 49 | 1.57 ± 0.79 (1.34 to 1.80) |
| Week 24 | 40 | 1.38 ± 0.59 (1.19 to 1.56) | 45 | 1.49 ± 0.87 (1.23 to 1.75) |
| EQ-5D-5L usual activities | ||||
| Baseline | 50 | 2.46 ± 0.73 (2.25 to 2.67) | 50 | 2.52 ± 0.91 (2.26 to 2.78) |
| Week 4 | 49 | 2.02 ± 0.75 (1.81 to 2.24) | 50 | 2.06 ± 0.87 (1.81 to 2.31) |
| Week 12 | 46 | 1.94 ± 0.85 (1.68 to 2.19) | 49 | 1.90 ± 0.80 (1.67 to 2.13) |
| Week 24 | 40 | 1.58 ± 0.64 (1.37 to 1.78) | 45 | 1.64 ± 0.83 (1.40 to 1.89) |
| EQ-5D-5L pain/discomfort | ||||
| Baseline | 50 | 3.06 ± 0.74 (2.85 to 3.27) | 50 | 3.08 ± 0.83 (2.84 to 3.32) |
| Week 4 | 49 | 2.45 ± 0.79 (2.22 to 2.68) | 50 | 2.52 ± 0.74 (2.31 to 2.73) |
| Week 12 | 46 | 2.44 ± 0.83 (2.19 to 2.68) | 49 | 2.16 ± 0.77 (1.94 to 2.39) |
| Week 24 | 40 | 2.13 ± 0.76 (1.88 to 2.37) | 45 | 2.18 ± 0.91 (1.90 to 2.45) |
| EQ-5D-5L anxiety/depression | ||||
| Baseline | 50 | 2.02 ± 0.87 (1.77 to 2.27) | 50 | 2.24 ± 1.19 (1.90 to 2.58) |
| Week 4 | 49 | 1.69 ± 0.77 (1.47 to 1.92) | 50 | 1.90 ± 1.05 (1.60 to 2.20) |
| Week 12 | 46 | 1.74 ± 0.95 (1.46 to 2.02) | 49 | 1.59 ± 0.81 (1.36 to 1.83) |
| Week 24 | 40 | 1.58 ± 0.71 (1.35 to 1.80) | 45 | 1.56 ± 0.81 (1.31 to 1.80) |
| EQ-5D-5L-based Level Sum Score (LSS) | ||||
| Baseline | 50 | 11.22 ± 2.74 (10.44 to 12.00) | 50 | 11.94 ± 3.97 (10.81 to 13.07) |
| Week 4 | 49 | 9.43 ± 2.94 (8.59 to 10.27) | 50 | 10.00 ± 3.70 (8.95 to 11.05) |
| Week 12 | 46 | 9.15 ± 3.61 (8.09 to 10.21) | 49 | 8.86 ± 3.28 (7.92 to 9.80) |
| Week 24 | 40 | 8.23 ± 2.67 (7.37 to 9.08) | 45 | 8.40 ± 3.74 (7.28 to 9.52) |
| EQ-5D-5L VAS | ||||
| Baseline | 50 | 66.14 ± 16.85 (61.35 to 70.93) | 50 | 60.14 ± 20.35 (54.36 to 65.92) |
| Week 4 | 49 | 69.94 ± 17.49 (64.92 to 74.96) | 50 | 71.70 ± 15.99 (67.16 to 76.25) |
| Week 12 | 46 | 71.74 ± 17.84 (66.44 to 77.04) | 49 | 74.67 ± 15.38 (70.26 to 79.09) |
| Week 24 | 40 | 74.75 ± 17.17 (69.26 to 80.24) | 45 | 77.62 ± 14.86 (73.16 to 82.09) |
| EQ-5D-5L Index | ||||
| Baseline | 50 | 0.805 ± 0.138 (0.766 to 0.844) | 50 | 0.754 ± 0.197 (0.698 to 0.810) |
| Week 4 | 49 | 0.875 ± 0.117 (0.842 to 0.909) | 50 | 0.862 ± 0.145 (0.821 to 0.903) |
| Week 12 | 46 | 0.872 ± 0.137 (0.832 to 0.913) | 49 | 0.902 ± 0.092 (0.876 to 0.928) |
| Week 24 | 40 | 0.913 ± 0.091 (0.884 to 0.942) | 45 | 0.895 ± 0.159 (0.847 to 0.943) |
| Epidural Administration | Perineural Administration | |||||||
|---|---|---|---|---|---|---|---|---|
| Difference to Baseline at | N | M ± SD (95% CI of M) | Cohen’s d | p-Value * | N | M ± SD (95% CI of M) | Cohen’s d | p-Value * |
| Numeric Rating Scale (NRS) | ||||||||
| Week 4 | 49 | −2.74 ± 2.24 (−3.38 to −2.09) | −1.22 | <0.0001 | 50 | −2.42 ± 2.04 (−3.00 to −1.84) | −1.19 | <0.0001 |
| Week 12 | 46 | −2.52 ± 2.51 (−3.27 to −1.78) | −1.00 | <0.0001 | 49 | −3.06 ± 2.49 (−3.77 to −2.35) | −1.23 | <0.0001 |
| Week 24 | 40 | −3.08 ± 2.12 (−3.75 to −2.40) | −1.45 | <0.0001 | 46 | −3.09 ± 2.49 (−3.83 to −2.35) | −1.24 | <0.0001 |
| Oswestry Disability Index (ODI) | ||||||||
| Week 4 | 49 | −5.82 ± 6.34 (−7.64 to −3.99) | −0.92 | <0.0001 | 50 | −5.82 ± 8.21 (−8.15 to −3.49) | −0.71 | <0.0001 |
| Week 12 | 46 | −6.26 ± 7.16 (−8.39 to −4.14) | −0.87 | <0.0001 | 49 | −8.96 ± 8.25 (−11.33 to −6.59) | −1.09 | <0.0001 |
| Week 24 | 40 | −7.80 ± 8.24 (−10.44 to −5.16) | −0.95 | <0.0001 | 46 | −8.78 ± 9.63 (−11.64 to −5.92) | −0.91 | <0.0001 |
| Roland Morris Questionnaire (RMQ) | ||||||||
| Week 4 | 49 | −2.53 ± 4.52 (−3.83 to −1.23) | −0.56 | 0.0003 | 50 | −3.34 ± 4.85 (−4.72 to −1.96) | −0.69 | <0.0001 |
| Week 12 | 46 | −2.94 ± 3.95 (−4.11 to −1.76) | −0.74 | <0.0001 | 49 | −4.76 ± 5.03 (−6.20 to −3.31) | −0.95 | <0.0001 |
| Week 24 | 40 | −3.55 ± 4.49 (−4.99 to −2.11) | −0.79 | <0.0001 | 46 | −3.94 ± 5.35 (−5.52 to −2.35) | −0.74 | <0.0001 |
| EQ-5D-5L mobility | ||||||||
| Week 4 | 49 | −0.22 ± 1.01 (−0.51 to 0.06) | −0.22 | 0.1247 | 50 | −0.38 ± 0.85 (−0.62 to −0.14) | −0.44 | 0.0028 |
| Week 12 | 46 | −0.22 ± 0.84 (−0.47 to 0.03) | −0.26 | 0.0864 | 49 | −0.59 ± 0.93 (−0.86 to −0.32) | −0.63 | 0.0001 |
| Week 24 | 40 | −0.38 ± 0.84 (−0.64 to −0.11) | −0.45 | 0.0073 | 45 | −0.60 ± 1.03 (−0.91 to −0.29) | −0.58 | 0.0003 |
| EQ-5D-5L self-care | ||||||||
| Week 4 | 49 | −0.14 ± 0.74 (−0.35 to 0.07) | −0.19 | 0.1806 | 50 | −0.20 ± 0.76 (−0.42 to 0.01) | −0.26 | 0.0673 |
| Week 12 | 46 | −0.24 ± 0.77 (−0.47 to −0.01) | −0.31 | 0.0397 | 49 | −0.22 ± 0.94 (−0.50 to 0.05) | −0.24 | 0.1015 |
| Week 24 | 40 | −0.30 ± 0.79 (−0.55 to −0.05) | −0.38 | 0.0213 | 45 | −0.29 ± 0.84 (−0.54 to −0.04) | −0.34 | 0.0263 |
| EQ-5D-5L usual activities | ||||||||
| Week 4 | 49 | −0.41 ± 0.96 (−0.68 to −0.13) | −0.43 | 0.0044 | 50 | −0.46 ± 0.81 (−0.69 to −0.23) | −0.57 | 0.0002 |
| Week 12 | 46 | −0.52 ± 0.94 (−0.80 to −0.24) | −0.56 | 0.0005 | 49 | −0.59 ± 0.98 (−0.87 to −0.31) | −0.61 | 0.0001 |
| Week 24 | 40 | −0.83 ± 0.81 (−1.09 to −0.57) | −1.01 | <0.0001 | 45 | −0.80 ± 0.94 (−1.08 to −0.52) | −0.85 | <0.0001 |
| EQ-5D-5L pain/discomfort | ||||||||
| Week 4 | 49 | −0.59 ± 0.93 (−0.86 to −0.32) | −0.63 | 0.0001 | 50 | −0.56 ± 0.95 (−0.83 to −0.29) | −0.59 | 0.0001 |
| Week 12 | 46 | −0.63 ± 0.77 (−0.86 to −0.40) | −0.82 | <0.0001 | 49 | −0.90 ± 0.96 (−1.17 to −0.62) | −0.93 | <0.0001 |
| Week 24 | 40 | −0.88 ± 0.91 (−1.17 to −0.58) | −0.96 | <0.0001 | 45 | −0.84 ± 1.09 (−1.17 to −0.52) | −0.78 | <0.0001 |
| EQ-5D-5L anxiety/depression | ||||||||
| Week 4 | 49 | −0.33 ± 0.99 (−0.61 to −0.04) | −0.33 | 0.0249 | 50 | −0.34 ± 1.00 (−0.63 to −0.06) | −0.34 | 0.0203 |
| Week 12 | 46 | −0.33 ± 1.01 (−0.63 to −0.03) | −0.32 | 0.0341 | 49 | −0.59 ± 0.96 (−0.87 to −0.32) | −0.62 | 0.0001 |
| Week 24 | 40 | −0.43 ± 1.01 (−0.75 to −0.10) | −0.42 | 0.0112 | 45 | −0.56 ± 1.20 (−0.92 to −0.20) | −0.46 | 0.0033 |
| EQ-5D-5L-based Level Sum Score (LSS) | ||||||||
| Week 4 | 49 | −1.69 ± 3.27 (−2.63 to −0.75) | −0.52 | 0.0007 | 50 | −1.94 ± 3.18 (−2.85 to −1.04) | −0.61 | 0.0001 |
| Week 12 | 46 | −2.04 ± 3.14 (−2.97 to −1.12) | −0.65 | 0.0001 | 49 | −2.90 ± 3.56 (−3.92 to −1.88) | −0.81 | <0.0001 |
| Week 24 | 40 | −2.80 ± 3.01 (−3.76 to −1.84) | −0.93 | <0.0001 | 45 | −3.09 ± 3.81 (−4.23 to −1.94) | −0.81 | <0.0001 |
| EQ-5D-5L VAS | ||||||||
| Week 4 | 49 | 3.57 ± 18.69 (−1.80 to 8.94) | 0.19 | 0.1873 | 50 | 11.56 ± 18.78 (6.22 to 16.90) | 0.62 | 0.0001 |
| Week 12 | 46 | 5.94 ± 17.47 (0.75 to 11.12) | 0.34 | 0.0259 | 49 | 14.22 ± 20.49 (8.34 to 20.11) | 0.69 | <0.0001 |
| Week 24 | 40 | 6.70 ± 20.70 (0.08 to 13.32) | 0.32 | 0.0474 | 45 | 15.02 ± 19.95 (9.03 to 21.02) | 0.75 | <0.0001 |
| EQ-5D-5L Index | ||||||||
| Week 4 | 49 | 0.0653 ± 0.150 (0.022 to 0.108) | 0.44 | 0.0037 | 50 | 0.108 ± 0.167 (0.060 to 0.155) | 0.65 | <0.0001 |
| Week 12 | 46 | 0.0688 ± 0.144 (0.026 to 0.112) | 0.48 | 0.0023 | 49 | 0.136 ± 0.163 (0.089 to 0.183) | 0.83 | <0.0001 |
| Week 24 | 40 | 0.0969 ± 0.138 (0.053 to 0.141) | 0.70 | 0.0001 | 45 | 0.119 ± 0.191 (0.062 to 0.177) | 0.62 | 0.0001 |
| Difference between Groups | |||||
|---|---|---|---|---|---|
| Difference at | Ne | Np | M ± SD (95% CI of M) | Cohen’s d | p-Value * |
| Numeric Rating Scale (NRS) | |||||
| Week 4 | 49 | 50 | 0.31 ± 2.14 (−0.54 to 1.17) | −0.15 | 0.4670 |
| Week 12 | 46 | 49 | −0.54 ± 2.50 (−1.55 to 0.47) | 0.22 | 0.2942 |
| Week 24 | 40 | 46 | −0.01 ± 2.33 (−1.01 to 0.99) | 0.01 | 0.9811 |
| Oswestry Disability Index (ODI) | |||||
| Week 4 | 49 | 50 | 0.00 ± 7.34 (−2.93 to 2.93) | 0.00 | 0.9980 |
| Week 12 | 46 | 49 | −2.70 ± 7.74 (−5.85 to 0.46) | 0.35 | 0.0929 |
| Week 24 | 40 | 46 | −0.98 ± 9.01 (−4.86 to 2.89) | 0.11 | 0.6155 |
| Roland Morris Questionnaire (RMQ) | |||||
| Week 4 | 49 | 50 | −0.81 ± 4.69 (−2.68 to 1.06) | 0.17 | 0.3927 |
| Week 12 | 46 | 49 | −1.82 ± 4.54 (−3.67 to 0.03) | 0.40 | 0.0538 |
| Week 24 | 40 | 46 | −0.38 ± 4.97 (−2.52 to 1.75) | 0.08 | 0.7211 |
| EQ-5D-5L mobility | |||||
| Week 4 | 49 | 50 | −0.16 ± 0.93 (−0.53 to 0.22) | 0.17 | 0.4087 |
| Week 12 | 46 | 49 | −0.37 ± 0.89 (−0.74 to −0.01) | 0.42 | 0.0432 |
| Week 24 | 40 | 45 | −0.23 ± 0.95 (−0.63 to 0.18) | 0.24 | 0.2766 |
| EQ-5D-5L self-care | |||||
| Week 4 | 49 | 50 | −0.06 ± 0.75 (−0.35 to 0.24) | 0.08 | 0.7040 |
| Week 12 | 46 | 49 | 0.01 ± 0.86 (−0.34 to 0.37) | −0.02 | 0.9341 |
| Week 24 | 40 | 45 | 0.01 ± 0.82 (−0.34 to 0.37) | −0.01 | 0.9504 |
| EQ-5D-5L usual activities | |||||
| Week 4 | 49 | 50 | −0.05 ± 0.89 (−0.41 to 0.30) | 0.06 | 0.7718 |
| Week 12 | 46 | 49 | −0.07 ± 0.96 (−0.46 to 0.32) | 0.07 | 0.7223 |
| Week 24 | 40 | 45 | 0.03 ± 0.88 (−0.36 to 0.41) | −0.03 | 0.8969 |
| EQ-5D-5L pain/discomfort | |||||
| Week 4 | 49 | 50 | 0.03 ± 0.94 (−0.34 to 0.41) | −0.03 | 0.8669 |
| Week 12 | 46 | 49 | −0.27 ± 0.88 (−0.62 to 0.09) | 0.31 | 0.1398 |
| Week 24 | 40 | 45 | 0.03 ± 1.01 (−0.41 to 0.47) | −0.03 | 0.8894 |
| EQ-5D-5L anxiety/depression | |||||
| Week 4 | 49 | 50 | −0.01 ± 0.99 (−0.41 to 0.38) | 0.01 | 0.9464 |
| Week 12 | 46 | 49 | −0.27 ± 0.98 (−0.67 to 0.14) | 0.27 | 0.1913 |
| Week 24 | 40 | 45 | −0.13 ± 1.11 (−0.61 to 0.35) | 0.12 | 0.5909 |
| EQ-5D-5L-based Level Sum Score (LSS) | |||||
| Week 4 | 49 | 50 | −0.25 ± 3.23 (−1.53 to 1.04) | 0.08 | 0.7054 |
| Week 12 | 46 | 49 | −0.86 ± 3.36 (−2.22 to 0.51) | 0.25 | 0.2158 |
| Week 24 | 40 | 45 | −0.29 ± 3.46 (−1.78 to 1.21) | 0.08 | 0.7019 |
| EQ-5D-5L VAS | |||||
| Week 4 | 49 | 50 | 7.99 ± 18.73 (0.51 to 15.46) | −0.43 | 0.0364 |
| Week 12 | 46 | 49 | 8.29 ± 19.09 (0.51 to 16.07) | −0.44 | 0.0370 |
| Week 24 | 40 | 45 | 8.32 ± 20.30 (−0.45 to 17.10) | −0.41 | 0.0628 |
| EQ-5D-5L Index | |||||
| Week 4 | 49 | 50 | 0.042 ± 0.159 (−0.021 to 0.106) | −0.27 | 0.1857 |
| Week 12 | 46 | 49 | 0.067 ± 0.154 (0.005 to 0.130) | −0.44 | 0.0359 |
| Week 24 | 40 | 45 | 0.022 ± 0.168 (−0.051 to 0.095) | −0.13 | 0.5452 |
| Epidural | Perineural | |||||
|---|---|---|---|---|---|---|
| Type of Hernia | N | M ± SD (95% CI of M) | Cohen’s d | N | M ± SD (95% CI of M) | Cohen’s d |
| Difference from baseline to week 24: Oswestry Disability Index (ODI) | ||||||
| Bulging | 13 | −7.31 ± 6.80 (−11.42 to −3.2) | −1.07 | 7 | −8.43 ± 9.11 (−16.85 to −0.01) | −0.93 |
| Extrusion | 6 | −5.83 ± 5.71 (−11.82 to 0.16) | −1.02 | 5 | −20 ± 10.75 (−33.34 to −6.66) | −1.86 |
| Prolapse | 11 | −8.82 ± 10.09 (−15.6 to −2.04) | −0.87 | 25 | −5.72 ± 9.08 (−9.47 to −1.97) | −0.63 |
| Residual | 8 | −7.63 ± 10.31 (−16.24 to 0.99) | −0.74 | 5 | −6.8 ± 2.77 (−10.25 to −3.36) | −2.45 |
| Difference from baseline to week 24: Roland Morris Questionnaire (RMQ) | ||||||
| Bulging | 13 | −3.23 ± 4.0652 (−5.69 to −0.77) | −0.79 | 7 | −2.43 ± 3.1 (−5.3 to 0.44) | −0.78 |
| Extrusion | 6 | −2.83 ± 2.86 (−5.83 to 0.17) | −0.99 | 5 | −10.6 ± 7.02 (−19.32 to −1.88) | −1.51 |
| Prolapse | 11 | −3.73 ± 4.15 (−6.52 to −0.94) | −0.90 | 25 | −2.88 ± 4.94 (−4.92 to −0.84) | −0.58 |
| Residual | 8 | −3.63 ± 5.88 (−8.54 to 1.29) | −0.62 | 5 | −3.4 ± 4.39 (−8.86 to 2.06) | −0.77 |
| Difference from baseline to week 24: EQ-5D-5L Index | ||||||
| Bulging | 13 | 0.07 ± 0.079 (0.022 to 0.118) | 0.88 | 7 | 0.147 ± 0.119 (0.022 to 0.271) | 1.24 |
| Extrusion | 6 | 0.101 ± 0.128 (−0.034 to 0.235) | 0.79 | 5 | 0.248 ± 0.238 (−0.048 to 0.543) | 1.04 |
| Prolapse | 11 | 0.103 ± 0.181 (−0.019 to 0.225) | 0.57 | 25 | 0.08 ± 0.214 (−0.008 to 0.169) | 0.37 |
| Residual | 8 | 0.082 ± 0.15 (−0.044 to 0.207) | 0.55 | 5 | 0.119 ± 0.105 (−0.011 to 0.249) | 1.14 |
| Event | Epidural Administration | Perineural Administration |
|---|---|---|
| Serious adverse reactions | Ø | Ø |
| Adverse reactions | ||
| Benign headache | 2 | Ø |
| Dizziness | Ø | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godek, P.; Szczepanowska-Wolowiec, B.; Golicki, D. Comparison of Analgesic Efficacy between Epidural and Perineural Administration of Autologous Conditioned Serum in the Conservative Treatment of Low Back Pain Due to Lumbar Degenerative Disc Disease: A Randomized, Open-Label, Controlled Clinical Trial. Brain Sci. 2023, 13, 749. https://doi.org/10.3390/brainsci13050749
Godek P, Szczepanowska-Wolowiec B, Golicki D. Comparison of Analgesic Efficacy between Epidural and Perineural Administration of Autologous Conditioned Serum in the Conservative Treatment of Low Back Pain Due to Lumbar Degenerative Disc Disease: A Randomized, Open-Label, Controlled Clinical Trial. Brain Sciences. 2023; 13(5):749. https://doi.org/10.3390/brainsci13050749
Chicago/Turabian StyleGodek, Piotr, Beata Szczepanowska-Wolowiec, and Dominik Golicki. 2023. "Comparison of Analgesic Efficacy between Epidural and Perineural Administration of Autologous Conditioned Serum in the Conservative Treatment of Low Back Pain Due to Lumbar Degenerative Disc Disease: A Randomized, Open-Label, Controlled Clinical Trial" Brain Sciences 13, no. 5: 749. https://doi.org/10.3390/brainsci13050749
APA StyleGodek, P., Szczepanowska-Wolowiec, B., & Golicki, D. (2023). Comparison of Analgesic Efficacy between Epidural and Perineural Administration of Autologous Conditioned Serum in the Conservative Treatment of Low Back Pain Due to Lumbar Degenerative Disc Disease: A Randomized, Open-Label, Controlled Clinical Trial. Brain Sciences, 13(5), 749. https://doi.org/10.3390/brainsci13050749





