AI Machine Learning Technique Characterizes Potential Markers of Depression in Two Animal Models of Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
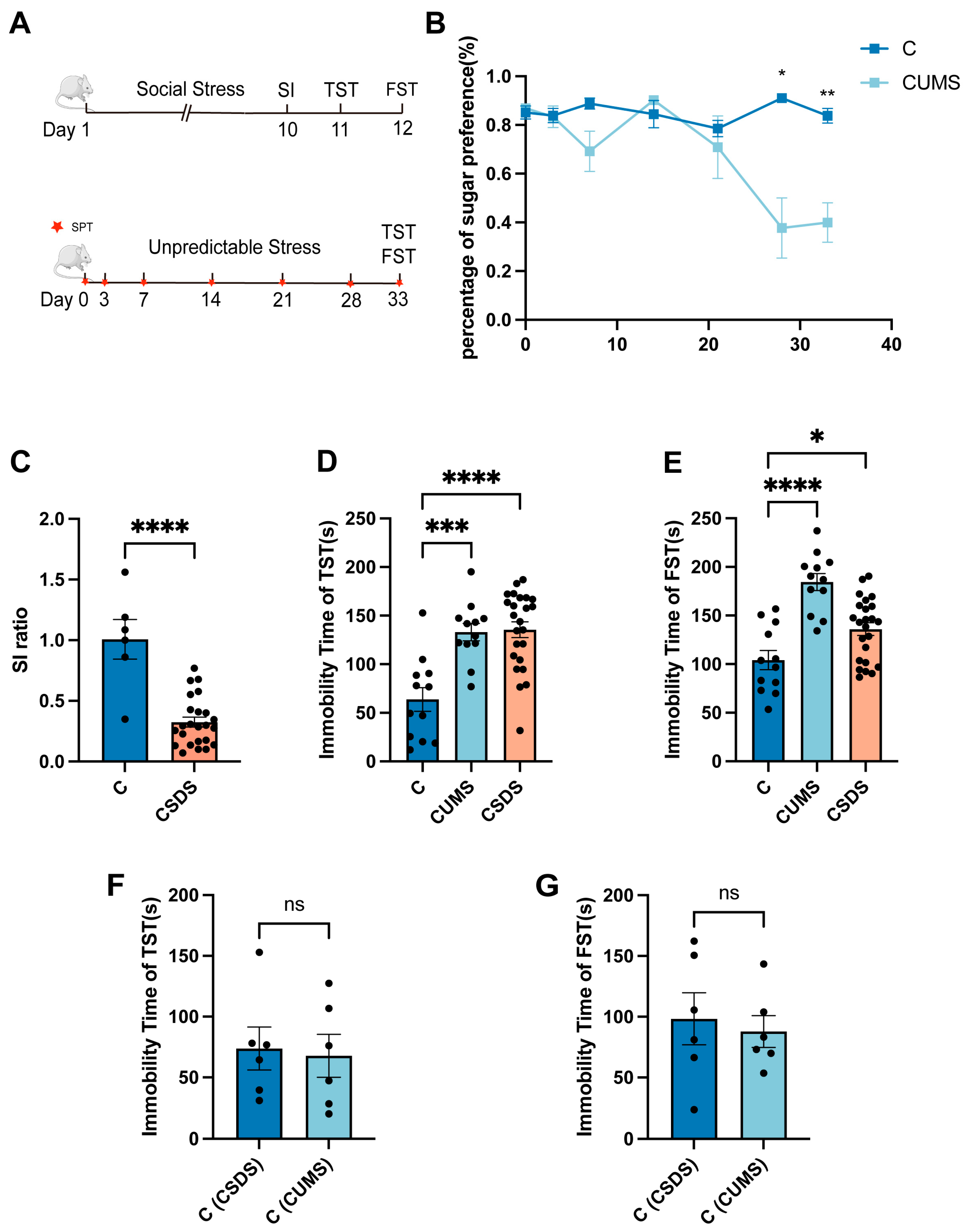
2.3. Depression Animal Models
2.3.1. Chronic Unpredictable Mild Stress Model (CUMS)
2.3.2. Chronic Social Defeat Stress Model (CSDS)
2.4. Behavioral Assays
2.4.1. Social Interaction Test (SI)
2.4.2. Sucrose Preference Test (SPT)
2.4.3. Tail Suspension Test (TST)
2.4.4. Forced Swim Test (FST)
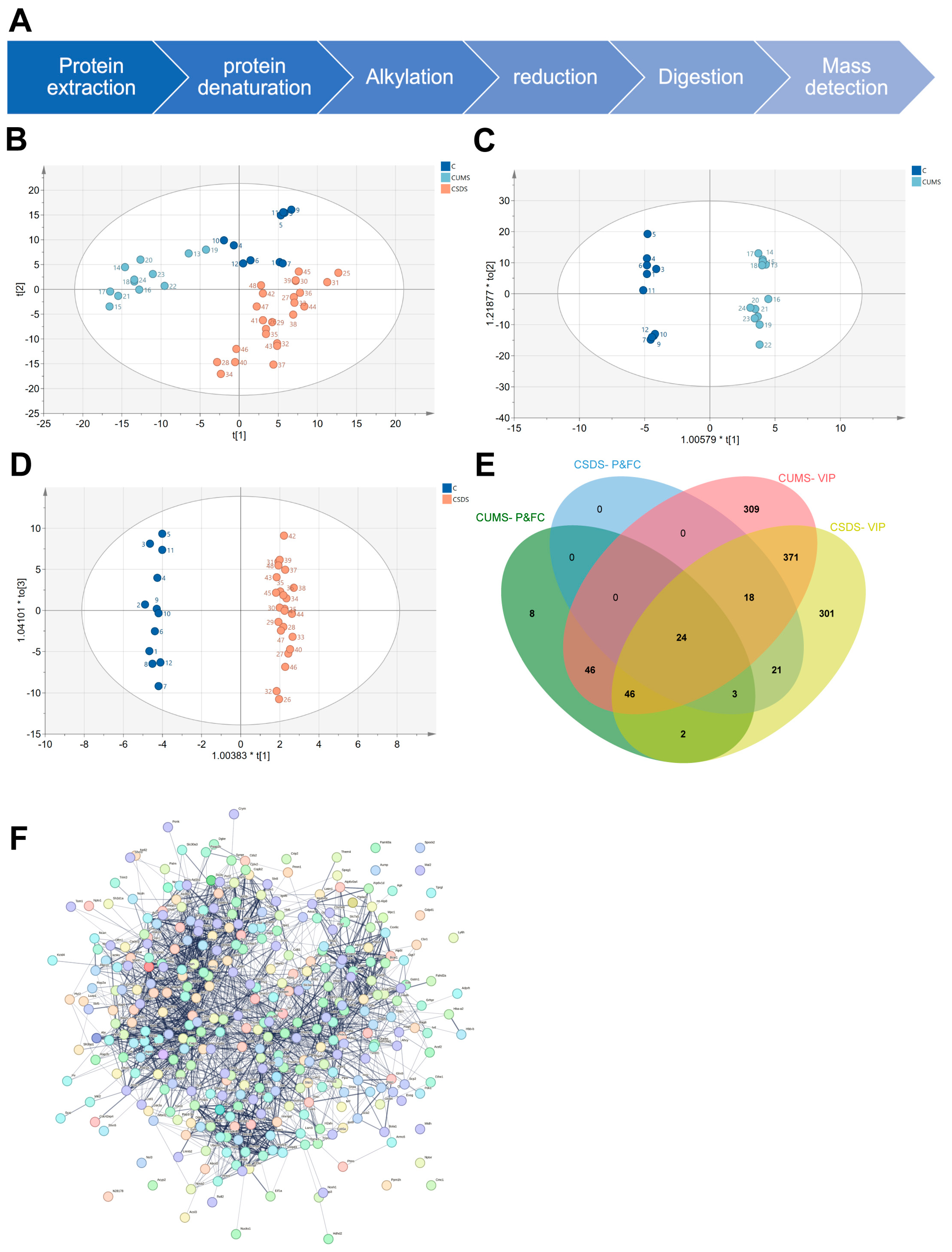
2.5. Untargeted Proteomics
2.5.1. Protein Sample Preparation
2.5.2. Project-Specific DDA Library Generation
2.5.3. SWATH Method Construction
2.5.4. Sample SWATH Detection
2.6. Data Analysis
2.6.1. Principal Component Analysis
2.6.2. Machine Learning
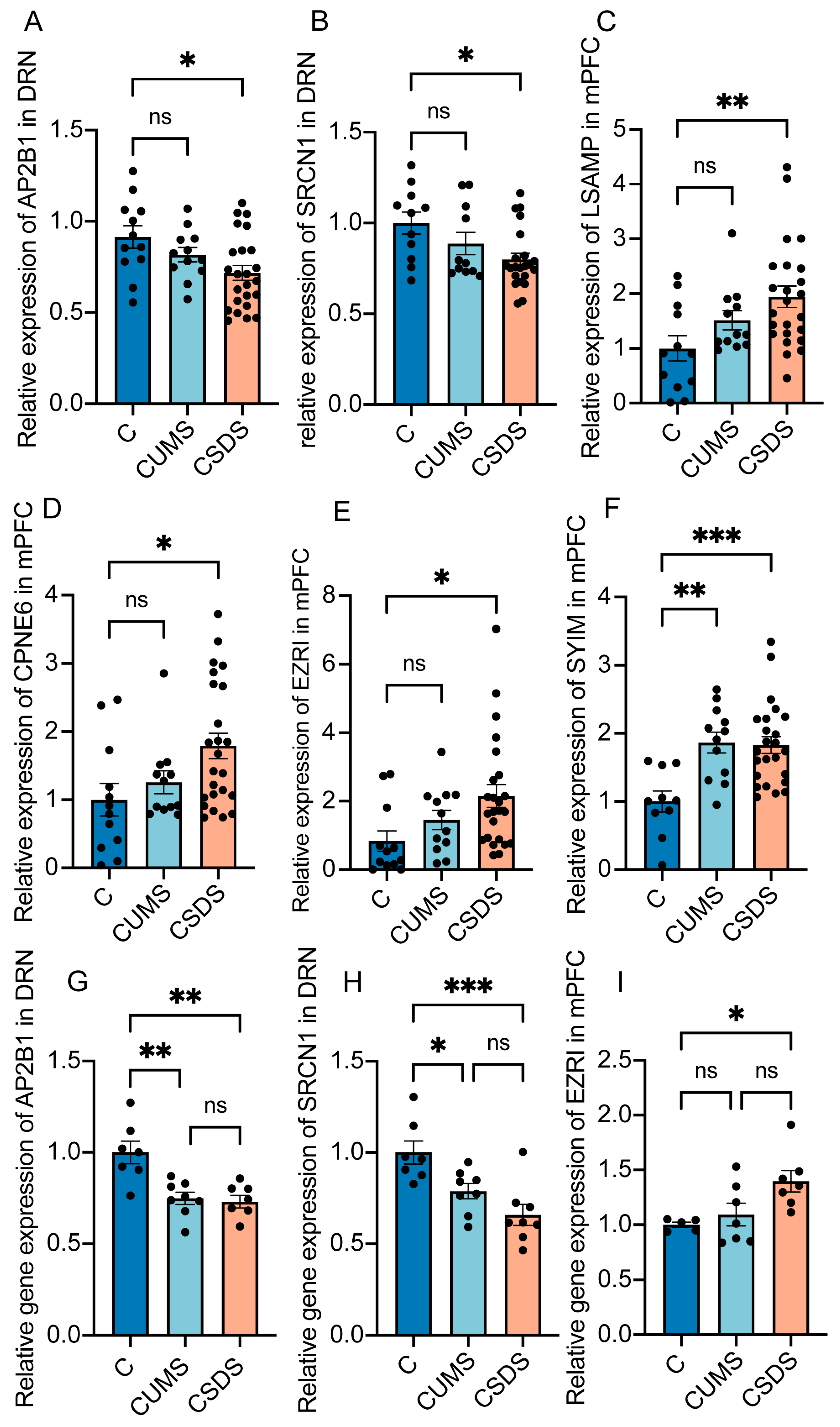
2.7. Quantitative Real-Time PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Two Different Kinds of Animal Depression Models
3.2. Analysis of Proteomic Data Using Principal Component Analysis
3.3. Proteomics Biomarker Panels Construction
3.4. Analysis and Verification of Expression Differences in Featured Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ge, H.; Sun, M.; Gao, Y. Selecting an Appropriate Animal Model of Depression. Int. J. Mol. Sci. 2019, 20, 4827. [Google Scholar] [CrossRef] [PubMed]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Hosp, F.; Mann, M. A Primer on Concepts and Applications of Proteomics in Neuroscience. Neuron 2017, 96, 558–571. [Google Scholar] [CrossRef]
- Fleuren, E.D.G.; Zhang, L.; Wu, J.; Daly, R.J. The kinome “at large” in cancer. Nat. Rev. Cancer 2016, 16, 83–98. [Google Scholar] [CrossRef]
- Lam, M.P.Y.; Ping, P.; Murphy, E. Proteomics Research in Cardiovascular Medicine and Biomarker Discovery. J. Am. Coll. Cardiol. 2016, 68, 2819–2830. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Lee, Y.M.; Kalesh, K.A.; Ong, Y.S.; Lim, J.; Jee, J.-E.; Sun, H.; Lee, S.S.; Hua, Z.-C.; et al. Target identification of natural and traditional medicines with quantitative chemical proteomics approaches. Pharmacol. Ther. 2016, 162, 10–22. [Google Scholar] [CrossRef]
- Mischak, H.; Delles, C.; Vlahou, A.; Vanholder, R. Proteomic biomarkers in kidney disease: Issues in development and implementation. Nat. Rev. Nephrol. 2015, 11, 221–232. [Google Scholar] [CrossRef]
- van Haeringen, M.; Milaneschi, Y.; Lamers, F.; Penninx, B.W.J.H.; Jansen, R. Dissection of depression heterogeneity using proteomic clusters. Psychol. Med. 2022, 1–9. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, Y.-Z.; Mu, R.-H.; Tang, S.-S.; Liu, X.; Xing, S.-Y.; Long, Y.; Yuan, D.-H.; Hong, H. Takeda G Protein–Coupled Receptor 5 Modulates Depression-like Behaviors via Hippocampal CA3 Pyramidal Neurons Afferent to Dorsolateral Septum. Biol. Psychiatry 2021, 89, 1084–1095. [Google Scholar] [CrossRef]
- Rupprechter, S.; Romaniuk, L.; Series, P.; Hirose, Y.; Hawkins, E.; Sandu, A.-L.; Waiter, G.D.; McNeil, C.J.; Shen, X.; Harris, M.A.; et al. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain J. Neurol. 2020, 143, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, M.V.; Wu, M.; Li, C.; Li, X.-Y.; Picciotto, M.R.; Duman, R.S. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol. Psychiatry 2021, 26, 3277–3291. [Google Scholar] [CrossRef]
- Ishimura, K.; Takeuchi, Y.; Fujiwara, K.; Tominaga, M.; Yoshioka, H.; Sawada, T. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci. Lett. 1988, 91, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Aa, J. Analysis of metabolomic data: Principal component analysis. Chin. J. Clin. Pharm. Therap. 2010, 15, 481–489. [Google Scholar]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef]
- Virreira Winter, S.; Karayel, O.; Strauss, M.T.; Padmanabhan, S.; Surface, M.; Merchant, K.; Alcalay, R.N.; Mann, M. Urinary proteome profiling for stratifying patients with familial Parkinson’s disease. EMBO Mol. Med. 2021, 13, e13257. [Google Scholar] [CrossRef]
- Hermsen, M.; de Bel, T.; den Boer, M.; Steenbergen, E.J.; Kers, J.; Florquin, S.; Roelofs, J.J.T.H.; Stegall, M.D.; Alexander, M.P.; Smith, B.H.; et al. Deep Learning–Based Histopathologic Assessment of Kidney Tissue. J. Am. Soc. Nephrol. 2019, 30, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Yin, C.-Y.; Zhu, L.-J.; Zhu, X.-H.; Xu, C.; Luo, C.-X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.-G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, X.; Kang, D.; Shen, B.; Zhu, Z.; Li, X.; Li, H.; Xie, L.; Wang, G.; Liang, Y. An integrated strategy for the quantitative analysis of endogenous proteins: A case of gender-dependent expression of P450 enzymes in rat liver microsome. Talanta 2017, 170, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, S.; Sun, R.; Jia, X.; Xu, C.; Aa, J.; Wang, G. Hirsutella sinensis Treatment Shows Protective Effects on Renal Injury and Metabolic Modulation in db/db Mice. Evid. Based Complement. Alternat. Med. 2019, 2019, 4732858. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, Q.; Gao, W.; Wu, Y.; Mu, R. Seeking for potential pathogenic genes of major depressive disorder in the Gene Expression Omnibus database. Asia-Pac. Psychiatry Off. J. Pac. Rim Coll. Psychiatr. 2020, 12, e12379. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ali, T.; Zheng, C.; Liu, Z.; He, K.; Shah, F.A.; Ren, Q.; Rahman, S.U.; Li, N.; Yu, Z.-J.; et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J. Neuroinflamm. 2021, 18, 38. [Google Scholar] [CrossRef]
- Koido, K.; Traks, T.; Balõtšev, R.; Eller, T.; Must, A.; Koks, S.; Maron, E.; Tõru, I.; Shlik, J.; Vasar, V.; et al. Associations between LSAMP gene polymorphisms and major depressive disorder and panic disorder. Transl. Psychiatry 2012, 2, e152. [Google Scholar] [CrossRef]
- Innos, J.; Koido, K.; Philips, M.-A.; Vasar, E. Limbic system associated membrane protein as a potential target for neuropsychiatric disorders. Front. Pharmacol. 2013, 4, 32. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.-P.; Wang, Q.; Wu, Q.; Hu, H.-H.; Zhang, M.; Fang, Y.-Y.; Zhang, J.; Li, S.-J.; Xiong, W.-C.; et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef]
- Fang, K.; Li, H.-R.; Chen, X.-X.; Gao, X.-R.; Huang, L.-L.; Du, A.-Q.; Jiang, C.; Li, H.; Ge, J.-F. Quercetin Alleviates LPS-Induced Depression-Like Behavior in Rats via Regulating BDNF-Related Imbalance of Copine 6 and TREM1/2 in the Hippocampus and PFC. Front. Pharmacol. 2019, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Sathyanesan, M.; Girgenti, M.J.; Banasr, M.; Stone, K.; Bruce, C.; Guilchicek, E.; Wilczak-Havill, K.; Nairn, A.; Williams, K.; Sass, S.; et al. A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl. Psychiatry 2012, 2, e139. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-M.; Wang, J.Q. Linkage of Non-receptor Tyrosine Kinase Fyn to mGlu5 Receptors in Striatal Neurons in a Depression Model. Neuroscience 2020, 433, 11–20. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, R.; Peng, Y.; Aa, J.; Wang, G. AI Machine Learning Technique Characterizes Potential Markers of Depression in Two Animal Models of Depression. Brain Sci. 2023, 13, 763. https://doi.org/10.3390/brainsci13050763
Zhang J, Zhang R, Peng Y, Aa J, Wang G. AI Machine Learning Technique Characterizes Potential Markers of Depression in Two Animal Models of Depression. Brain Sciences. 2023; 13(5):763. https://doi.org/10.3390/brainsci13050763
Chicago/Turabian StyleZhang, Jing, Ran Zhang, Ying Peng, Jiye Aa, and Guangji Wang. 2023. "AI Machine Learning Technique Characterizes Potential Markers of Depression in Two Animal Models of Depression" Brain Sciences 13, no. 5: 763. https://doi.org/10.3390/brainsci13050763
APA StyleZhang, J., Zhang, R., Peng, Y., Aa, J., & Wang, G. (2023). AI Machine Learning Technique Characterizes Potential Markers of Depression in Two Animal Models of Depression. Brain Sciences, 13(5), 763. https://doi.org/10.3390/brainsci13050763








