Attachment Trauma Is Associated with White Matter Fiber Microstructural Alterations in Adolescents with Anorexia Nervosa before and after Exposure to Psychotherapeutic and Nutritional Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Eating Disorder Diagnosis and Symptomatology
2.2.2. Attachment Classification
- The secure attachment pattern, which is characterized by mutual enjoyment in relationships and thoughtful self-exploration.
- The insecure-dismissing pattern, which is characterized by individuals using primarily deactivating elements (i.e., authoritarian orientation, distance, normalization) to create a distance to attachment relationships.
- The insecure-preoccupied pattern, which is characterized by narratives with a lot of confusing material and negative emotions such as anger, guilt and shame.
- The unresolved/disorganized attachment or attachment trauma, which refers to narratives characterized by an inability to protect oneself or seek protection or care from attachment figures when confronted with traumatic attachment-related themes such as death, abuse, emptiness or isolation [3].
2.2.3. MRI Acquisition and Processing
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic and Clinical Values
3.2. Cross-Sectional Comparisons of FA
3.3. Longitudinal Comparisons of FA
3.4. Cross-Sectional and Longitudinal Analyses of MD
3.5. Cross-Sectional Comparisons of Patients with Attachment Trauma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smink, F.R.E.; van Hoeken, D.; Hoek, H.W. Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, H.; Tasca, G.A.; Grenon, R.; Ritchie, K.; Bissada, H.; Balfour, L. Change in attachment states of mind of women with binge-eating disorder. Clin. Psychol. Psychother. 2017, 24, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.; George, C. The disorganized attachment-caregiving system: Dysregulation of adaptive processes at multiple levels. In Disorganized Attachment and Caregiving; Solomon, J., George, C., Eds.; The Guilford Press: New York, NY, USA, 2011; pp. 3–24. [Google Scholar]
- Lenhart, L.; Gander, M.; Steiger, R.; Dabkowska-Mika, A.; Mangesius, S.; Haid-Stecher, N.; Fuchs, M.; Buchheim, A.; Sevecke, K.; Gizewski, E.R. Attachment status is associated with grey matter recovery in adolescent anorexia nervosa: Findings from a longitudinal study. Eur. J. Neurosci. 2022, 55, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- von Schwanenflug, N.; Müller, D.K.; King, J.A.; Ritschel, F.; Bernardoni, F.; Mohammadi, S.; Geisler, D.; Roessner, V.; Biemann, R.; Marxen, M.; et al. Dynamic changes in white matter microstructure in anorexia nervosa: Findings from a longitudinal study. Psychol. Med. 2019, 49, 1555–1564. [Google Scholar] [CrossRef]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef]
- Caminiti, R.; Carducci, F.; Piervincenzi, C.; Battaglia-Mayer, A.; Confalone, G.; Visco-Comandini, F.; Pantano, P.; Innocenti, G.M. Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: Comparing data from histology and magnetic resonance imaging diffusion tractography. J. Neurosci. 2013, 33, 14501–14511. [Google Scholar] [CrossRef]
- Vos, S.B.; Jones, D.K.; Jeurissen, B.; Viergever, M.A.; Leemans, A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage 2012, 59, 2208–2216. [Google Scholar] [CrossRef]
- Dehaene, S.; Changeux, J.P. Reward-dependent learning in neuronal networks for planning and decision making. Prog. Brain Res. 2000, 126, 217–229. [Google Scholar] [CrossRef]
- Martin Monzon, B.; Hay, P.; Foroughi, N.; Touyz, S. White matter alterations in anorexia nervosa: A systematic review of diffusion tensor imaging studies. World J. Psychiatry 2016, 6, 177–186. [Google Scholar] [CrossRef]
- King, J.A.; Frank, G.K.W.; Thompson, P.M.; Ehrlich, S. Structural Neuroimaging of Anorexia Nervosa: Future Directions in the Quest for Mechanisms Underlying Dynamic Alterations. Biol. Psychiatry 2018, 83, 224–234. [Google Scholar] [CrossRef]
- Kazlouski, D.; Rollin, M.D.; Tregellas, J.; Shott, M.E.; Jappe, L.M.; Hagman, J.O.; Pryor, T.; Yang, T.T.; Frank, G.K. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011, 192, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Barona, M.; Brown, M.; Clark, C.; Frangou, S.; White, T.; Micali, N. White matter alterations in anorexia nervosa: Evidence from a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2019, 100, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Frieling, H.; Fischer, J.; Wilhelm, J.; Engelhorn, T.; Bleich, S.; Hillemacher, T.; Dörfler, A.; Kornhuber, J.; de Zwaan, M.; Peschel, T. Microstructural abnormalities of the posterior thalamic radiation and the mediodorsal thalamic nuclei in females with anorexia nervosa--a voxel based diffusion tensor imaging (DTI) study. J. Psychiatr. Res. 2012, 46, 1237–1242. [Google Scholar] [CrossRef]
- Frank, G.K.; Shott, M.E.; Hagman, J.O.; Mittal, V.A. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am. J. Psychiatry 2013, 170, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.Y.; Bischoff-Grethe, A.; Theilmann, R.J.; Torres, L.; Wagner, A.; Kaye, W.H.; Fennema-Notestine, C. Alterations in white matter microstructure in women recovered from anorexia nervosa. Int. J. Eat. Disord. 2013, 46, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, Y.; Nakamae, T.; Nishizawa, S.; Mizuhara, Y.; Moritoki, Y.; Wada, Y.; Sakai, Y.; Yamashita, T.; Narumoto, J.; Miyata, J.; et al. A tract-based spatial statistics study in anorexia nervosa: Abnormality in the fornix and the cerebellum. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.J.; Lipsman, N.; Chen, D.Q.; Woodside, D.B.; Davis, K.D.; Lozano, A.M.; Hodaie, M. Subcallosal Cingulate Connectivity in Anorexia Nervosa Patients Differs From Healthy Controls: A Multi-tensor Tractography Study. Brain Stimul. 2015, 8, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Travis, K.E.; Golden, N.H.; Feldman, H.M.; Solomon, M.; Nguyen, J.; Mezer, A.; Yeatman, J.D.; Dougherty, R.F. Abnormal white matter properties in adolescent girls with anorexia nervosa. Neuroimage Clin. 2015, 9, 648–659. [Google Scholar] [CrossRef]
- Gaudio, S.; Quattrocchi, C.C.; Piervincenzi, C.; Zobel, B.B.; Montecchi, F.R.; Dakanalis, A.; Riva, G.; Carducci, F. White matter abnormalities in treatment-naive adolescents at the earliest stages of Anorexia Nervosa: A diffusion tensor imaging study. Psychiatry Res. Neuroimaging 2017, 266, 138–145. [Google Scholar] [CrossRef]
- Via, E.; Zalesky, A.; Sánchez, I.; Forcano, L.; Harrison, B.J.; Pujol, J.; Fernández-Aranda, F.; Menchón, J.M.; Soriano-Mas, C.; Cardoner, N.; et al. Disruption of brain white matter microstructure in women with anorexia nervosa. J. Psychiatry Neurosci. 2014, 39, 367–375. [Google Scholar] [CrossRef]
- Griffiths, K.R.; Martin Monzon, B.; Madden, S.; Kohn, M.R.; Touyz, S.; Sachdev, P.S.; Clarke, S.; Foroughi, N.; Hay, P. White matter microstructural differences in underweight adolescents with anorexia nervosa and a preliminary longitudinal investigation of change following short-term weight restoration. Eat. Weight Disord. 2021, 26, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Joos, A.; Tebartz van Elst, L.; Ebert, D.; Endres, D.; Domschke, K.; Lahmann, C.; Zeeck, A.; Runge, K.; Denzel, D.; et al. Reduced structural connectivity in the corpus callosum in patients with anorexia nervosa. Eur. Eat. Disord. Rev. 2022, 30, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Walter, M.; Mainz, V.; Herpertz-Dahlmann, B.; Konrad, K.; von Polier, G. Brain volume reduction predicts weight development in adolescent patients with anorexia nervosa. J. Psychiatr. Res. 2015, 68, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.; Herpertz-Dahlmann, B.; Konrad, K. Brain morphological changes in adolescent and adult patients with anorexia nervosa. J. Neural Transm. 2016, 123, 949–959. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Leunissen, C.; Kempermann, J.; Etdöger, S.; Kas, M.J.; Tolba, R.; Heussen, N.; Neulen, J.; et al. The reduction of astrocytes and brain volume loss in anorexia nervosa-the impact of starvation and refeeding in a rodent model. Transl. Psychiatry 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.M.; Yeatman, J.D.; Lee, E.S.; Barde, L.H.; Gaman-Bean, S. Diffusion tensor imaging: A review for pediatric researchers and clinicians. J. Dev. Behav. Pediatr. 2010, 31, 346–356. [Google Scholar] [CrossRef]
- Lampinen, B.; Szczepankiewicz, F.; Mårtensson, J.; van Westen, D.; Sundgren, P.C.; Nilsson, M. Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. Neuroimage 2017, 147, 517–531. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Sullivan, E.V. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology 2005, 30, 423–432. [Google Scholar] [CrossRef]
- Cha, J.; Ide, J.S.; Bowman, F.D.; Simpson, H.B.; Posner, J.; Steinglass, J.E. Abnormal reward circuitry in anorexia nervosa: A longitudinal, multimodal MRI study. Hum. Brain Mapp. 2016, 37, 3835–3846. [Google Scholar] [CrossRef]
- George, C.; West, M.L. The Adult Attachment Projective Picture System: Attachment Theory and Assessment in Adults; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Bizzi, F.; Cavanna, D.; Castellano, R.; Pace, C.S. Children’s mental representations with respect to caregivers and post-traumatic symptomatology in Somatic Symptom Disorders and Disruptive Behavior Disorders. Front. Psychol. 2015, 6, 1125. [Google Scholar] [CrossRef]
- Laible, D. Attachment with parents and peers in late adolescence: Links with emotional competence and social behavior. Personal. Individ. Differ. 2007, 43, 1185–1197. [Google Scholar] [CrossRef]
- Buchheim, A.; Diamond, D. Attachment and Borderline Personality Disorder. Psychiatr. Clin. N. Am. 2018, 41, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.; George, C. Attachment disorganization in borderline personality disorder and anxiety disorder. In Disorganized Attachment and Caregiving; Solomon, J., George, C., Eds.; Guilford Press: New York, NY, USA, 2011; pp. 343–382. [Google Scholar]
- George, C.; West, M. The development and preliminary validation of a new measure of adult attachment: The adult attachment projective. Attach. Hum. Dev. 2001, 3, 30–61. [Google Scholar] [CrossRef] [PubMed]
- George, C.; West, M. The Adult Attachment Projective Picture System: Integrating Attachment Into Clinical Assessment. J. Personal. Assess. 2011, 93, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Levy, K.N.; Kivity, Y.; Johnson, B.N.; Gooch, C.V. Adult attachment as a predictor and moderator of psychotherapy outcome: A meta-analysis. J. Clin. Psychol. 2018, 74, 1996–2013. [Google Scholar] [CrossRef]
- Burns, E.E.; Fischer, S.; Jackson, J.L.; Harding, H.G. Deficits in emotion regulation mediate the relationship between childhood abuse and later eating disorder symptoms. Child. Abus. Negl. 2012, 36, 32–39. [Google Scholar] [CrossRef]
- Ringer, F.; Crittenden, P.M. Eating disorders and attachment: The effects of hidden family processes on eating disorders. Eur Eat. Disord. Rev. 2007, 15, 119–130. [Google Scholar] [CrossRef]
- Gander, M.; Sevecke, K.; Buchheim, A. Disorder-specific attachment characteristics and experiences of childhood abuse and neglect in adolescents with anorexia nervosa and a major depressive episode. Clin. Psychol. Psychother. 2018, 25, 894–906. [Google Scholar] [CrossRef]
- Latzer, Y.; Hochdorf, Z. Dying to be thin: Attachment to death in anorexia nervosa. Sci. World J. 2005, 5, 820–827. [Google Scholar] [CrossRef]
- Unterrainer, H.F.; Hiebler-Ragger, M.; Koschutnig, K.; Fuchshuber, J.; Tscheschner, S.; Url, M.; Wagner-Skacel, J.; Reininghaus, E.Z.; Papousek, I.; Weiss, E.M.; et al. Addiction as an Attachment Disorder: White Matter Impairment Is Linked to Increased Negative Affective States in Poly-Drug Use. Front. Hum. Neurosci. 2017, 11, 208. [Google Scholar] [CrossRef]
- Serra, M.; De Pisapia, N.; Rigo, P.; Papinutto, N.; Jager, J.; Bornstein, M.H.; Venuti, P. Secure attachment status is associated with white matter integrity in healthy young adults. Neuroreport 2015, 26, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Riem, M.M.E.; van Hoof, M.J.; Garrett, A.S.; Rombouts, S.A.R.B.; van der Wee, N.J.A.; van IJzendoorn, M.H.; Vermeiren, R.R.J.M. General psychopathology factor and unresolved-disorganized attachment uniquely correlated to white matter integrity using diffusion tensor imaging. Behav. Brain Res. 2019, 359, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benetti, S.; McCrory, E.; Arulanantham, S.; De Sanctis, T.; McGuire, P.; Mechelli, A. Attachment style, affective loss and gray matter volume: A voxel-based morphometry study. Hum. Brain Mapp. 2010, 31, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Baldaçara, L.; Borgio, J.G.; Lacerda, A.L.; Jackowski, A.P. Cerebellum and psychiatric disorders. Braz. J. Psychiatry 2008, 30, 281–289. [Google Scholar] [CrossRef]
- Wittchen, H.-U.; Zaudig, M.; Fydrich, T. Strukturiertes klinisches Interview für DSM-IV (SKID); Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Petermann, F.; Petermann, U. Hamburg-Wechsler-Intelligenztest für Kinder IV (HAWIK-IV); Huber: Bern, Germany, 2008. [Google Scholar]
- Engelhardt, C.; Föcker, M.; Bühren, K.; Dahmen, B.; Becker, K.; Weber, L.; Correll, C.U.; Egberts, K.M.; Ehrlich, S.; Roessner, V.; et al. Age dependency of body mass index distribution in childhood and adolescent inpatients with anorexia nervosa with a focus on DSM-5 and ICD-11 weight criteria and severity specifiers. Eur. Child. Adolesc. Psychiatry 2021, 30, 1081–1094. [Google Scholar] [CrossRef]
- Zanarini, M.C.; Skodol, A.E.; Bender, D.; Dolan, R.; Sanislow, C.; Schaefer, E.; Morey, L.C.; Grilo, C.M.; Shea, M.T.; McGlashan, T.H.; et al. The Collaborative Longitudinal Personality Disorders Study: Reliability of axis I and II diagnoses. J. Pers. Disord. 2000, 14, 291–299. [Google Scholar] [CrossRef]
- Kappel, V.; Thiel, A.; Holzhausen, M.; Jaite, C.; Schneider, N.; Pfeiffer, E.; Lehmkuhl, U.; Salbach-Andrae, H. Eating Disorder Inventory-2 (EDI-2) Normierung an einer stichprobe normalgewichtiger schüler im alter von 10 bis 20 jahren und an patientinnen mit anorexia nervosa = Eating Disorder Inventory (EDI-2): Normative data among 10 to 20 year old German girls and boys. Diagnostica 2012, 58, 127–144. [Google Scholar] [CrossRef]
- Gander, M.; Buchheim, A.; Bock, A.; Steppan, M.; Sevecke, K.; Goth, K. Unresolved Attachment Mediates the Relationship Between Childhood Trauma and Impaired Personality Functioning in Adolescence. J. Pers Disord. 2020, 34, 84–103. [Google Scholar] [CrossRef]
- Gander, M.; Fuchs, M.; Franz, N.; Jahnke-Majorkovits, A.C.; Buchheim, A.; Bock, A.; Sevecke, K. Non-suicidal self-injury and attachment trauma in adolescent inpatients with psychiatric disorders. Compr. Psychiatry 2021, 111, 152273. [Google Scholar] [CrossRef]
- Friston, K.J.; Ashburner, C.D.; Frith, C.D.; Poline, J.-B.; Heather, J.D.; Frackowiak, R.S.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Kaufmann, L.K.; Baur, V.; Hänggi, J.; Jäncke, L.; Piccirelli, M.; Kollias, S.; Schnyder, U.; Pasternak, O.; Martin-Soelch, C.; Milos, G. Fornix Under Water? Ventricular Enlargement Biases Forniceal Diffusion Magnetic Resonance Imaging Indices in Anorexia Nervosa. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.E.; Kaplan, A.S.; French, L.; Voineskos, A.N. White matter microstructure in women with acute and remitted anorexia nervosa: An exploratory neuroimaging study. Brain Imaging Behav. 2020, 14, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Geisler, D.; King, J.A.; Bahnsen, K.; Bernardoni, F.; Doose, A.; Müller, D.K.; Marxen, M.; Roessner, V.; van den Heuvel, M.; Ehrlich, S. Altered White Matter Connectivity in Young Acutely Underweight Patients With Anorexia Nervosa. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Phillipou, A.; Carruthers, S.P.; Di Biase, M.A.; Zalesky, A.; Abel, L.A.; Castle, D.J.; Gurvich, C.; Rossell, S.L. White matter microstructure in anorexia nervosa. Hum. Brain Mapp. 2018, 39, 4385–4392. [Google Scholar] [CrossRef]
- Zhang, A.; Leow, A.; Zhan, L.; GadElkarim, J.; Moody, T.; Khalsa, S.; Strober, M.; Feusner, J.D. Brain connectome modularity in weight-restored anorexia nervosa and body dysmorphic disorder. Psychol. Med. 2016, 46, 2785–2797. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.J.; Lee, S.K.; Seok, J.H.; Chun, J.; Kim, D.I.; Lee, J.D. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapp. 2008, 29, 503–516. [Google Scholar] [CrossRef]
- Fitsiori, A.; Nguyen, D.; Karentzos, A.; Delavelle, J.; Vargas, M.I. The corpus callosum: White matter or terra incognita. Br. J. Radiol. 2011, 84, 5–18. [Google Scholar] [CrossRef]
- Mettler, L.N.; Shott, M.E.; Pryor, T.; Yang, T.T.; Frank, G.K. White matter integrity is reduced in bulimia nervosa. Int. J. Eat. Disord. 2013, 46, 264–273. [Google Scholar] [CrossRef]
- Burke, T.; Gleeson, C.; Holleran, L.; Mothersill, D.; Holland, J.; Costello, L.; Kane, R.; McKernan, D.P.; Morris, D.W.; Kelly, J.P.; et al. Corpus Callosum Microstructural Tract Integrity Relates to Longer Emotion Recognition Reaction Time in People with Schizophrenia. Brain Sci. 2022, 12, 1208. [Google Scholar] [CrossRef]
- Nowakowski, M.E.; McFarlane, T.; Cassin, S. Alexithymia and eating disorders: A critical review of the literature. J. Eat. Disord. 2013, 1, 21. [Google Scholar] [CrossRef]
- Paul, L.K.; Pazienza, S.R.; Brown, W.S. Alexithymia and somatization in agenesis of the corpus callosum. Soc. Cogn. Affect. Neurosci. 2021, 16, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Shott, M.E.; Pryor, T.L.; Yang, T.T.; Frank, G.K. Greater Insula White Matter Fiber Connectivity in Women Recovered from Anorexia Nervosa. Neuropsychopharmacology 2016, 41, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Rinne-Albers, M.A.; van der Werff, S.J.; van Hoof, M.J.; van Lang, N.D.; Lamers-Winkelman, F.; Rombouts, S.A.; Vermeiren, R.R.; van der Wee, N.J. Abnormalities of white matter integrity in the corpus callosum of adolescents with PTSD after childhood sexual abuse: A DTI study. Eur. Child. Adolesc. Psychiatry 2016, 25, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, P.; Mancini, C.; Terlizzi, S.; Sales, C.; Francesconi, M.F.; Todisco, P. Urinary free cortisol and childhood maltreatments in eating disorder patients: New evidence for an ecophenotype subgroup. Eur. Eat. Disord. Rev. 2022, 30, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, P.; Collantoni, E.; Solmi, M.; Tenconi, E.; Favaro, A. Anorexia nervosa and diffusion weighted imaging: An open methodological question raised by a systematic review and a fractional anisotropy anatomical likelihood estimation meta-analysis. Int. J. Eat. Disord. 2019, 52, 1237–1250. [Google Scholar] [CrossRef]
- Kochunov, P.; Glahn, D.C.; Lancaster, J.; Thompson, P.M.; Kochunov, V.; Rogers, B.; Fox, P.; Blangero, J.; Williamson, D.E. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage 2011, 58, 41–49. [Google Scholar] [CrossRef]
- Gander, M.; Diamond, D.; Buchheim, A.; Sevecke, K. Use of the Adult Attachment Projective Picture System in the formulation of a case of an adolescent refugee with PTSD. J. Trauma Dissociation 2018, 19, 572–595. [Google Scholar] [CrossRef]
- Kobak, R.R.; Kerig, P.K. Introduction to the special issue: Attachment-based treatments for adolescents. Attach. Hum. Dev. 2015, 17, 111–118. [Google Scholar] [CrossRef]

| Criteria | Description |
|---|---|
| Population | Adolescent inpatients between 14 and 18 years, with diagnosed anorexia nervosa (restrictive type) and attachment trauma |
| Clinical exposure | Standardized psychotherapeutic and nutritional treatment in patients with anorexia nervosa |
| Environmental exposure | Attachment trauma |
| Comparison | Adolescents between 14 and 18 years with no present or history of anorexia nervosa and no attachment trauma; adolescent inpatients between 14 and 18 years with diagnosed anorexia nervosa (restrictive type) and no attachment trauma |
| Outcome | White matter fiber microstructural alterations in anorexia nervosa assessed by diffusion tensor imaging |
| acAN Cohort | HC Cohort | |||
|---|---|---|---|---|
| Whole Cohort | Resolved Attachment | Unresolved Attachment | ||
| Sample size | 22 | 10 | 12 | 18 |
| Age (years) | 15.8 (1.2) * | 16 (1.2) * | 15.6 (1.3) * | 17.7 (0.7) * |
| BMI Tp1 (kg/m2) | 15.4 (1.4) * | 15.5 (0.9) * | 15.5 (1.4) * | 21.2 (1) *° |
| BMI Tp2 (kg/m2) | 17.8 (1) ° | 17.8 (1.2) ° | 17.7 (0.7) ° | - |
| Duration of illness (months) | 9.4 (6.8) | 9.6 (4.8) | 9.3 (8.3) | - |
| EDI-2 (total score) | 298.6 (63) * | 316 (44) * | 293.4 (69) * | 217.7 (58.4) * |
| Months between 1st and 2nd MRI scan | 2.6 (0.9) | 2.9 (1) | 2.4 (0.8) | - |
| TIV (mm3) | 1336.5 (114.1) | 1366.6 (122.3) | 1309.9 (116.8) | 1369.8 (93.9) |
| AN Group | HC Group | χ2 | Φ | p | |
|---|---|---|---|---|---|
| n = 22 (%) | n = 18 (%) | ||||
| Number of siblings | |||||
| Single child | 4 (18.2) | 2 (11.8) | 4.296 | 0.33 | 0.231 |
| One sibling | 9 (40.9) | 6 (35.3) | |||
| Two siblings | 3 (13.6) | 7 (41.2) | |||
| More than two siblings | 6 (27.3) | 2 (11.8) | |||
| Marital status of parents | |||||
| Married/partnership | 9 (40.9) | 11 (61.1) | 1.616 | −2.01 | 0.204 |
| Single/divorced | 13 (59.1) | 7 (38.9) | |||
| Occupation | |||||
| Attending school | 21 (95.5) | 17 (94.4) | 0.021 | 0.02 | 0.884 |
| Employed/trainee | 1 (4.5) | 1 (5.6) | |||
| Attachment classifications | |||||
| Resolved/organized | 10 (45.5) | 14 (77.8) | 4.310 | −0.33 | 0.038 |
| Unresolved/disorganized | 12 (54.5) | 4 (22.2) |
| Significant regional differences in FA values in the 21 AN patients compared to the 18 HC participants | |||||||
| Overlap of cluster region | kE | MNI coordinates | t value | p-value corrected at the cluster level (FWE) | Height Threshold | ||
| x | y | z | |||||
| Significant FA decreases in the 21 acute AN patients compared to the 18 HC participants at Tp1 | |||||||
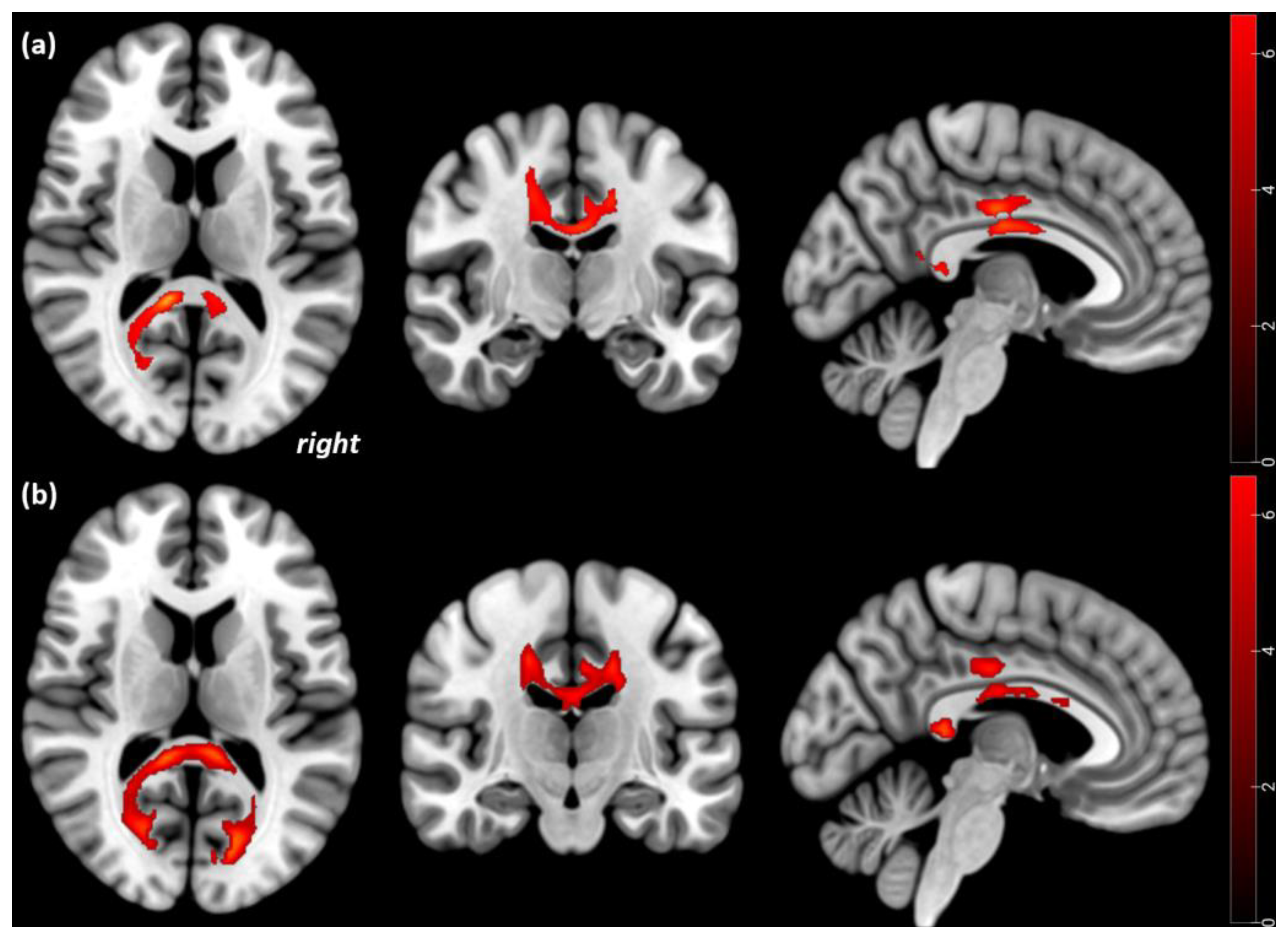
| Corpus callosum and cingulum bilaterally spreading to the fornix, and the corticospinal tract | 1485 | 18 −20 14 | −20 −36 0.34 | 26 4 9 | 6.4 | <0.001 | 0.001 |
| Inferior occipitofrontal and longitudinal fasciculus left | 156 | −32 | −42 | −4 | 5.6 | 0.029 | |
| Significant FA increases in the 17 AN patients from Tp1 to Tp2 | |||||||
| Corpus callosum and cingulum bilaterally spreading to the fornix | 885 | 2 −4 4 | −12 −26 2 | 14 22 −1 | 6.7 | <0.001 | 0.001 |
| Inferior occipitofrontal and longitudinal fasciculus left | 165 | −34 | −42 | −4 | 4.5 | 0.008 | |
| Significant FA decreases in the 17 AN patients from Tp1 to Tp2 | |||||||
| Corticospinal tract, right | 125 | 26 | −24 | 30 | 8.1 | <0.001 | 0.001 |
| Significant regional differences in FA values in the 11 AN patients with attachment trauma compared to the 18 HC participants when corrected for BMI | |||||||
| Overlap of cluster region | kE | MNI coordinates | t value | p-value corrected at the cluster level (FWE) | Height Threshold | ||
| x | y | z | |||||
| Significant FA decreases in the 11 acute AN patients with attachment trauma compared to the 18 HC participants at Tp1 | |||||||
| Corpus callosum and cingulum bilaterally | 3035 | −10 14 16 | −40 −6 −18 | 12 40 40 | 5.2 | <0.001 | 0.01 |
| Significant FA decreases in the 11 AN patients with attachment trauma compared to the 18 HC participants at Tp2 | |||||||
| Corpus callosum and cingulum, left | 1393 | −12 | −40 | 12 | 4.4 | 0.005 | 0.01 |
| Corpus callosum and cingulum, right | 1312 | 14 | 12 | 38 | 3.8 | 0.007 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gander, M.; Lenhart, L.; Steiger, R.; Buchheim, A.; Mangesius, S.; Birkl, C.; Haid-Stecher, N.; Fuchs, M.; Libal, A.; Dabkowska-Mika, A.; et al. Attachment Trauma Is Associated with White Matter Fiber Microstructural Alterations in Adolescents with Anorexia Nervosa before and after Exposure to Psychotherapeutic and Nutritional Treatment. Brain Sci. 2023, 13, 798. https://doi.org/10.3390/brainsci13050798
Gander M, Lenhart L, Steiger R, Buchheim A, Mangesius S, Birkl C, Haid-Stecher N, Fuchs M, Libal A, Dabkowska-Mika A, et al. Attachment Trauma Is Associated with White Matter Fiber Microstructural Alterations in Adolescents with Anorexia Nervosa before and after Exposure to Psychotherapeutic and Nutritional Treatment. Brain Sciences. 2023; 13(5):798. https://doi.org/10.3390/brainsci13050798
Chicago/Turabian StyleGander, Manuela, Lukas Lenhart, Ruth Steiger, Anna Buchheim, Stephanie Mangesius, Christoph Birkl, Nina Haid-Stecher, Martin Fuchs, Anna Libal, Agnieszka Dabkowska-Mika, and et al. 2023. "Attachment Trauma Is Associated with White Matter Fiber Microstructural Alterations in Adolescents with Anorexia Nervosa before and after Exposure to Psychotherapeutic and Nutritional Treatment" Brain Sciences 13, no. 5: 798. https://doi.org/10.3390/brainsci13050798
APA StyleGander, M., Lenhart, L., Steiger, R., Buchheim, A., Mangesius, S., Birkl, C., Haid-Stecher, N., Fuchs, M., Libal, A., Dabkowska-Mika, A., Gizewski, E. R., & Sevecke, K. (2023). Attachment Trauma Is Associated with White Matter Fiber Microstructural Alterations in Adolescents with Anorexia Nervosa before and after Exposure to Psychotherapeutic and Nutritional Treatment. Brain Sciences, 13(5), 798. https://doi.org/10.3390/brainsci13050798








