Circulating Serum VEGF, IGF-1 and MMP-9 and Expression of Their Genes as Potential Prognostic Markers of Recovery in Post-Stroke Rehabilitation—A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Presentation
2.2. Clinical Parameter Determination
2.2.1. Cognitive Assessment
2.2.2. Depressive Symptom Assessment
2.2.3. Physical and Motor Condition Assessment
2.2.4. Blood Sample Collection
2.3. Determination of IGF1, VEGF and MMP-9 Level in Plasma
2.4. Determination of MMP-9, VEGF-A and IGF1 Expression in Whole Blood Samples
2.5. Data Analysis
3. Results
4. Discussion
5. Limitations and Future Perspective
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Action Plan for the Prevention and Control of NCDs 2013–2020. Available online: https://www.who.int/publications/i/item/9789241506236 (accessed on 1 January 2020).
- Global, regional, and national burden of stroke, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [CrossRef] [PubMed]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.M.; Cramer, S.C. Spontaneous and Therapeutic-Induced Mechanisms of Functional Recovery After Stroke. Transl. Stroke Res. 2017, 8, 33–46. [Google Scholar] [CrossRef]
- Wlodarczyk, L.; Szelenberger, R.; Cichon, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. Biomarkers of Angiogenesis and Neuroplasticity as Promising Clinical Tools for Stroke Recovery Evaluation. Int. J. Mol. Sci. 2021, 22, 3949. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Cai, H.; Ma, Y.; Jiang, L.; Mu, Z.; Jiang, Z.; Chen, X.; Wang, Y.; Yang, G.-Y.; Zhang, Z. Hypoxia Response Element-Regulated MMP-9 Promotes Neurological Recovery via Glial Scar Degradation and Angiogenesis in Delayed Stroke. Mol. Ther. 2017, 25, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Liu, H.J.; Liu, X.F. VEGF promotes angiogenesis and functional recovery in stroke rats. J. Investig. Surg. 2010, 23, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Zhu, Y.; Li, Y.; Liu, P.; Gao, B.; Tian, S.; Du, L.; Bai, Y. Post-stroke Constraint-induced Movement Therapy Increases Functional Recovery, Angiogenesis, and Neurogenesis with Enhanced Expression of HIF-1α and VEGF. Curr. Neurovasc. Res. 2017, 14, 368–377. [Google Scholar] [CrossRef]
- Zhu, W.; Fan, Y.; Frenzel, T.; Gasmi, M.; Bartus, R.T.; Young, W.L.; Yang, G.Y.; Chen, Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke 2008, 39, 1254–1261. [Google Scholar] [CrossRef]
- Okoreeh, A.K.; Bake, S.; Sohrabji, F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia 2017, 65, 1043–1058. [Google Scholar] [CrossRef]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Abdelnaseer, M.M.; Elfauomy, N.M.; Esmail, E.H.; Kamal, M.M.; Elsawy, E.H. Matrix Metalloproteinase-9 and Recovery of Acute Ischemic Stroke. J. Stroke Cereb. Dis. 2017, 26, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Yang, J.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Ju, Z.; et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology 2017, 89, 805–812. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Luo, S.; Huang, H.; Huang, X.; Li, M.; Huang, Y.; Chen, Y.; Wu, Z. Predictive value of longitudinal changes of serum matrix metalloproteinase-9 and brain-derived neurotrophic factor in acute ischemic stroke. Front. Aging Neurosci. 2022, 14, 952038. [Google Scholar] [CrossRef]
- Kowalski, R.G.; Ledreux, A.; Violette, J.E.; Neumann, R.T.; Ornelas, D.; Yu, X.; Griffiths, S.G.; Lewis, S.; Nash, P.; Monte, A.A.; et al. Rapid Activation of Neuroinflammation in Stroke: Plasma and Extracellular Vesicles Obtained on a Mobile Stroke Unit. Stroke 2023, 54, e52–e57. [Google Scholar] [CrossRef]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Crola Da Silva, C.; Buisson, M.; Amaz, C.; Bouin, M.; Derex, L.; Ong, E.; Berthezene, Y.; et al. Matrix Metalloproteinase-9 Relationship With Infarct Growth and Hemorrhagic Transformation in the Era of Thrombectomy. Front. Neurol. 2020, 11, 473. [Google Scholar] [CrossRef]
- Pu, M.; You, Y.; Wang, X. Predictive value of serum matrix metalloproteinase 9 combined with tissue inhibitor of metalloproteinase 1 for post-stroke cognitive impairment. J. Clin. Neurosci. 2022, 105, 103–108. [Google Scholar] [CrossRef]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, N.; Bijak, M.; Czarny, P.; Miller, E.; Synowiec, E.; Sliwinski, T.; Saluk-Bijak, J. Increase in Blood Levels of Growth Factors Involved in the Neuroplasticity Process by Using an Extremely Low Frequency Electromagnetic Field in Post-stroke Patients. Front. Aging Neurosci. 2018, 10, 294. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Pang, Q.; Zhang, H.; Chen, Z.; Wu, Y.; Bai, M.; Liu, Y.; Zhao, Y.; Tu, F.; Liu, C.; Chen, X. Role of caveolin-1/vascular endothelial growth factor pathway in basic fibroblast growth factor-induced angiogenesis and neurogenesis after treadmill training following focal cerebral ischemia in rats. Brain Res. 2017, 1663, 9–19. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Carl, D.; Khoury, J.C.; Gerson, M.; Kissela, B.; et al. Effects of Exercise Intensity on Acute Circulating Molecular Responses Poststroke. Neurorehabil. Neural Repair 2020, 34, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, R.; Ago, T.; Kamouchi, M.; Kuroda, J.; Kuwashiro, T.; Hata, J.; Sugimori, H.; Fukuda, K.; Gotoh, S.; Makihara, N.; et al. Clinical significance of plasma VEGF value in ischemic stroke—Research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Mattlage, A.E.; Rippee, M.A.; Sandt, J.; Billinger, S.A. Decrease in Insulin-Like Growth Factor-1 and Insulin-Like Growth Factor-1 Ratio in the First Week of Stroke Is Related to Positive Outcomes. J. Stroke Cereb. Dis. 2016, 25, 1800–1806. [Google Scholar] [CrossRef]
- Åberg, N.D.; Åberg, D.; Lagging, C.; Holmegaard, L.; Redfors, P.; Jood, K.; Nilsson, M.; Åberg, M.; Blomstrand, C.; Svensson, J.; et al. Association Between Levels of Serum Insulin-like Growth Factor I and Functional Recovery, Mortality, and Recurrent Stroke at a 7-year Follow-up. Exp. Clin. Endocrinol. Diabetes 2020, 128, 303–310. [Google Scholar] [CrossRef]
- Åberg, N.D.; Åberg, D.; Jood, K.; Nilsson, M.; Blomstrand, C.; Kuhn, H.G.; Svensson, J.; Jern, C.; Isgaard, J. Altered levels of circulating insulin-like growth factor I (IGF-I) following ischemic stroke are associated with outcome—A prospective observational study. BMC Neurol. 2018, 18, 106. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bour, A.; Rasquin, S.; Boreas, A.; Limburg, M.; Verhey, F. How predictive is the MMSE for cognitive performance after stroke? J. Neurol. 2010, 257, 630–637. [Google Scholar] [CrossRef]
- Chiti, G.; Pantoni, L. Use of Montreal Cognitive Assessment in patients with stroke. Stroke 2014, 45, 3135–3140. [Google Scholar] [CrossRef] [PubMed]
- Abzhandadze, T.; Rafsten, L.; Lundgren Nilsson, Å.; Palstam, A.; Sunnerhagen, K.S. Very Early MoCA Can Predict Functional Dependence at 3 Months After Stroke: A Longitudinal, Cohort Study. Front. Neurol. 2019, 10, 1051. [Google Scholar] [CrossRef]
- Salvadori, E.; Cova, I.; Mele, F.; Pomati, S.; Pantoni, L. Prediction of post-stroke cognitive impairment by Montreal Cognitive Assessment (MoCA) performances in acute stroke: Comparison of three normative datasets. Aging Clin. Exp. Res. 2022, 34, 1855–1863. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Marc, L.G.; Raue, P.J.; Bruce, M.L. Screening performance of the 15-item geriatric depression scale in a diverse elderly home care population. Am. J. Geriatr. Psychiatry 2008, 16, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Steer, R.A.; Rissmiller, D.J.; Beck, A.T. Use of the Beck Depression Inventory-II with depressed geriatric inpatients. Behav. Res Ther. 2000, 38, 311–318. [Google Scholar] [CrossRef]
- Beck, A.T. Beck Depression Inventory–II. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft00742-000 (accessed on 1 January 2022).
- Scopus Preview. Available online: https://www.scopus.com/home.uri (accessed on 1 January 2022).
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.T.; Collin, C. The Barthel ADL Index: A standard measure of physical disability? Int. Disabil. Stud. 1988, 10, 64–67. [Google Scholar] [CrossRef]
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201–211. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of Stroke Recovery: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil. Neural Repair 2017, 31, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Naylor, S. Biomarkers: Current perspectives and future prospects. Expert Rev. Mol. Diagn. 2003, 3, 525–529. [Google Scholar] [CrossRef]
- Simpkins, A.N.; Janowski, M.; Oz, H.S.; Roberts, J.; Bix, G.; Doré, S.; Stowe, A.M. Biomarker Application for Precision Medicine in Stroke. Transl. Stroke Res. 2020, 11, 615–627. [Google Scholar] [CrossRef]
- Ferrer-Ferrer, M.; Dityatev, A. Shaping Synapses by the Neural Extracellular Matrix. Front. Neuroanat. 2018, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Stawarski, M.; Stefaniuk, M.; Wlodarczyk, J. Matrix metalloproteinase-9 involvement in the structural plasticity of dendritic spines. Front. Neuroanat. 2014, 8, 68. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix Metalloproteinase-9 Is Required for Hippocampal Late-Phase Long-Term Potentiation and Memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011, 124, 3369–3380. [Google Scholar] [CrossRef]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Nakade, J.; Tachibana, M.; Ibi, D.; Someya, E.; Koike, H.; Kamei, H.; Nabeshima, T.; Itohara, S.; Takuma, K.; et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J. Neurosci. 2011, 31, 12963–12971. [Google Scholar] [CrossRef]
- Cojocarui, I.M.; Cojocaru, M.; Sapira, V.; Socoliuc, G.; Hertea, C.; Paveliu, S. Changes in plasma matrix metalloproteinase-9 levels in patients with acute ischemic stroke. Rom. J. Intern. Med. 2012, 50, 155–158. [Google Scholar] [PubMed]
- Brouns, R.; Wauters, A.; De Surgeloose, D.; Mariën, P.; De Deyn, P.P. Biochemical Markers for Blood-Brain Barrier Dysfunction in Acute Ischemic Stroke Correlate with Evolution and Outcome. Eur. Neurol. 2011, 65, 23–31. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, Z.; Zhong, C.; Wang, A.; Xie, X.; Xu, T.; Peng, Y.; Peng, H.; Li, Q.; Ju, Z.; et al. Prognostic Metrics Associated with Inflammation and Atherosclerosis Signaling Evaluate the Burden of Adverse Clinical Outcomes in Ischemic Stroke Patients. Clin. Chem. 2020, 66, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Bu, X.; Xu, T.; Guo, L.; Wang, X.; Zhang, J.; Cui, Y.; Li, D.; Zhang, J.; Ju, Z.; et al. Serum Matrix Metalloproteinase-9 and Cognitive Impairment After Acute Ischemic Stroke. J. Am. Heart Assoc. 2018, 7, e007776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Meng, L.; Wang, F.; Li, Q.; Yang, F.; Wang, M.; Yu, M.; Zhang, J.; Li, S.; et al. Relationship between MMP-9 serum levels and tHcy levels and total imaging load and cognitive dysfunction. J. Stroke Cerebrovasc. Dis. 2022, 31, 106759. [Google Scholar] [CrossRef]
- Castellanos, M.; Sobrino, T.; Millán, M.; García, M.; Arenillas, J.; Nombela, F.; Brea, D.; Perez de la Ossa, N.; Serena, J.; Vivancos, J.; et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: A multicenter confirmatory study. Stroke 2007, 38, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.; Tyson, S.F. Screening for cognitive impairment after stroke: A systematic review of psychometric properties and clinical utility. J. Rehabil. Med. 2015, 47, 193–203. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, Y.T.; Chiu, E.C. A comparison of test-retest reliability of four cognitive screening tools in people with dementia. Disabil. Rehabil. 2022, 44, 4090–4095. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Harvey, P.D.; Wesnes, K.A.; Snyder, P.J.; Schneider, L.S. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement. 2015, 1, 103–111. [Google Scholar] [CrossRef]
- Galasko, D.; Abramson, I.; Corey-Bloom, J.; Thal, L.J. Repeated exposure to the Mini-Mental State Examination and the Information-Memory-Concentration Test results in a practice effect in Alzheimer’s disease. Neurology 1993, 43, 1559–1563. [Google Scholar] [CrossRef]
- Feeney, J.; Savva, G.M.; O’Regan, C.; King-Kallimanis, B.; Cronin, H.; Kenny, R.A. Measurement Error, Reliability, and Minimum Detectable Change in the Mini-Mental State Examination, Montreal Cognitive Assessment, and Color Trails Test among Community Living Middle-Aged and Older Adults. J. Alzheimers Dis. 2016, 53, 1107–1114. [Google Scholar] [CrossRef]
- Che, B.; Zhong, C.; Ge, J.; Li, R.; Zhu, Z.; Bu, X.; Xu, T.; Ju, Z.; Liu, J.; Zhang, J.; et al. Serum Matrix Metalloproteinase-9 Is Associated With Depression After Acute Ischemic Stroke. Circ. J. 2019, 83, 2303–2311. [Google Scholar] [CrossRef]
- Saban, M.R.; Davis, C.A.; Avelino, A.; Cruz, F.; Maier, J.; Bjorling, D.E.; Sferra, T.J.; Hurst, R.E.; Saban, R. VEGF signaling mediates bladder neuroplasticity and inflammation in response to BCG. BMC Physiol. 2011, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zheng, X.R.; Zhang, S.S.; Wang, X.; Yu, X.H.; Tan, J.L.; Yang, Y.J. Transplantation of vascular endothelial growth factor-modified neural stem/progenitor cells promotes the recovery of neurological function following hypoxic-ischemic brain damage. Neural Regen. Res. 2016, 11, 1456–1463. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Chodobski, A.; Chung, I.; Koźniewska, E.; Ivanenko, T.; Chang, W.; Harrington, J.F.; Duncan, J.A.; Szmydynger-Chodobska, J. Early neutrophilic expression of vascular endothelial growth factor after traumatic brain injury. Neuroscience 2003, 122, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-W.; Duan, C.-L.; Chen, X.-H.; Wang, Y.-Q.; Sun, X.; Zhang, Q.-W.; Cui, H.-R.; Sun, F.-Y. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology 2016, 108, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Chen, H.; Zhang, T.; Chen, J.; Geng, Z.; Zhao, Y. Changes in serum vascular endothelial growth factor and endostatin concentrations associated with circulating endothelial progenitor cells after acute ischemic stroke. Metab. Brain Dis. 2017, 32, 641–648. [Google Scholar] [CrossRef]
- Nunes, F.D.D.; Ferezin, L.P.; Pereira, S.C.; Figaro-Drumond, F.V.; Pinheiro, L.C.; Menezes, I.C.; Baes, C.V.W.; Coeli-Lacchini, F.B.; Tanus-Santos, J.E.; Juruena, M.F.; et al. The Association of Biochemical and Genetic Biomarkers in VEGF Pathway with Depression. Pharmaceutics 2022, 14, 2757. [Google Scholar] [CrossRef]
- Bhasin, A.; Srivastava, M.V.P.; Vivekanandhan, S.; Moganty, R.; Talwar, T.; Sharma, S.; Kuthiala, N.; Kumaran, S.; Bhatia, R. Vascular Endothelial Growth Factor as Predictive Biomarker for Stroke Severity and Outcome; An Evaluation of a New Clinical Module in Acute Ischemic Stroke. Neurol. India 2019, 67, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Acurio, J.; López, E.; Rodríguez, A.; Benavente, A.; Lara, E.; Korzeniewski, S.J. Vascular endothelial growth factor and poor prognosis after ischaemic stroke. Eur. J. Neurol. 2021, 28, 1759–1764. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, T.; Hu, H.; Wang, J.; Zhou, S. Serum vascular endothelial growth factor as a biomarker for prognosis of minor ischemic stroke. Clin. Neurol. Neurosurg. 2020, 196, 106060. [Google Scholar] [CrossRef]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The role of Insulin-Like Growth Factor 1 (IGF-1) in brain development, maturation and neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ashpole, N.M.; Sanders, J.E.; Hodges, E.L.; Yan, H.; Sonntag, W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015, 68, 76–81. [Google Scholar] [CrossRef]
- Saber, H.; Himali, J.J.; Beiser, A.S.; Shoamanesh, A.; Pikula, A.; Roubenoff, R.; Romero, J.R.; Kase, C.S.; Vasan, R.S.; Seshadri, S. Serum Insulin-Like Growth Factor 1 and the Risk of Ischemic Stroke: The Framingham Study. Stroke 2017, 48, 1760–1765. [Google Scholar] [CrossRef]
- Hunt, K.J.; Lukanova, A.; Rinaldi, S.; Lundin, E.; Norat, T.; Palmqvist, R.; Stattin, P.; Riboli, E.; Hallmans, G.; Kaaks, R. A potential inverse association between insulin-like growth factor I and hypertension in a cross-sectional study. Ann. Epidemiol. 2006, 16, 563–571. [Google Scholar] [CrossRef]
- Teppala, S.; Shankar, A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care 2010, 33, 2257–2259. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhao, J.; Mou, L. Expression and clinical value of miR-128 and IGF-1 in patients with acute ischemic stroke. Minerva Med. 2020, 111, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, M.; Ipsen, N.; Leonards, C.O.; Empl, L.; Hanne, L.; Liman, T.; Mai, K.; Strasburger, C.J.; Spranger, J.; Endres, M. Circulating insulin-like growth factor binding protein-3 predicts one-year outcome after ischemic stroke. Exp. Clin. Endocrinol. Diabetes 2015, 123, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, M.; Worthmann, H.; Dengler, R.; Schumacher, H.; Lichtinghagen, R.; Eschenfelder, C.C.; Endres, M.; Ebinger, M. Circulating Insulin-like Growth Factor-1 and Insulin-like Growth Factor Binding Protein-3 predict Three-months Outcome after Ischemic Stroke. Exp. Clin. Endocrinol. Diabetes 2017, 125, 485–491. [Google Scholar] [CrossRef]
- Ploughman, M.; Eskes, G.A.; Kelly, L.P.; Kirkland, M.C.; Devasahayam, A.J.; Wallack, E.M.; Abraha, B.; Hasan, S.M.M.; Downer, M.B.; Keeler, L.; et al. Synergistic Benefits of Combined Aerobic and Cognitive Training on Fluid Intelligence and the Role of IGF-1 in Chronic Stroke. Neurorehabil. Neural Repair 2019, 33, 199–212. [Google Scholar] [CrossRef]
- Mattlage, A.E.; Rippee, M.A.; Abraham, M.G.; Sandt, J.; Billinger, S.A. Estimated Prestroke Peak VO2 Is Related to Circulating IGF-1 Levels During Acute Stroke. Neurorehabil. Neural Repair 2017, 31, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Meschia, J.F.; Gottesman, R.; Wruck, L.; Helmer, K.; Greenberg, S.M. Cognitive Impairment and Dementia After Stroke: Design and Rationale for the DISCOVERY Study. Stroke 2021, 52, e499–e516. [Google Scholar] [CrossRef] [PubMed]
- Drag, L.L.; Mlynash, M.; Nassar, H.; Osborn, E.; Kim, D.E.; Angst, M.S.; Aghaeepour, N.; Buckwalter, M.; Lansberg, M.G. A longitudinal study of the post-stroke immune response and cognitive functioning: The StrokeCog study protocol. BMC Neurol. 2020, 20, 313. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Doubal, F.; Brown, R.; Backhouse, E.; Woodhouse, L.; Bath, P.; Quinn, T.J.; Robinson, T.; Markus, H.S.; McManus, R.; et al. Rates, risks and routes to reduce vascular dementia (R4vad), a UK-wide multicentre prospective observational cohort study of cognition after stroke: Protocol. Eur. Stroke J. 2021, 6, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Brännmark, C.; Klasson, S.; Stanne, T.M.; Samuelsson, H.; Alt Murphy, M.; Sunnerhagen, K.S.; Åberg, N.D.; Jalnefjord, O.; Björkman-Burtscher, I.; Jood, K.; et al. FIND Stroke Recovery Study (FIND): Rationale and protocol for a longitudinal observational cohort study of trajectories of recovery and biomarkers poststroke. BMJ Open 2023, 13, e072493. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Mean (SD) or Number (Frequency) |
|---|---|
| Sociodemographic | |
| Sex | |
| Female | 14 (44%) |
| Male | 18 (56%) |
| Age [years] | 68.3 (9.1) |
| Comorbidity and treatment | |
| Hypertension | 17 (53%) |
| Diabetes | 10 (31%) |
| Atherosclerosis | 4 (13%) |
| Trombolytic treatment | 4 (13%) |
| Blood parameters | |
| Sodium | 139.2 (3.0) |
| Potassium | 4.2 (0.7) |
| WBC | 7.46 (2.16) |
| RBC | 4.30 (0.63) |
| Hb | 12.9 (1.7) |
| HCT | 40.2 (4.0) |
| Urea | 29.8 (13.8) |
| Creatinine | 0.7 (0.2) |
| CRP | 9.0 (10.1) 6.0 (1.3–11.2) a |
| Parameter | Mean Estimate ± SEM | p-Value | |
|---|---|---|---|
| Before | After | ||
| Physical | |||
| ADL | 8.8 ± 0.8 | 13.0 ± 0.9 | 0.0008 |
| Rankin | 3.9 ± 0.2 | 3.1 ± 0.2 | 0.0024 |
| NIHSS | 9.0 ± 0.5 | 6.6 ± 0.7 | 0.0059 |
| Psychological | |||
| Cognitive function | 0.0004 a,b | ||
| MOCA | 20.0 ± 1.9 | 24.6 ± 1.9 | |
| MMSE | 23.4 ± 0.6 | 25.2 ± 0.8 | |
| Depressive symptoms | 0.0162 a,c | ||
| BDI | 7.6 ± 1.5 | 7.3 ± 2.4 | |
| GDS | 11.8 ± 0.9 | 6.8 ± 1.0 | |
| Change in the Level of Measurand over the Rehabilitation Process | Cognitive Improvement | Improvement in Depression | ||
|---|---|---|---|---|
| Beta Coefficient ± SEM | p-Value | Beta Coefficient ± SEM | p-Value | |
| MMP9 protein | 0.49 ± 0.15 | 0.0034 | −0.02 ± 0.17 | 0.8878 |
| VEGF protein | 0.01 ± 0.18 | 0.9333 | −0.34 ± 0.16 | 0.0427 |
| IGF1 protein | −0.03 ± 0.18 | 0.8721 | −0.07 ± 0.17 | 0.7083 |
| MMP9 mRNA | −0.02 ± 0.19 | 0.8974 | 0.29 ± 0.18 | 0.1076 |
| VEGF-A mRNA | −0.22 ± 0.18 | 0.2257 | 0.42 ± 0.16 | 0.0117 |
| Pre-Rehabilitation Level of Measurand | Cognitive Improvement | Improvement in Depression | ||
|---|---|---|---|---|
| Beta Coefficient ± SEM | p-Value | Beta Coefficient ± SEM | p-Value | |
| MMP9 protein | 0.26 ± 0.17 | 0.1402 | −0.16 ± 0.17 | 0.3504 |
| VEGF protein | 0.03 ± 0.19 | 0.8603 | −0.41 ± 0.17 | 0.0218 |
| IGF1 protein | 0.08 ± 0.18 | 0.6575 | 0.11 ± 0.17 | 0.5225 |
| MMP9 mRNA | −0.45 ± 0.17 | 0.0127 | 0.14 ± 0.18 | 0.4319 |
| VEGF-A mRNA | −0.15 ± 0.18 | 0.4084 | −0.25 ± 0.17 | 0.1499 |
| Predictor | Odds Ratio | Wald Statistics | p-Value | |
|---|---|---|---|---|
| Point Estimate | 95% CI | |||
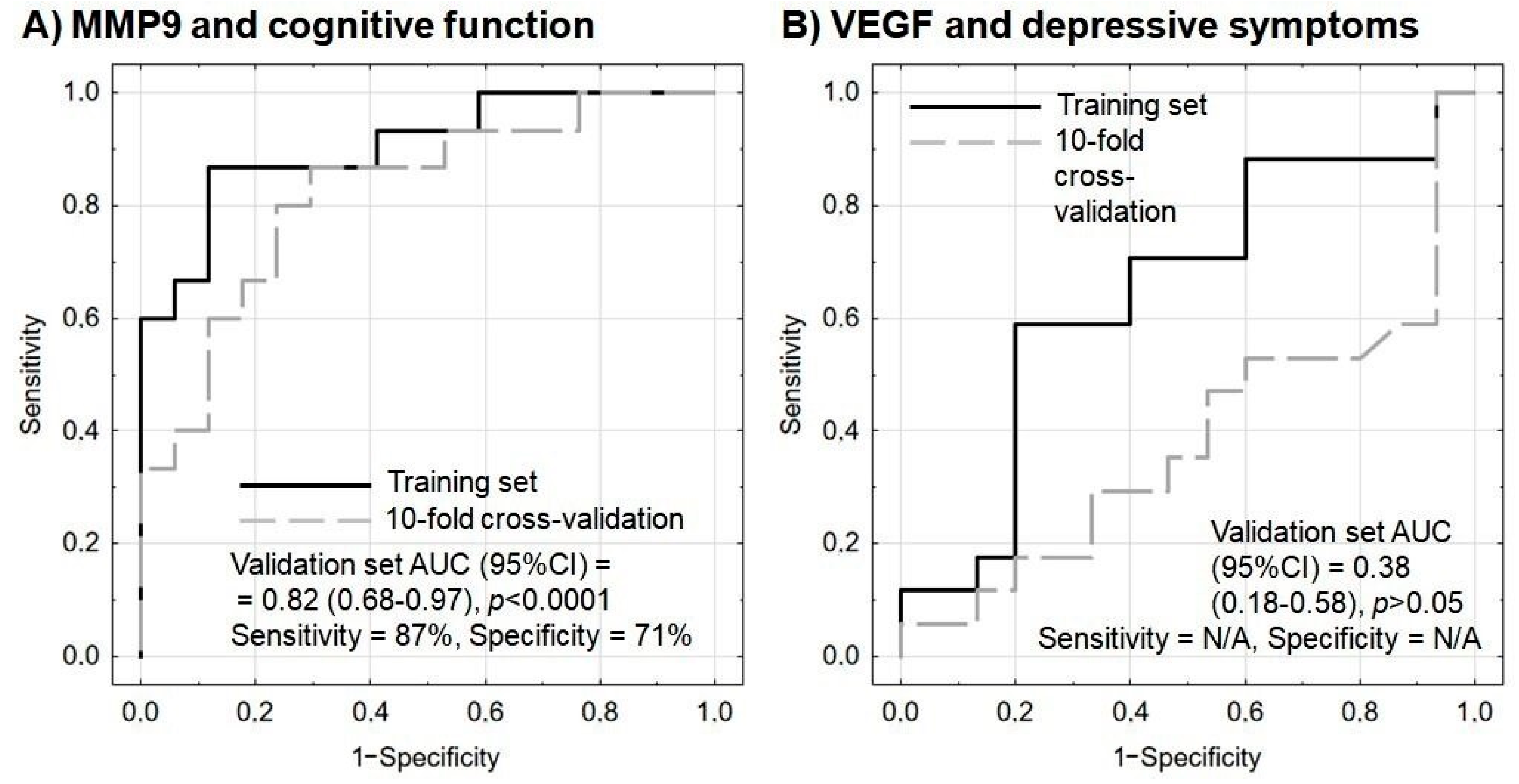
| MMP9 protein a | 1.34 | 1.04–1.73 | 5.2 | 0.0225 |
| MMP9 mRNA b | 0.25 | 0.08–0.76 | 6.0 | 0.0147 |
| Age c | 0.90 | 0.78–1.04 | 2.0 | 0.1547 |
| Sex d | 4.26 | 0.22–82.92 | 0.9 | 0.3391 |
| Predictor | Odds Ratio | Wald Statistics | p-Value | |
|---|---|---|---|---|
| Point Estimate | 95% CI | |||
| VEGF protein a | 0.84 | 0.61–1.15 | 1.2 | 0.2693 |
| VEGF-A mRNA b | 0.82 | 0.60–1.12 | 1.6 | 0.2109 |
| Age c | 1.01 | 0.92–1.11 | 0.1 | 0.7922 |
| Sex d | 1.17 | 0.24–5.76 | <0.1 | 0.8438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, L.; Cichoń, N.; Karbownik, M.S.; Saso, L.; Saluk, J.; Miller, E. Circulating Serum VEGF, IGF-1 and MMP-9 and Expression of Their Genes as Potential Prognostic Markers of Recovery in Post-Stroke Rehabilitation—A Prospective Observational Study. Brain Sci. 2023, 13, 846. https://doi.org/10.3390/brainsci13060846
Włodarczyk L, Cichoń N, Karbownik MS, Saso L, Saluk J, Miller E. Circulating Serum VEGF, IGF-1 and MMP-9 and Expression of Their Genes as Potential Prognostic Markers of Recovery in Post-Stroke Rehabilitation—A Prospective Observational Study. Brain Sciences. 2023; 13(6):846. https://doi.org/10.3390/brainsci13060846
Chicago/Turabian StyleWłodarczyk, Lidia, Natalia Cichoń, Michał Seweryn Karbownik, Luciano Saso, Joanna Saluk, and Elżbieta Miller. 2023. "Circulating Serum VEGF, IGF-1 and MMP-9 and Expression of Their Genes as Potential Prognostic Markers of Recovery in Post-Stroke Rehabilitation—A Prospective Observational Study" Brain Sciences 13, no. 6: 846. https://doi.org/10.3390/brainsci13060846








