Abstract
The relevance of formal hypnotic induction to the experience of trance and its neural correlates is not clear, in that hypnotizability, beliefs and expectation of hypnosis may play a major role. The aim of the study was assessing the EEG brain activity of participants with high (highs) or low hypnotizability scores (lows), aware of their hypnotizability level and informed that the session will include simple relaxation, formal hypnotic induction and neutral hypnosis. A total of 16 highs and 15 lows (according to the Stanford Hypnotic Susceptibility Scale, form A) were enrolled. Their EEGs were recorded during consecutive conditions of open/closed-eyes relaxation, hypnotic induction, neutral hypnosis and post hypnosis not interrupted by interviews. The studied variables were theta, alpha and gamma power spectral density (PSD), and the Determinism (DET) and Entropy (ENT) of the EEG signal Multidimensional Recurrence Plot (mRP). Highs reported significantly greater changes in their state of consciousness than lows across the session. The theta, alpha and gamma PSD did not exhibit condition-related changes in both groups. The Alpha PSD was larger in highs than in lows on midline sites, and the different sides/regions’ theta and gamma PSD were observed in the two groups independently from conditions. ENT showed no correlation with hypnotizability, while DET positively correlated with hypnotizability during hypnosis. In conclusion, the relevance of formal hypnotic induction to the experience of trance may be scarce in highs, as they are aware of their hypnotizability scores and expecting hypnosis. Cognitive processing varies throughout the session depending on the hypnotizability level.
1. Introduction
According to the American Psychological Association (APA), hypnosis is a state of focused attention, reduced contact with the environment and increased proneness to accept suggestions [1]. Despite being subjectively reported as different from normal wakefulness [2,3], hypnosis cannot be regarded as a distinct physiological state, as it cannot be defined independently from self-reports, unlike sleep stages [4]. Nonetheless, neuroimaging highlighted changes in the activity and connectivity of the Default, Salience and Executive Networks [5,6,7,8]. In particular, the hypnotic state has been associated with an increase in the functional connectivity between the anterior cingulate cortex and the dorsolateral prefrontal cortex, and with a reduced activation of the anterior part or of the entire Default Mode Network [8]. Specific neurotransmitters (dopamine, oxytocin, serotonin), in contrast, have been associated with hypnotizability rather than hypnosis [5]. In contrast, EEG studies based on the analysis of spectral frequencies [9,10,11,12,13] were often inconsistent with each other. Recent studies [14], in fact, revealed that changes in information processing during hypnosis are not necessarily associated with changes in local power and can be better measured with time series analyses [15]. Moreover, different connectivity could be responsible for different information processing not implying power changes, like the segregated mechanisms described by complexity indices during hypnosis [16]. The analyses of the EEG complexity [17,18,19], however, were often focused on the correlates of specific suggestions (like those administered during hypnotic assessment by standard scales) rather than solely on neutral hypnosis [13,20], which is the state following hypnotic induction not associated with any suggestion other than relaxation and pleasantness included in standard induction procedures [21].
The difficulty to physiologically characterize the experience of neutral hypnosis, which can be self-induced or achieved after hypnotic induction performed by a hypnotist [1], could be accounted for by the great individual variability, which makes it difficult to propose a unique model of “hypnotic state”. Indeed, spontaneous hypnotic states occur like normal fluctuations of the state of consciousness. Moreover, beliefs, the expectation of hypnosis and hypnotizability may show greater relevance than formal induction in the experience of trance [22,23,24,25,26,27]. In line with these observations, the physiological modifications induced by suggestions (requests to imagine sensory–motor and/or cognitive–emotional experiences different from the actual one and experiencing them as real) during hypnosis are not very different from those induced by the suggestions administered out of hypnosis [28,29,30].
In healthy participants with high hypnotizability scores (highs), informed about their level of hypnotizability and aware that the experimental session will include hypnotic induction, the effects of expectation, hypnotizability and beliefs may prevail over those of formal hypnotic induction, so that the EEG changes during the session might not be time-locked to it. Thus, the present study was aimed at identifying possible changes in the EEG brain activity of participants with high or low (lows) hypnotizability scores during a session including simple relaxation, formal hypnotic induction and neutral hypnosis.
2. Participants and Study Design
2.1. Participants
The study was conducted according to the Declaration of Helsinki and approved by the Bioethics Committee of the University of Pisa (n.17/2021). For the power spectral density (PSD) repeated measures ANOVA the minimum number of participants required to obtain α = 0.05 and (1-β) = 90% with the participants divided in 2 groups was 32, according to G*Power 3.1 analysis [31]. After their written informed consent, 198 healthy right-handed (Edinburgh Handedness Inventory, score > 16) students at the University of Pisa were submitted to hypnotic assessment by the Italian version of the Stanford Hypnotic Susceptibility Scale, form A (SHSS: A (range: 0–12) [21]). A total of 16 highs (SHSS score (mean ± sd): 10.18 ± 1.19; 9 females, age: 25.14 ± 3.82 years) and 16 lows (SHSS score: 0.20 ± 0.56; 7 females, age: 26.47 ± 4.82 years) were enrolled in the study. They had negative anamneses of medical, neurological and psychiatric disorders, attention/sleep disturbance and drug intake in the latest 6 months. Six highs and two lows reported an episodic practice of relaxation/meditation. Their EEG traces were analyzed in another study with different aims and methods [32].
2.2. Experimental Procedure
As previously described [32], on the day of the experimental session, the participants were invited to sit in an armchair and were prepared for EEG recording. The experimental sessions took place in the early afternoon, after the intake of the last meal and caffeine-containing beverages, in a sound- and light-attenuated, temperature-controlled (22 °C) room. They were invited to relax and informed that, after a few minutes, they will have to listen to a pre-recorded hypnotic induction (SHSS: A induction, modified by the direct request made to participants to close their eyes at a certain point of the session). The EEG traces were recorded during consecutive 3 min conditions: open-eyes relaxation (ROE), closed-eyes relaxation (RCE), earliest and latest part of the induction (IND1, IND2), neutral hypnosis (NH) and recovery after hypnosis (POST). The same experimenter invited all participants to relax (before ROE), to close their eyes (before RCE), to listen to the pre-recorded voice (after RCE) and to open their eyes after NH.
Finally, the participants scored the experienced change in their state of consciousness (∆SoC) throughout the session (“How much did your state of consciousness change during the session?”; range: min 0–max 10). Questions about the expectancy of ∆SoC during hypnosis were not asked before starting the session to prevent the participants from unintentionally reporting ∆SoC congruent with the declared expectancy, at its end, owing to expectation/motivation-induced placebo response [33,34]. Specific questionnaires (i.e., [2]) to characterize the state of consciousness were not administered after each condition to avoid altering the participants’ flow of the state of consciousness by interrupting the session.
2.3. Signal Acquisition and Analysis
ECG and EEG signals were recorded with a telemetric Nautilus EEG system (g.tec, Schiedlberg). Leads were placed in F3, F7, C3, T7, P3, PO3, F4, F8, C4, T8, P4, PO4, Fz, Cz, Pz, Oz positions according to the International 10–20 System and referred to Cz. In addition, 2 electrodes were placed on the lateral cantus of the left eye and in correspondence to the left orbital ridge to detect eye movements. Another electrode, referred to Cz, was placed in at the level of the left shoulder for ECG acquisition. All impedances were kept below 5 kΩ. To clean the EEG signals from unwanted noise and artefacts, we used EEGLAB [35] and MATLAB 2020b [36] custom scripts. Specifically, all EEG signals were downsampled to a sampling frequency of 125 Hz, after applying a proper low-pass anti-aliasing filter. Then, a high-pass filter of 0.1 Hz was applied. Bad channels were removed through a semi-automatic procedure (for details see [32]) and successively recovered through a spline–spherical interpolation method. Signals were re-referenced to the numerical average of all channels and decomposed through independent component analysis (ICA) [37]. Finally, we reconstructed the signal on the scalp with the only contribution of independent components related to brain activity [38].
We estimated the PSD on cleaned EEG signals in a time-varying fashion. To this aim, we used a sliding window. Within each three-minute-long window (i.e., ROE, RCE, IND1, IND2, NH, POST), we estimated the PSD with the Welch method using five-second-long windows with an overlap of 80%. The PSD was then integrated in the canonical EEG frequency bands, i.e., delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (13–30 Hz), and gamma (30–45 Hz).
In a similar fashion, we performed a time-varying Multidimensional Recurrence Quantification Analysis (MdRQA) of the EEG signal. QA measures have been used to characterize EEG signals because their evaluation does not require any assumptions on stationarity, length or noise of time series [38]. Here, we used one-minute-long sliding windows with no overlap. For each window, we used the MdRQA as implemented in [39]. This method extends the original implementation of the RQA [40] to multidimensional data and is thus especially suited for the analysis of the EEG signal. Particularly, it allows us to characterize the behavior of multivariate time-series that are the result of multiple interdependent variables, potentially exhibiting non-linear behavior over time [39,40,41]. To determine the MdRQA, it is necessary to first perform a phase-space reconstruction using the method of time-delayed embedding. This latter method needs two parameters to be estimated: the delay parameter τ, which is the lag at which the time series has to be plotted against itself, and the embedding dimension parameter D, where D − 1 is the number of times that the time series has to be plotted against itself using the delay τ [42]. Then, if these two parameters are known, one can reconstruct an approximation of the original phase-space dynamics from a one-dimensional time series [43]. The two methods that are typically used to estimate these parameters in one-dimensional time series are the Average Mutual Information (AMI) function and the False Nearest Neighbor (FNN) function. Here, we used their optimized versions for the multidimensional data implemented in [44]. Finally, we extracted Determinism (DET) and Entropy (ENT) from the obtained recurrence plots (RP) as the variables of interest. In fact, these variables had been studied in not-hypnotized highs, showing a significant group difference in DET at Pz [44,45]
2.4. Variables
Variables include scores of the change in the state of consciousness (∆SoC); EEG theta, alpha and gamma PSD, which are modulated during relaxation and hypnosis [9,46]; EEG Multidimensional Recurrence Plot (mRP); and DET and ENT, which have been found different between highs and lows during relaxation [44,45].
2.5. Statistical Analysis
One subject of the lows group was excluded from analysis owing to the many artefacts in the recorded signals. The Kolmogorov–Smirnov test was used to study the variables’ distribution.
The Spearman correlation coefficient between the change in state of consciousness (∆SoC) and the SHSS scores was computed. Log transformed theta, alpha and gamma hemispheric PSD were analyzed through a design of 2 Groups (highs, lows) × 2 Sides (left, right) × 3 Regions [(frontal (F), central-temporal (C-T), parieto-occipital (P-O) × 6 Conditions (ROE, RCE, IND1, IND2, NH, Post). Midline sites (Fz, Cz, Pz) were analyzed for the same frequency bands and time domain indices through a 2 Groups × 3 Regions (Fz, Cz, Pz) × 6 Conditions (ROE, RCE, IND1, IND2, NH, Post) repeated measures ANOVA. The Greenhouse–Geisser ε correction for non-sphericity was applied when necessary. Post hoc comparisons were conducted by paired t-tests between conditions and regions. The level of significance was set at p = 0.05.
To observe group and condition significant effects/interactions for DET (d = 0.203) and ENT (d = 0.127), a larger sample (N ≥ 96 for DET, N ≥ 86 for ENT) would be required. Since we could not increase the sample size within the time approved for the study by the Bioethics Committee, we limited the analysis to the bivariate Spearman correlations between SHSS scores and DET/ENT.
3. Results
None of the participants showed EEG sleep episodes during the session, but two lows reported sleepiness (∆SoC = 7). The mean values of ∆SoC, significantly higher in highs (mean ± SD; 6.90 ± 1.17) than in lows (1.57 ± 2.21), as well as the satisfaction of their last night’s sleep, similar in highs and lows, were reported in [32]. A significant Spearman correlation between SHSS scores and the ∆SoC experienced over the entire session was observed (ρ = 0.810, p < 0.001).
3.1. EEG Spectral Analysis
3.1.1. Theta PSD
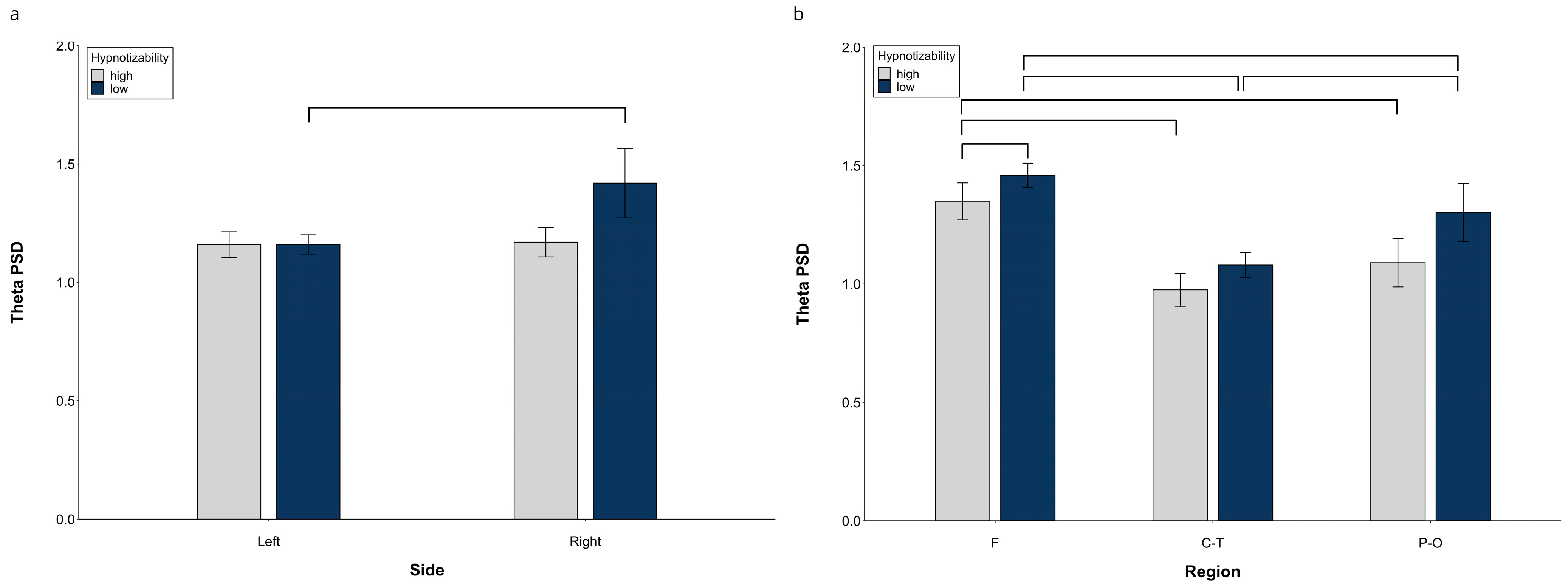
At hemispheric level, decomposition of the Side × Group (F(1, 29) = 13.15, p = 0.0001, η2 = 0.312, α = 0.939) revealed significant side differences in lows (right > left, F(1, 14) = 24.06, p = 0.0001) and no significant difference between groups on each side (Figure 1a).
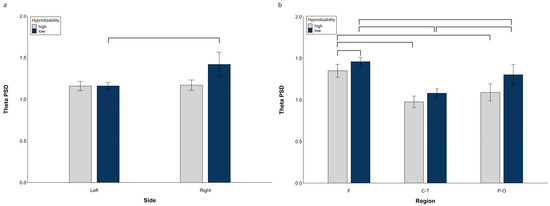
Figure 1.
Theta PSD, raw data. (a) Hemispheric Side × Group interaction, (b) Midline Region × Group interaction. Lines indicate significant differences.
Significant Side × Region (F(2, 28) = 26.14, p = 0.001, η2 = 0.474, α = 1.00) and Region × Condition (F(10, 140) = 2.17, p = 0.039, η2 = 0.070, α = 0.908) interactions were also observed. Decomposition of the Side × Region interaction (Table S1) showed significant differences between all regions within the same side and between the corresponding regions of the two sides, except for the similar right and left P-O regions. Decomposition of the Region × Condition interaction indicated significant differences between almost all regions, with the F and P-O PSD larger than the C-T PSD in all conditions, and the F PSD larger than the P-O PSD only during Roe and IND1 (Table S2).
At midline sites, a significant Region × Group interaction F(2, 58) = 5.84, p = 0.005, η2 = 0.168 α = 0.855) showed a Region effect in both highs (F(2, 30) = 38.61, p = 0.0001) and lows (F(2, 28) = 55.36, p = 0.0001, η2 = 0.798, α = 1.00). Highs exhibited a lower theta PSD than lows (Figure 1b) at the frontal region (t(29) = 2.58, p = 0.015). Moreover, in highs, the frontal PSD was larger than the central-temporal (t(15) = 7.41, p = 0.0001) and parieto-occipital PSD (t(15) = 9.59, p = 0.0001), whereas there was no difference between the central and parieto-occipital sites (Figure 1b). In contrast, in lows, all regions were different between each other (F > C-T, (t(14) = 10.99, p = 0.0001) and P-O (t(14) = 5.70, p = 0.0001), C-T < P-O (t(14) = 4.42, p = 0.001).
Raw data for all regions in each experimental condition are reported in Figure S1.
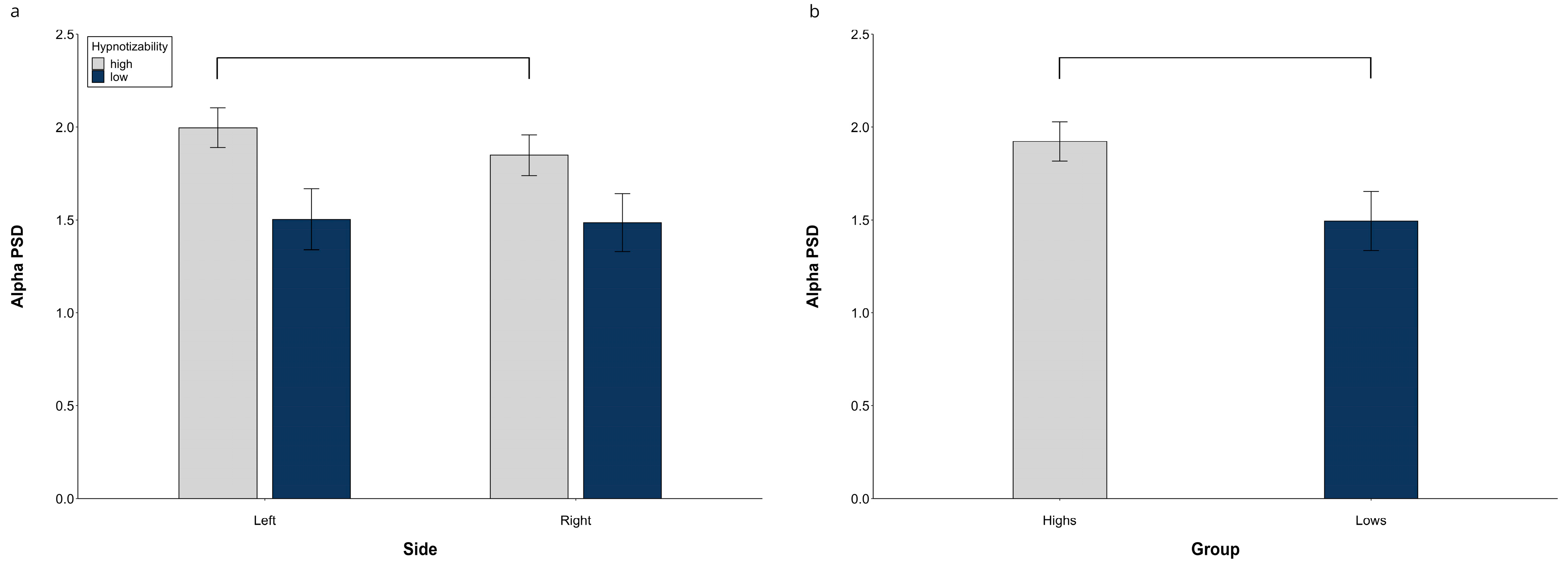
3.1.2. Alpha PSD
At hemispheric level, the alpha PSD exhibited a significant Group effect (F(1, 29) = 4.19, p = 0.037, η2 = 0.141, α = 0.560), with the highs’ value larger than lows’, and significant Side × Group (F(1, 29) = 9.30, p = 0.004, η2 = 0.255, α = 0.861) and Side × Region interactions (F(1, 58) = 21.47, p = 0.001, η2 = 0.427, α = 1.00). Decomposition of the former revealed a higher PSD on the left than on the right side in highs (F(1, 15) = 21.06, p = 0.001) and no side difference in lows. The latter interaction (Figure 2a) was sustained, in averaged groups, by a significantly larger PSD on both sides at the frontal than central-temporal regions (left, t(30) = 7.98, p = < 0.0001; right, t(30) = 20.49, p = < 0.0001) and at the central than posterior regions (left, t(30) = 7.97, p = < 0.0001; right, t(30) = 17.83, p < 0.0001). Moreover, the right PSD was lower than the left one at the central-temporal level (t(30) = 5.77, p = < 0.0001).
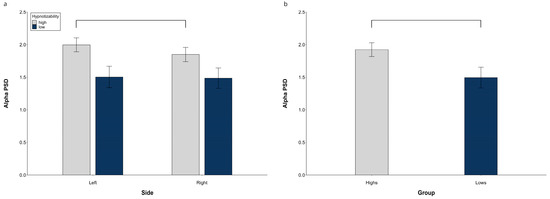
Figure 2.
Alpha PSD, raw data. (a) Hemispheric Side × Group interaction, (b) Midline group difference. Lines indicate significant differences.
The midline alpha PSD also exhibited a significant Group effect (F(1, 29) = 5.25, p = 0.029, η2 = 0.153, α = 0.601) with a higher PSD in the highs than in the lows (Figure 2b).
Raw data for all regions in each experimental condition are reported in Figure S2.
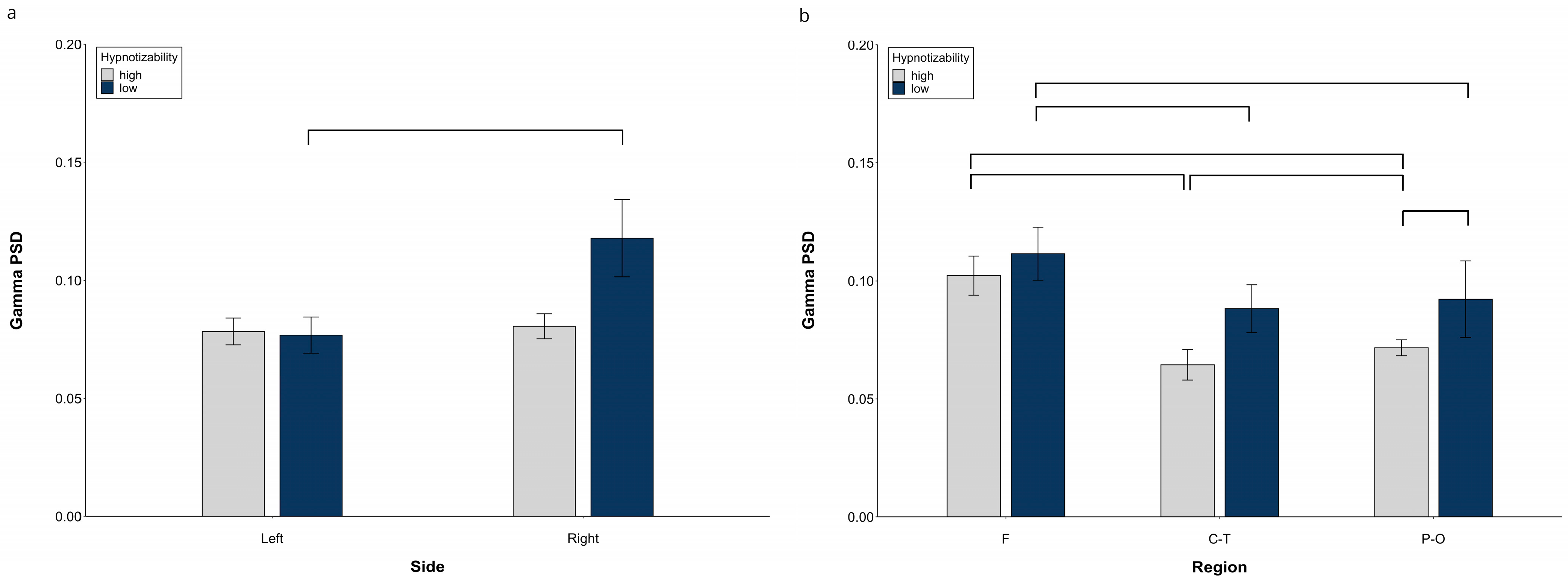
3.1.3. Gamma PSD
ANOVA revealed a significant Side × Group interaction (F(1, 29) = 8.14, p = 0.008, η2 = 0.219, α = 0.712), whose decomposition (Figure 3a) indicated no Side difference in highs and a higher gamma PSD on the right than on the left side in lows (F(1, 14) = 8.34, p = 0.012). Moreover, we observed a significant Region × Group interaction (F(2, 58) = 13.46, p = 0.001, η2 = 0.317, α = 0.942) with the highs’ PSD lower than lows’ (t(29) = 3.43, p = 0.021) in the parieto-occipital region (Figure 3b).
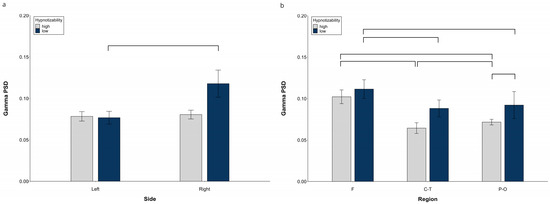
Figure 3.
Gamma PSD, raw data. (a) Hemispheric Side × Group interaction, (b) Hemispheric Region × Group interaction. Lines indicate significant differences.
Decomposition of the significant Group × Region × Condition interaction (F(10, 290) = 2.23, p = 0.047, η2 = 0.071, α = 0.735) revealed a significant Region effect in highs (F(2, 30) = 120.44, p = 0.001), whose regions were all different between each other (F > P-O, t(15) = 7.17, p < 0.0001; P-O > C-T, t(15) = 6.39, p = 0.0001; F > C-T, t(15) = 26.83, p = 0.0001), and a significant Region effect (F(2, 28) = 85.44, p = 0.001) and Region × Condition interaction in lows (F(10, 140) = 4.64, p = 0.006). Decomposition of the latter revealed a higher gamma PSD in the frontal than central-temporal (except Post) and parieto-occipital regions (except Rce and NH), and no difference between the central-temporal and parieto-occipital PSD in all conditions. (Table S3).
Decomposition of the significant Side × Region interaction (F(2, 58) = 11.46, p = 0.0001, η2 = 0.283, α = 0.958) showed a higher PSD in the F than C-T and P-O regions on both sides, a higher PSD in the P-O than C-T only on the left side, and a left PSD lower than the right PSD at the P-O level (Table S4).
At midline sites, we observed only a significant Region effect (F(2, 58) = 125.40, p = 0.0001, η2 = 0.812, α = 0.999), with Cz > Fz (t(30) = 7.88, p = 0.0001), Cz > Pz (t(30) = 13.85, p = 0.0001), Fz > Pz (t(30) = 15.19, p = 0.0001).
Raw data for all regions in each experimental condition are reported in Figure S3.
3.2. EEG Recurrence Quantification Analysis
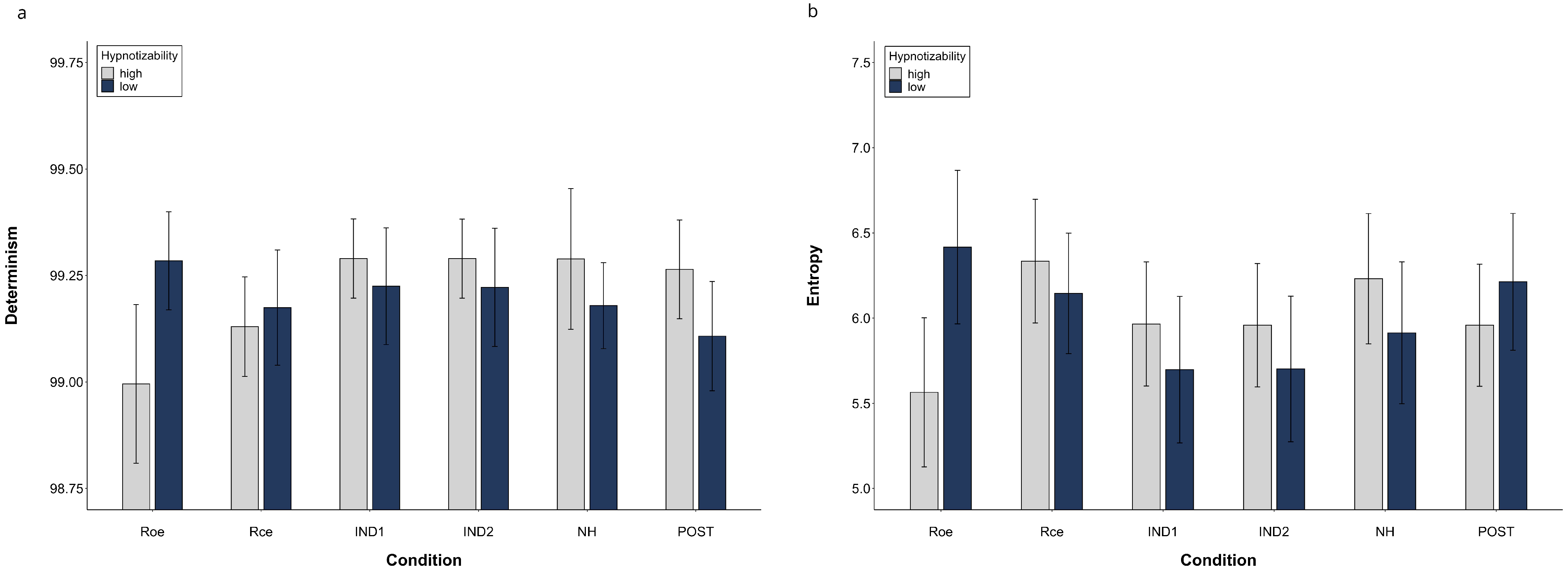
Figure 4 illustrates DET and ENT mean values of highs and lows in different conditions.
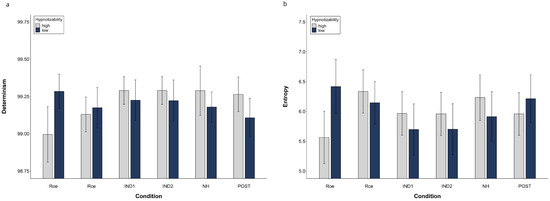
Figure 4.
Determinism (a) and Entropy (b) of the Multidimensional Recurrence Plot. Raw data (mean, SEM).
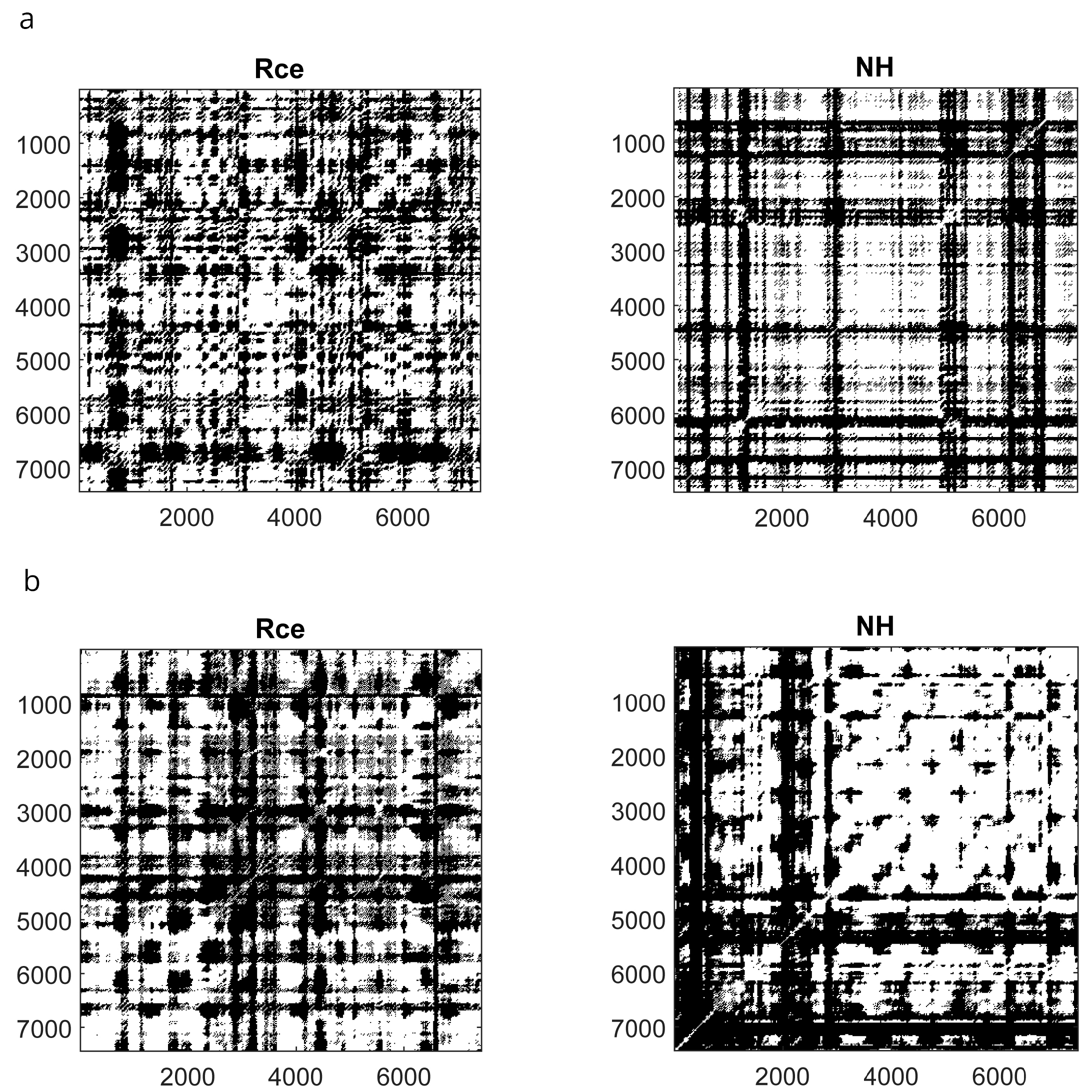
The SHSS score correlated with DET during NH (ρ = 0.431, p = 0.016) and Post (ρ = 0.368, p = 0.041) and did not correlate with ENT. Thus, the cognitive processing of highs and lows across the session seems to be different, as shown in Figure 5 which illustrates the mRP of a low and a high hypnotizable subject during closed-eyes relaxation and hypnosis.
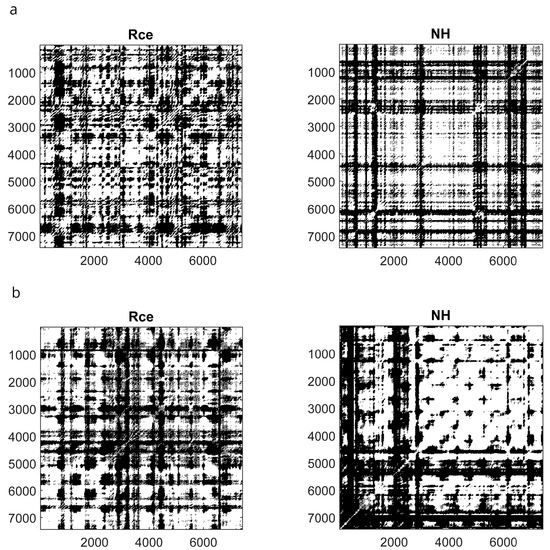
Figure 5.
Recurrence Plots of a low ((a) upper panels) and a high ((b) lower panels) hypnotizable participant. Rce, closed eyes relaxation; NH, neutral hypnosis.
To support the relevance of the hypnotizability trait in the mRP differences across the session, we correlated the values of DET and ENT observed during hypnosis with those found during closed-eyes relaxation, which is the condition most similar to NH except for hypnotic induction. We observed, in fact, a significant positive correlation between RCE and NH (DET, ρ = 0.646, p = 0.0001; ENT, ρ = 0.910, p = 0.0001).
4. Discussion
Several studies described EEG correlates of hypnosis, but their results were often inconsistent owing to methodological differences [9,17,18,19,46]. The aim of the present study was to detect the role of formal hypnotic induction in the EEG activity of highs that are aware of their hypnotic score and waiting for hypnotic induction. Our study provides evidence against a major role of hypnotic induction in the EEG correlates of hypnosis despite the highs’ experience of changes in their state of consciousness.
In the frequency domain, we limited our investigation to theta, alpha and gamma PSD. Theta and alpha are, in fact, the EEG frequency bands most studied during relaxation and hypnosis, although inconsistent findings of their hypnotizability-related and hypnosis-induced difference have been reported [9,46], and high gamma activity has been considered a marker of high hypnotizability and hypnosis [9].
Present findings indicate that formal hypnotic induction did not influence the EEG spectral correlates of neutral hypnosis. In fact, lows exhibited a larger theta and gamma PSD on the right than on the left side, whereas highs did not exhibit any hemispheric difference, suggesting cognitive processing different from the highs from the very beginning of the session.
The stability of theta, alpha and gamma PSD observed in both groups across the session suggests greater relevance of the expectation of hypnosis (likely sustained by different hypnotic level) rather than induction to the experience of trance in highs, while in lows, it accords with their scarce change in the state of consciousness.
The lower theta PSD exhibited by highs on the frontal midline with respect to lows may indicate lower cognitive effort, as the frontal-midline theta increases during cognitive tasks [47]. The theta source has been localized on the anterior cingulate and medial prefrontal cortices [48,49,50], both parts of the Default Mode Network, which is less activated during hypnosis [7,51].
The highs’ larger alpha PSD on the midline sites accords with a reduced activation of the Default Mode Network [8]. Higher alpha power has been associated with internally directed attention [52], which is likely to occur in highs during and/or when expecting hypnosis. Moreover, it has been considered responsible for top–down inhibitory processes for the exclusion of irrelevant input [53,54], as occurs in highs during hypnotic state/induction [1]. Finally, the alpha PSD, which did not differ between the right and left side in lows, was larger on the left than on the right side in highs throughout the session, according with the observation of pre-eminent right side activation during hypnosis in highs [55].
Our findings of gamma PSD do not accord with earlier reports of increased PSD over both hemispheres in the early hypnotic induction, decreased activity in the left and increased activity in the right hemisphere in the late hypnotic induction in highs, and reduction of the gamma PSD in lows in both hemispheres [9].
The findings cannot be compared to the EEG activities recorded during a long-lasting simple relaxation session [56]—increasing alpha, theta and gamma absolute power throughout the session—as in that case, alpha and theta were divided into sub-bands, the instructions for relaxation were repeated every 3 min and the cortical activities were measured by averaging the EEG activity all over the brain.
The method we used to characterize the EEG Recurrence Plot [42] provides a multidimensional description of the brain activity considering the simultaneous recording from all electrodes. This prevented the assessment of hemispheric/regional differences, although other measures of the EEG complexity did not reveal hemispheric differences [13]. We showed that the cognitive processes developing in highs and lows across the experimental session were qualitatively different, and that those occurring during hypnosis were closely related to those observed during closed-eyes relaxation. Such a finding should be supported, however, by the investigation of further mRP dimensions in a larger sample. In not-hypnotized highs [44,45], the original RQA method [40] revealed higher Determinism compared to lows on the midline parietal site (Pz) during the earliest 3 min of simple relaxation, which roughly corresponds to the present closed-eyes relaxation condition. They were followed by progressive increases in the lows’ DET, likely depending on relaxation, so that in the following 9 min there was no significant group difference. Those participants were aware of their hypnotizability score but had been explicitly told that no hypnotic induction will be administered. In the present study, the open-eyes relaxation preceding the closed-eyes condition may have buffered the difference observed during closed-eyes relaxation in the participants not expecting hypnosis.
5. Limitations
The episodic experience of relaxation/meditation reported by a few participants may have biased the results. Other limitations are that our protocol did not include the direct assessment of the participants’ changes in the state of consciousness [2] during/soon after each experimental condition, and that a preliminary assessment of the expected changes was not performed [33,34]. We are aware that, in the absence of a direct assessment of expectations, the pre-eminent role of the expectation of hypnosis and hypnotizability over hypnotic induction in the highs’ experience of trance can only be inferred and that the reported changes in the state of consciousness over the entire session could be inaccurate. Nonetheless, we intentionally chose to prevent expectation-induced (placebo) responses and to allow the participants’ state of consciousness to flow freely throughout the session without interruptions. Moreover, spectral analysis may have failed to detect task-related differences in highs owing to their largely distributed and less-pronounced local changes in brain activity during sensory, motor and cognitive tasks [57,58,59]. Finally, we must consider that the effect sizes of the non-significant group × conditions interactions were quite low (below 0.1); thus, investigation in a larger sample is mandatory.
From a methodological point of view, present findings, together with current evidence, suggest that multidisciplinary (psychological, neurophysiological) and multiparametric approaches (power spectra, coherence, functional connectivity, topology) may assist in the characterization of the states of consciousness. Further improvement of the related research may be obtained by enrolling medium hypnotizable participants [60].
6. Conclusions
The main findings of the present study were that the power spectrum density of alpha, theta and gamma bands does not support the relevance of the hypnotic induction to the highs’ experience of hypnosis. For lows, who reported negligible changes in their state of consciousness despite a few changes in the EEG, a strategy of disengagement from the task can be hypothesized. The different regional power observed in highs and lows, however, may indicate different modes of information processing in the two groups. These results are consistent with earlier reports not showing significant differences between conditions in the parasympathetic heart rate variability of both groups—as measured by the root mean square of successive differences between normal heartbeats (RMSSD, ms)—whose increase is associated with heightened experience of altered state of consciousness [61]. The multidimensional Recurrence Plot, however, highlighted qualitative differences in the cognitive processes of highs and lows and a close relation between awake (RCE) and post-induction conditions (NH).
The theoretical significance of the described findings is in their support for socio-cognitive theories of hypnosis, in that they provide EEG evidence in line with behavioral/experiential findings indicating a major role of expectation and hypnotizability in the experience of hypnosis [23]. Moreover, the findings highlight the relevance of the experimental setting in hypnotizability/hypnosis-related research.
Despite the apparent negligible role of formal induction in our experimental condition, we must underline that the induction procedure may have greater importance in clinical contexts. Specifically, it could reinforce the interpersonal relation between the hypnotist and the subject though gestures, posture, tone and rhythm of voice, choice of words and grammar construction [62], which can modulate the activation of the right orbitofrontal cortex and central oxytocin system [63].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci13060875/s1, Table S1: Theta PSD (log10). Region × Side interaction; Table S2: Theta PSD (log10). Region × Condition interaction in lows; Table S3: Gamma PSD (log10). Region × Condition interaction in lows; Table S4: Gamma PSD (log10). Side × Region interaction; Figure S1: Theta PSD in the frontal, central-temporal and parieto-occipital regions during each experimental condition (ROE, RCE, IND1, IND2, NH, Post). Side and regions averaged; Figure S2: Alpha PSD in the frontal, central-temporal and parieto-occipital regions during each experimental condition (ROE, RCE, IND1, IND2, NH, Post). Side and regions averaged; Figure S3: Gamma PSD in the frontal, central-temporal and parieto-occipital regions during each experimental condition (ROE, RCE, IND1, IND2, NH, Post). Side and regions averaged.
Author Contributions
Conceptualization, E.L.S. and L.S.; methodology, A.G., A.L.C. and E.L.S.; formal analysis, A.L.C. and Ž.Z.; investigation, L.F.; data curation, L.F.; writing—original draft preparation, E.L.S., L.S., A.L.C. and Ž.Z.; writing—review and editing, A.G., E.L.S., L.S., A.L.C. and Ž.Z.; visualization, Ž.Z. and A.L.C.; supervision, E.L.S.; funding acquisition, A.G., E.L.S. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Horizon 2020 Research and Innovation Program, Grant Number 801715-PUPILTRAITS; University of Pisa Fondi Ateneo 2021.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the University of Pisa (n.17/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available upon request after the paper acceptance.
Acknowledgments
We wish to acknowledge the contribution of Iacopo Belcari to data acquisition during his training at the Cognitive and Behavioral Neuroscience Lab., Dept Translational Research and NTMS as a student at the Italian School of Psychotherapy and Ericksonian Hypnosis. Research was partly funded by European Commission under the Next Generation EU program, Grant Number PNRR-M4C2-Investment1.3, PE00000013-FAIR; European Union—Next Generation EU, in the context of The National Recovery and Resilience Plan, Investment 1.5 Ecosystems of Innovation, Project Tuscany Health Ecosystem (THE), Spoke 3 “Advanced technologies, methods, materials and heath analytics” CUP: I53C22000780001.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elkins, G.R.; Barabasz, A.F.; Council, J.R.; Spiegel, D. Advancing Research and Practice: The Revised APA Division 30 Definition of Hypnosis. Am. J. Clin. Hypn. 2015, 57, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pekala, R.J.; Kumar, V.K.; Maurer, R.; Elliott-Carter, N.; Moon, E.; Mullen, K. Suggestibility, Expectancy, Trance State Effects, and Hypnotic Depth: I. Implications for Understanding Hypnotism. Am. J. Clin. Hypn. 2010, 52, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Pekala, R.J.; Maurer, R.; Kumar, V.K.; Elliott-Carter, N.; Mullen, K. Trance State Effects and Imagery Vividness Before and During a Hypnotic Assessment: A Preliminary Study. Int. J. Clin. Exp. Hypn. 2010, 58, 383–416. [Google Scholar] [CrossRef] [PubMed]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S. The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Cardeña, E.; Jönsson, P.; Terhune, D.B.; Marcusson-Clavertz, D. The Neurophenomenology of Neutral Hypnosis. Cortex 2013, 49, 375–385. [Google Scholar] [CrossRef]
- Halligan, P.W.; Oakley, D.A. Hypnosis and Cognitive Neuroscience: Bridging the Gap. Cortex 2013, 49, 359–364. [Google Scholar] [CrossRef]
- Jiang, H.; White, M.P.; Greicius, M.D.; Waelde, L.C.; Spiegel, D. Brain Activity and Functional Connectivity Associated with Hypnosis. Cereb. Cortex 2017, 27, 4083–4093. [Google Scholar] [CrossRef]
- Landry, M.; Lifshitz, M.; Raz, A. Brain Correlates of Hypnosis: A Systematic Review and Meta-Analytic Exploration. Neurosci. Biobehav. Rev. 2017, 81, 75–98. [Google Scholar] [CrossRef]
- De Pascalis, V.; Santarcangelo, E.L. Hypnotizability-Related Asymmetries: A Review. Symmetry 2020, 12, 1015. [Google Scholar] [CrossRef]
- Dunham, C.M.; Burger, A.J.; Hileman, B.M.; Chance, E.A.; Hutchinson, A.E. Bispectral Index Alterations and Associations With Autonomic Changes During Hypnosis in Trauma Center Researchers: Formative Evaluation Study. JMIR Form. Res. 2021, 5, e24044. [Google Scholar] [CrossRef]
- Jensen, M.P.; Adachi, T.; Hakimian, S. Brain Oscillations, Hypnosis, and Hypnotizability. Am. J. Clin. Hypn. 2015, 57, 230–253. [Google Scholar] [CrossRef]
- Rho, G.; Callara, A.L.; Petri, G.; Nardelli, M.; Scilingo, E.P.; Greco, A.; Pascalis, V.D. Linear and Nonlinear Quantitative EEG Analysis during Neutral Hypnosis Following an Opened/Closed Eye Paradigm. Symmetry 2021, 13, 1423. [Google Scholar] [CrossRef]
- Keshmiri, S.; Alimardani, M.; Shiomi, M.; Sumioka, H.; Ishiguro, H.; Hiraki, K. Higher Hypnotic Suggestibility Is Associated with the Lower EEG Signal Variability in Theta, Alpha, and Beta Frequency Bands. PLoS ONE 2020, 15, e0230853. [Google Scholar] [CrossRef]
- Wutz, A.; Loonis, R.; Roy, J.E.; Donoghue, J.A.; Miller, E.K. Different Levels of Category Abstraction by Different Dynamics in Different Prefrontal Areas. Neuron 2018, 97, 716–726.e8. [Google Scholar] [CrossRef]
- Florian, G.; Andrew, C.; Pfurtscheller, G. Do Changes in Coherence Always Reflect Changes in Functional Coupling? Electroencephalogr. Clin. Neurophysiol. 1998, 106, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, J.; Kallio, S.; Kaasinen, V.; Railo, H. Segregated Brain State during Hypnosis. Neurosci. Conscious. 2021, 2021, niab002. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, G.; Nasrabadi, A.M. EEG Phase Synchronization during Hypnosis Induction. J. Med. Eng. Technol. 2012, 36, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Yargholi, E.; Nasrabadi, A.M. The Impacts of Hypnotic Susceptibility on Chaotic Dynamics of EEG Signals during Standard Tasks of Waterloo-Stanford Group Scale. J. Med. Eng. Technol. 2013, 37, 273–281. [Google Scholar] [CrossRef]
- Yargholi, E.; Nasrabadi, A.M. Chaos–Chaos Transition of Left Hemisphere EEGs during Standard Tasks of Waterloo-Stanford Group Scale of Hypnotic Susceptibility. J. Med. Eng. Technol. 2015, 39, 281–285. [Google Scholar] [CrossRef]
- Benson, H.; Arns, P.A.; Hoffman, J.W. The Relaxation Response and Hypnosis. Int. J. Clin. Exp. Hypn. 1981, 29, 259–270. [Google Scholar] [CrossRef]
- Weitzenhoffer, A.M.; Hilgard, E.R. Scala Stanford di Suscettibilità Ipnotica, forme A e B [Stanford Hypnotic Susceptibility Scale, Form A]; Organizzazioni Speciali: Firenze, Italy, 1962. Original work published 1959. [Google Scholar]
- Cardeña, E.; Terhune, D.B. The Roles of Response Expectancies, Baseline Experiences, and Hypnotizability in Spontaneous Hypnotic Experiences. Int. J. Clin. Exp. Hypn. 2019, 67, 1–27. [Google Scholar] [CrossRef]
- Lynn, S.J.; Laurence, J.-R.; Kirsch, I. Hypnosis, Suggestion, and Suggestibility: An Integrative Model. Am. J. Clin. Hypn. 2015, 57, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Lynn, S.J.; Green, J.P.; Polizzi, C.P.; Ellenberg, S.; Gautam, A.; Aksen, D. Hypnosis, Hypnotic Phenomena, and Hypnotic Responsiveness: Clinical and Research Foundations—A 40-Year Perspective. Int. J. Clin. Exp. Hypn. 2019, 67, 475–511. [Google Scholar] [CrossRef]
- Myga, K.A.; Kuehn, E.; Azanon, E. Autosuggestion: A Cognitive Process That Empowers Your Brain? Exp. Brain Res. 2022, 240, 381–394. [Google Scholar] [CrossRef]
- Reategui, R. The Relationship between Expectation and Hypnotic Susceptibility: A Literature Review. Am. J. Clin. Hypn. 2020, 63, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, G.F. Hypnosis and the Relationship between Trance, Suggestion, Expectancy and Depth: Some Semantic and Conceptual Issues. Am. J. Clin. Hypn. 2010, 53, 47–59. [Google Scholar] [CrossRef]
- Derbyshire, S.W.G.; Whalley, M.G.; Oakley, D.A. Fibromyalgia Pain and Its Modulation by Hypnotic and Non-Hypnotic Suggestion: An FMRI Analysis. Eur. J. Pain 2009, 13, 542–550. [Google Scholar] [CrossRef]
- Maxwell, R.; Lynn, S.J.; Condon, L. Hypnosis, Hypnotic Suggestibility, Memory, and Involvement in Films. Conscious. Cogn. 2015, 33, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Parris, B.A.; Dienes, Z. Hypnotic Suggestibility Predicts the Magnitude of the Imaginative Word Blindness Suggestion Effect in a Non-Hypnotic Context. Conscious. Cogn. 2013, 22, 868–874. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Callara, A.L.; Fontanelli, L.; Belcari, I.; Rho, G.; Greco, A.; Zelič, Ž.; Sebastiani, L.; Santarcangelo, E.L. Modulation of the heartbeat evoked cortical potential by hypnotizability and hypnosis. Psychophysiology 2023, e014309. [Google Scholar] [CrossRef]
- Colloca, L.; Miller, F.G. Role of Expectations in Health. Curr. Opin. Psychiatry 2011, 24, 149–155. [Google Scholar] [CrossRef]
- Frisaldi, E.; Piedimonte, A.; Benedetti, F. Placebo and Nocebo Effects: A Complex Interplay Between Psychological Factors and Neurochemical Networks. Am. J. Clin. Hypn. 2015, 57, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- The MathWorks Inc. MATLAB, version 2020b; The MathWorks Inc.: Natick, MA, USA, 2020. [Google Scholar]
- Makeig, S.; Bell, A.J.; Jung, T.-P.; Sejnowski, T.J. Independent Component Analysis of Electroencephalographic Data. In Advances in Neural Information Processing Systems 8; Touretzky, D., Mozer, M.C., Hasselmo, M., Eds.; MIT Press: Cambridge, MA, USA, 1995; pp. 145–151. [Google Scholar]
- Onton, J.; Westerfield, M.; Townsend, J.; Makeig, S. Imaging Human EEG Dynamics Using Independent Component Analysis. Neurosci. Biobehav. Rev. 2006, 30, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Wallot, S.; Roepstorff, A.; Mønster, D. Multidimensional Recurrence Quantification Analysis (MdRQA) for the Analysis of Multidimensional Time-Series: A Software Implementation in MATLAB and Its Application to Group-Level Data in Joint Action. Front. Psychol. 2016, 7, 1835. [Google Scholar] [CrossRef] [PubMed]
- Webber, C.L.; Zbilut, J.P. Dynamical Assessment of Physiological Systems and States Using Recurrence Plot Strategies. J. Appl. Physiol. 1994, 76, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Marwan, N.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Kurths, J. Recurrence-Plot-Based Measures of Complexity and Their Application to Heart-Rate-Variability Data. Phys. Rev. E 2002, 66, 026702. [Google Scholar] [CrossRef] [PubMed]
- Wallot, S.; Mønster, D. Calculation of Average Mutual Information (AMI) and False-Nearest Neighbors (FNN) for the Estimation of Embedding Parameters of Multidimensional Time Series in Matlab. Front. Psychol. 2018, 9, 1679. [Google Scholar] [CrossRef]
- Buzug, T.; Pfister, G. Optimal Delay Time and Embedding Dimension for Delay-Time Coordinates by Analysis of the Global Static and Local Dynamical Behavior of Strange Attractors. Phys. Rev. A 1992, 45, 7073–7084. [Google Scholar] [CrossRef]
- Madeo, D.; Castellani, E.; Santarcangelo, E.L.; Mocenni, C. Hypnotic Assessment Based on the Recurrence Quantification Analysis of EEG Recorded in the Ordinary State of Consciousness. Brain Cogn. 2013, 83, 227–233. [Google Scholar] [CrossRef]
- Chiarucci, R.; Madeo, D.; Loffredo, M.I.; Castellani, E.; Santarcangelo, E.L.; Mocenni, C. Cross-Evidence for Hypnotic Susceptibility through Nonlinear Measures on EEGs of Non-Hypnotized Subjects. Sci. Rep. 2014, 4, 5610. [Google Scholar] [CrossRef]
- Hiltunen, S.; Karevaara, M.; Virta, M.; Makkonen, T.; Kallio, S.; Paavilainen, P. No Evidence for Theta Power as a Marker of Hypnotic State in Highly Hypnotizable Subjects. Heliyon 2021, 7, e06871. [Google Scholar] [CrossRef] [PubMed]
- Snipes, S.; Krugliakova, E.; Meier, E.; Huber, R. The Theta Paradox: 4–8 Hz EEG Oscillations Reflect Both Sleep Pressure and Cognitive Control. J. Neurosci. 2022, 42, 8569–8586. [Google Scholar] [CrossRef]
- Ishii, R.; Canuet, L.; Ishihara, T.; Aoki, Y.; Ikeda, S.; Hata, M.; Katsimichas, T.; Gunji, A.; Takahashi, H.; Nakahachi, T.; et al. Frontal Midline Theta Rhythm and Gamma Power Changes during Focused Attention on Mental Calculation: An MEG Beamformer Analysis. Front. Hum. Neurosci. 2014, 8, 406. [Google Scholar] [CrossRef]
- Michels, L.; Bucher, K.; Lüchinger, R.; Klaver, P.; Martin, E.; Jeanmonod, D.; Brandeis, D. Simultaneous EEG-FMRI during a Working Memory Task: Modulations in Low and High Frequency Bands. PLoS ONE 2010, 5, e10298. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.; Delorme, A.; Makeig, S. Frontal Midline EEG Dynamics during Working Memory. NeuroImage 2005, 27, 341–356. [Google Scholar] [CrossRef] [PubMed]
- McGeown, W.J.; Mazzoni, G.; Vannucci, M.; Venneri, A. Structural and Functional Correlates of Hypnotic Depth and Suggestibility. Psychiatry Res. Neuroimaging 2015, 231, 151–159. [Google Scholar] [CrossRef]
- Cooper, N.R.; Burgess, A.P.; Croft, R.J.; Gruzelier, J.H. Investigating Evoked and Induced Electroencephalogram Activity in Task-Related Alpha Power Increases during an Internally Directed Attention Task. NeuroReport 2006, 17, 205–208. [Google Scholar] [CrossRef]
- Başar, E. The Theory of the Whole-Brain-Work. Int. J. Psychophysiol. 2006, 60, 133–138. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG Alpha Oscillations: The Inhibition–Timing Hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Gruzelier, J.H. Frontal Functions, Connectivity and Neural Efficiency Underpinning Hypnosis and Hypnotic Susceptibility. Contemp. Hypnosis 2006, 23, 15–32. [Google Scholar] [CrossRef]
- Sebastiani, L.; Simoni, A.; Gemignani, A.; Ghelarducci, B.; Santarcangelo, E.L. Relaxation as a Cognitive Task. Arch. Ital. Biol. 2005, 143, 1–12. [Google Scholar] [PubMed]
- Cavallaro, F.I.; Cacace, I.; Del Testa, M.; Andre, P.; Carli, G.; De Pascalis, V.; Rocchi, R.; Santarcangelo, E.L. Hypnotizability-Related EEG Alpha and Theta Activities during Visual and Somesthetic Imageries. Neurosci. Lett. 2010, 470, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Marcelo, E.; Campioni, L.; Phinyomark, A.; Petri, G.; Santarcangelo, E.L. Topology Highlights Mesoscopic Functional Equivalence between Imagery and Perception: The Case of Hypnotizability. NeuroImage 2019, 200, 437–449. [Google Scholar] [CrossRef]
- Ruggirello, S.; Campioni, L.; Piermanni, S.; Sebastiani, L.; Santarcangelo, E.L. Does Hypnotic Assessment Predict the Functional Equivalence between Motor Imagery and Action? Brain Cogn. 2019, 136, 103598. [Google Scholar] [CrossRef]
- Jensen, M.P.; Jamieson, G.A.; Lutz, A.; Mazzoni, G.; McGeown, W.J.; Santarcangelo, E.L.; Demertzi, A.; De Pascalis, V.; Bányai, É.I.; Rominger, C.; et al. New Directions in Hypnosis Research: Strategies for Advancing the Cognitive and Clinical Neuroscience of Hypnosis. Neurosci. Conscious. 2017, 2017, nix004. [Google Scholar] [CrossRef]
- Zaccaro, A.; Piarulli, A.; Melosini, L.; Menicucci, D.; Gemignani, A. Neural Correlates of Non-Ordinary States of Consciousness in Pranayama Practitioners: The Role of Slow Nasal Breathing. Front. Syst. Neurosci. 2022, 16, 803904. [Google Scholar] [CrossRef]
- Zeig, J.; Tanev, K.S. Advancing Hypnotic Inductions: An Ericksonian Perspective. Eur. J. Psychother. Couns. 2022, 24, 457–472. [Google Scholar] [CrossRef]
- Varga, K. Possible Mechanisms of Hypnosis from an Interactional Perspective. Brain Sci. 2021, 11, 903. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).