3.1. In Abstract
There was an error in the original abstract stated that “TBR also protected against neuron loss, reduced activation of astrocytes and microglia, primarily in 6-month-old mice, and attenuated Aβ deposition.” Upon reanalysis, we found that TBR did not protect against neuron loss or attenuate Aβ deposition in treated 5xFAD mice. The original article has been updated. Corrected paragraph/abstract below.
Abstract: Combined treatments using polyphenols and omega fatty acids provide several therapeutic benefits for a variety of age-related disorders, including Alzheimer’s disease (AD). Previously, we found a commercial product, Total Body Rhythm (TBR), consisting of tart cherry extract, a potent polyphenol, and omega fatty acids, significantly reduced memory, and neuropathological deficits in the 192 IgG-saporin mouse model of AD. The present study assessed the efficacy of TBR for treating behavioral and neuropathological deficits in the 5xFAD model of AD. Both 6- and 12-month-old 5xFAD mice and age-matched wild-type controls received TBR (60 mg/kg) or the equivalent dose of vehicle (0.5% methylcellulose) via oral administration, every other day for two months. All mice were tested in the open field (OF), novel object recognition (NOR), and the Morris water maze (MWM) tasks. In addition, neuronal morphology, neurodegeneration, Aβ plaque load, and glial activation were assessed. TBR treatment reduced memory deficits in the MWM and NOR tests and lessened anxiety levels in the OF task, mostly in the 6-month-old male mice. TBR also protected and reduced activation of astrocytes and microglia, primarily in 6-month-old mice. These results suggest that the combination of tart cherry extract and omega fatty acids in TBR can reduce AD-like deficits in 5xFAD mice.
3.2. In the Results Part
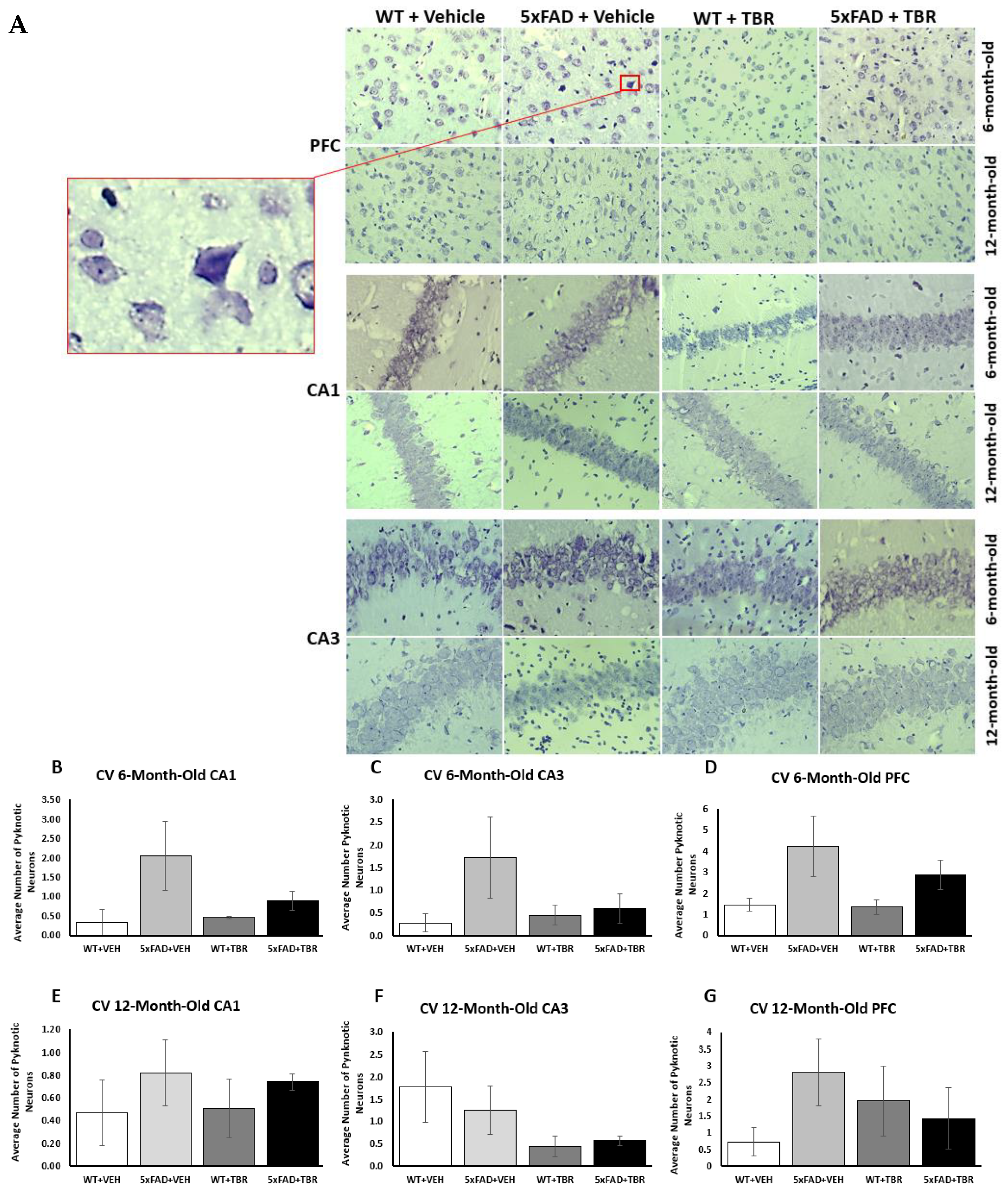
There was an error in the original article, in the first paragraph in Section 3.5. Cresyl-Violet Imaging for Neuronal Morphology, that stated: “a significant decrease in the number of pyknotic cells in 6-month-old 5xFAD mice treated with TBR”. Upon reanalysis, we found that TBR did not decrease the number of pyknotic neurons.
Corrected paragraph: Neuron morphology was assessed by staining sections with 0.1% cresyl-violet and counting the number of pyknotic neurons in the CA1 and CA3 areas of the hippocampus, as well as in the pyramidal layer 5 of the PFC (
Figure 9A). Using a one-way ANOVA, it was revealed that there was a significant decrease in the number of pyknotic cells in 6-month-old 5xFAD mice treated with TBR.
There was an error in the original article, in the first paragraph in
Section 3.6. Amyloid β-Plaque Staining, that stated: “A two-way independent
t-test revealed a significant difference in the average number of Aβ plaque for the 6-month-old mice in the retro-splenial cortex between the four AD 6-month-old mice treated with TBR and the five AD mice treated with vehicle (
Figure 10B). There was a significant difference in the average number of β-amyloid peptide between the five AD 12-month-old mice treated with TBR and the six AD mice treated with vehicle (
Figure 10C). There were no significant differences in the dentate gyrus for either age group in the number of Aβ plaques. However, subsequent analyses indicated that this difference was due primarily to how the male mice responded to TBR treatment (
Figure 11).” Upon reanalysis, we found that TBR did not protect against Aβ deposition.
Corrected paragraph: A two-way independent t-test revealed there was no significant difference in the average number of Aβ plaque for the 6-month-old or 12-month-old mice (
Figure 10B–G).
There was an error in the original article, in the first paragraph in
Section 3.7. Sex Differences in Number of Amyloid-β Plaques in 6-Month-Old Mice, that stated: “One-way ANOVAs revealed significant sex differences in the average number of Aβ plaques for the 6-month-old 5xFAD mice. There was a significant difference in the number of Aβ plaques retrosplenial cortex (RSC) (
F(3, 68) = 17.7579,
p < 0.05), dentate gyrus (DG) (
F(3, 72) = 10.4856,
p < 0.05), and entorhinal area (EC) (
F(3, 67) = 8.4006,
p < 0.05) (
Figure 11A–C). Using the Scheffè post hoc analysis, it was found that there was a significant difference in the number of Aβ plaques between the male 5xFAD mice treated with TBR and the male 5xFAD+VEH, female 5xFAD+VEH, and the female 5xFAD+TBR (
p < 0.05). The 6-month-old female 5xFAD mice treated with TBR were not significantly different than the female mice treated with vehicle (
Figure 11A,B). Using the Scheffè post hoc analysis, it was found that there was a significant difference between the male 5xFAD mice treated with TBR, female 5xFAD+VEH, and female 5xFAD+TBR (
p < 0.05) in the DG. The 6-month-old female 5xFAD mice treated with TBR were not significantly different than the female mice treated with vehicle (
Figure 11C).” Upon reanalysis, we found that TBR did not protect against sex-dependent Aβ deposition.
Corrected paragraph: One-way ANOVAs revealed no significant sex differences in the average number of Aβ plaques for the 6-month-old or 12-month-old 5xFAD mice (
Figure 11A–F).
3.3. In the Discussion Part
There was an error in the original Discussion section. In the first paragraph, it stated: “Our findings confirmed our hypotheses that mice receiving TBR would have fewer cognitive deficits, increased neuron protection, changes in glial cell functioning, and reduced β-amyloid load.” Upon reanalysis, we found that TBR did not protect against Aβ deposition.
Corrected paragraph: The current study tested the combinatorial treatment of tart cherry extract and omega fatty acids (TBR) in 5xFAD AD mice at both 6 and 12 months of age. Our findings confirmed our hypotheses that mice receiving TBR would have fewer cognitive deficits and changes in glial cell functioning. Previous work from our lab has demonstrated that TBR can prevent loss of body weight in a cholinergic-toxin (192 IgG-saporin) mouse model of AD [22]. The finding that there were no significant differences between the 5xFAD animals and age-matched WT controls in weight or of activity in our study underscores the argument that the differences seen in the NOR and MWM tasks were due to disturbances in cognition rather than overall decrements in health or activity levels [28]. However, the findings of an increased level of anxiety in untreated 5xFAD mice may have influenced the outcome of the NOR tasks, although differences in the number of approaches to the objects were not observed, suggesting that the differences in the amount of time investigating the novel object were likely due to recognition memory differences rather than differences in anxiety levels or motivation.
In the fourth paragraph in the Discussion section, it stated: “In addition to cognitive sparing, we found that TBR was able to reduce the number of pyknotic cells in the hippocampus and prefrontal cortex of the mouse brain (
Figure 9). Alzheimer’s disease, unlike normal aging, is associated with a large loss of neurons [35]. Furthermore, the total number of lost neurons is positively correlated with severity of symptoms [36]. Pyknotic cells are those which are undergoing necrosis or apoptosis. For both 6-and 12-month-old mice, there were reduced numbers of pyknotic cells in those animals that received TBR treatment. It is possible that TBR, through reducing oxidative stress and correction of fatty acid profiles was able to protect both neurons and glial cells.” Upon reanalysis, we found that TBR did not protect against neuron loss or attenuate Aβ deposition in treated 5xFAD mice.
Corrected paragraph: Although TBR reduced cognitive deficits, it was unable to reduce the number of pyknotic cells in the hippocampus and prefrontal cortex of the mouse brain (
Figure 9). Alzheimer’s disease, unlike normal aging, is associated with a large loss of neurons [32]. Furthermore, the total number of lost neurons is positively correlated with the severity of symptoms [33]. Pyknotic cells are those which are undergoing necrosis or apoptosis. For both 6-and 12-month-old mice there was only an insignificant trend toward reduced numbers of pyknotic cells in those animals which received TBR treatment. Further research is needed to determine whether higher doses or newer formulations with higher amounts of omega-3 fatty acids might provide a neuroprotective effect in rodent models of AD.
In the sixth paragraph in the Discussion section, it stated: “We demonstrated that TBR was able to reduce amyloid beta levels within the retrosplenial cortex of the 6-month-old AD mice treated with TBR and the entorhinal cortex of 12-month-old mice.” Upon reanalysis, we found that TBR did not attenuate Aβ deposition in treated 5xFAD mice.
Corrected paragraph: The 5xFAD mouse model is known to be an aggressive model, with rapid formation of amyloid plaques. TBR was not able to reduce amyloid beta levels within the 6-and 12-month-old AD mice, although a non-significant trend was observed in the retrosplenial cortex (RSC). The RSC constitutes a large portion of cortex in rodents, which corresponds to Broadman’s area 29 and 30 [37]. Although the RSC was identified by Broadman over 90 years ago, the structure and function of this region remain elusive. It is known that in sporadic AD there are volumetric differences in the RSC that are comparable with the atrophy seen within the hippocampus of humans [38]. It has been demonstrated that there are dramatic differences in size between species, and there is now a strong connection between the RSC cortex and a range of cognitive functions. According to an extensive review conducted by Vann and colleagues, the RSC has emerged as a key member of a network of brain regions, including the hippocampus and limbic system, and is involved in episodic memory, navigation, imagination, and planning for the future [37]. All of these functions are crucial for performing the memory tasks assessed in our study and could explain why we found such a significant difference at 48 h post-training in the MWM.
In the seventh paragraph in the Discussion section, it stated: “When exploring the impact that sex has on AD symptoms, we found a difference between the 6-month-old male and female 5xFAD mice on the NOR task and the number of amyloid β deposits. We found that male mice responded better to treatment on the NOR (Figure 6) than the female mice, in that they spent increased time exploring the novel object during testing.” Upon reanalysis, we found that TBR did not attenuate sex dependent Aβ deposition in treated 5xFAD mice.
Corrected paragraph: When exploring the impact that sex has on AD symptoms, we found a difference between the 6-month-old male and female 5xFAD mice on the NOR task. We found that male mice responded better to treatment on the NOR (Figure 6) than the female mice, in that they spent increased time exploring the novel object during testing. Understanding the relationship between amyloid β and cognitive impairment in AD is complex. Some argue that there is very little connection; indeed, patients can have varying degrees of cognitive deficits, even when having similar amyloid beta loads [39].
In the eighth paragraph in the Discussion section, it stated: “Our results show that TBR influences amyloid beta deposition in a sex-dependent fashion.” It also stated: “However, the degree to which amyloid β load is related to cognitive deficits is debatable, but our findings that males had reduced levels of amyloid β and responded more strongly to TBR therapy suggests that increased amyloid β may have contributed to the memory deficits observed in this study.” Upon reanalysis, we found that TBR did not attenuate sex-dependent Aβ deposition in treated 5xFAD mice.
Corrected paragraph: Our results show that TBR does not influence amyloid beta deposition in a sex-dependent fashion. It has been demonstrated in multiple transgenic mice, including APPswe, PS1 double transgenic, and 3xTg mice, that female mice have increased amyloid β burden compared with male mice [40,41]. This increased burden of amyloid-β was also found to coincide with worse cognitive outcomes for female mice as well [41]. In addition, it has been demonstrated for over two decades that both macaque and human males respond preferentially to donepezil, one of the most common cholinesterase inhibitors [42,43]. Both polyphenols and omega fatty acids, through different mechanisms, have been shown to reduce amyloid β load. However, the degree to which amyloid β load is related to cognitive deficits is debatable and our results suggest that TBR can reduce cognitive deficits without significantly reducing amyloid beta levels.
In the ninth paragraph in the Discussion section, it stated: “Manipulation of sex hormone levels in the mice could be contributing to the sex differences observed in the behavioral and amyloid-β data of our study. It has been demonstrated that 5xFAD female mice can have increasing amyloid burden until 14 months of age, possibly due to influences of the Thy-1 promoter used to express the transgenes in this model of AD [11]. We hypothesize that either the tart cherry extract or the fatty acids influenced sex hormone differences occurring earlier in disease development, which then influenced outcomes; it is well known that within the human population and in animal models that females have higher rates of AD and that this difference cannot be attributed to simply living longer [47]. A recent comprehensive review using over 275 articles and meta-analysis found that women are more likely to suffer increased cognitive deterioration than men who are at the same AD disease stage [48]. Indeed, the same group who found a disparity between men and women in AD have found that episodic memory is a key factor in this discrepancy [49]. However, if the difference seen in the NOR in our study was primarily because of amyloid-β load, it could be argued that 12-month-old 5xFAD mice would be expected to have more amyloid-β than a 6-month-old female.” Upon reanalysis, we found that TBR did not attenuate sex dependent Aβ deposition in treated 5xFAD mice.
Corrected paragraph: Manipulation of sex hormone levels in the mice could be contributing to the sex differences observed in the behavioral data of our study. It has been demonstrated that 5xFAD female mice can have an increasing amyloid burden until 14 months of age, possibly due to influences of the Thy-1 promoter used to express the transgenes in this model of AD [11]. We hypothesize that either the tart cherry extract or the fatty acids influenced sex hormone differences occurring earlier in disease development, which then influenced outcomes; it is well known that within the human population and in animal models that females have higher rates of AD and that this difference cannot be attributed to simply living longer [44]. A recent comprehensive review using over 275 articles and meta-analysis found that women are more likely to suffer increased cognitive deterioration than men who are at the same AD disease stage [45]. Indeed, the same group who found a disparity between men and women in AD have found that episodic memory is a key factor in this discrepancy [46].
3.4. In the Materials and Methods Part
There was an error in the original Section 2.4.1. Open-Field Testing, which stated: “The OF testing was used to assess spontaneous motor activity and anxiety [23,24]. Additionally, fecal boli counts were taken during each trial. Mice were tested in one 30 min trial at both pre- and post-treatment. For details, see [24].”
Corrected paragraph: The OF testing was used to assess spontaneous motor activity and anxiety [23]. Additionally, fecal boli counts were taken during each trial. Mice were tested in one 30 min trial at both pre- and post-treatment.
There was an error in the original Section 2.4.2. Novel Object Recognition, which stated: “The novel object recognition (NOR) task allows for the assessment of recognition memory in an environment with minimal stress [22,25–27]. We have published details of NOR procedure [24].”
Corrected sentence: The novel object recognition (NOR) task allows for the assessment of recognition memory in an environment with minimal stress [22,24,25].
There was an error in the original Section 2.4.3. Morris Water Maze (MWM), which stated: “The MWM task measures the ability to find a hidden platform in a pool of water and is used as an assessment of procedural learning and spatial memory [24,28,29].”
Corrected sentence: The MWM task measures the ability to find a hidden platform in a pool of water and is used as an assessment of procedural learning and spatial memory [26,27].
There was an error in the original Section 2.6.2. Amyloid β-Plaque Count, which stated: “For both 6- and 12-month-old mice, coronal sections were sampled from bregma at –1.28 mm and −2.92 mm. Sections were stained using curcumin, which labels plaques as efficiently as Aβ-specific antibodies [30]”.
Corrected sentence: For both 6- and 12-month-old mice, coronal sections were sampled from bregma at –1.28 mm and −2.92 mm. Sections were stained using curcumin.