The Oxygen and Glucose Deprivation of Immature Cells of the Nervous System Exerts Distinct Effects on Mitochondria, Mitophagy, and Autophagy, Depending on the Cells’ Differentiation Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Differentiation of Neural Stem Cells
2.2. Oxygen–Glucose Deprivation Model
2.3. Immunocytochemistry
2.4. Western Blot
2.5. Quantitative Polymerase Chain Reaction (qPCR)
2.6. Lactate Dehydrogenase (LDH) Detection Kit
2.7. MitoSOX
2.8. TMRE
2.9. LC3-GFP/Lysotracker Colocalization
2.10. Mitochondrial Morphology
2.11. Statistical Analyses
3. Results
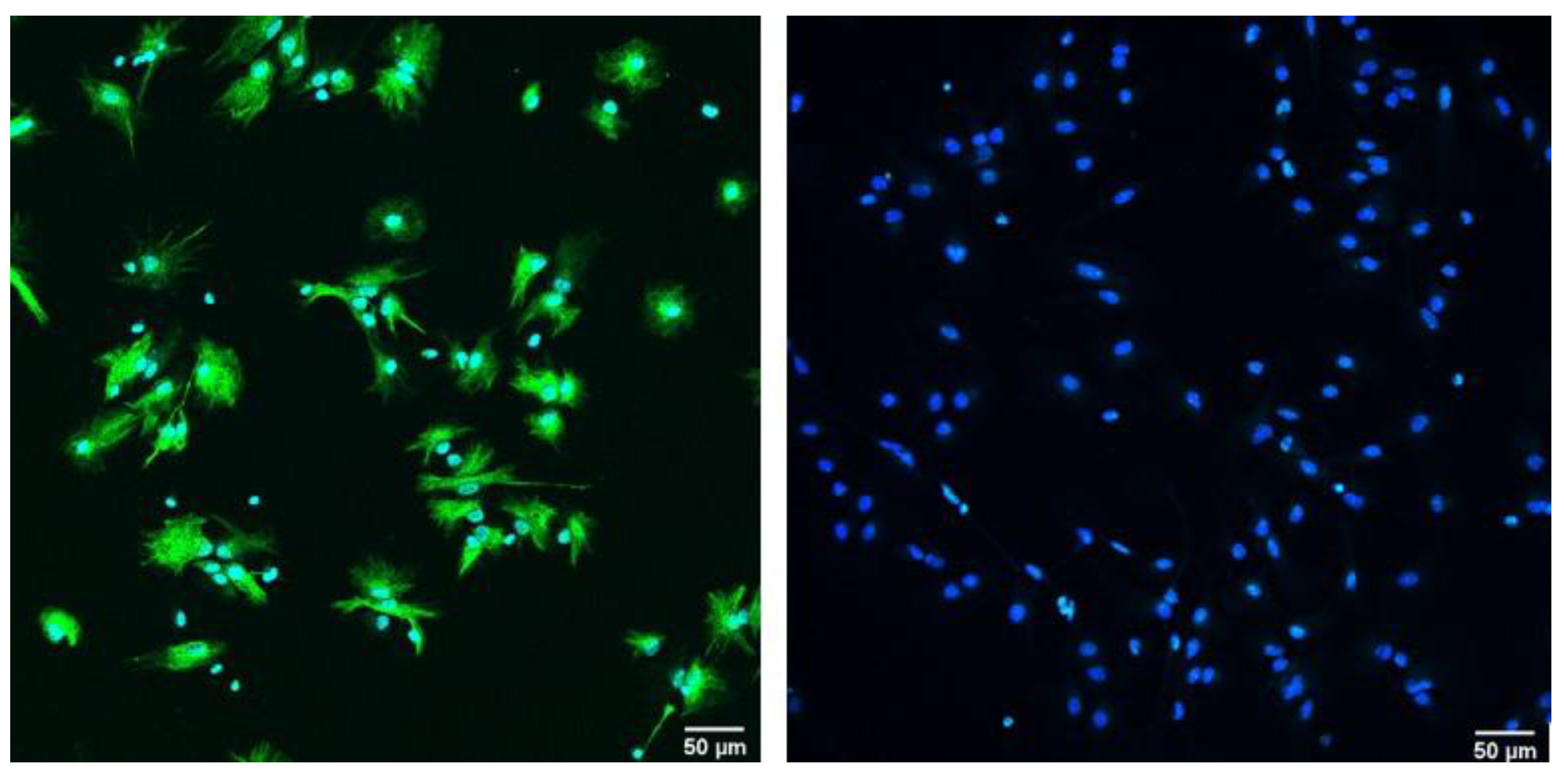
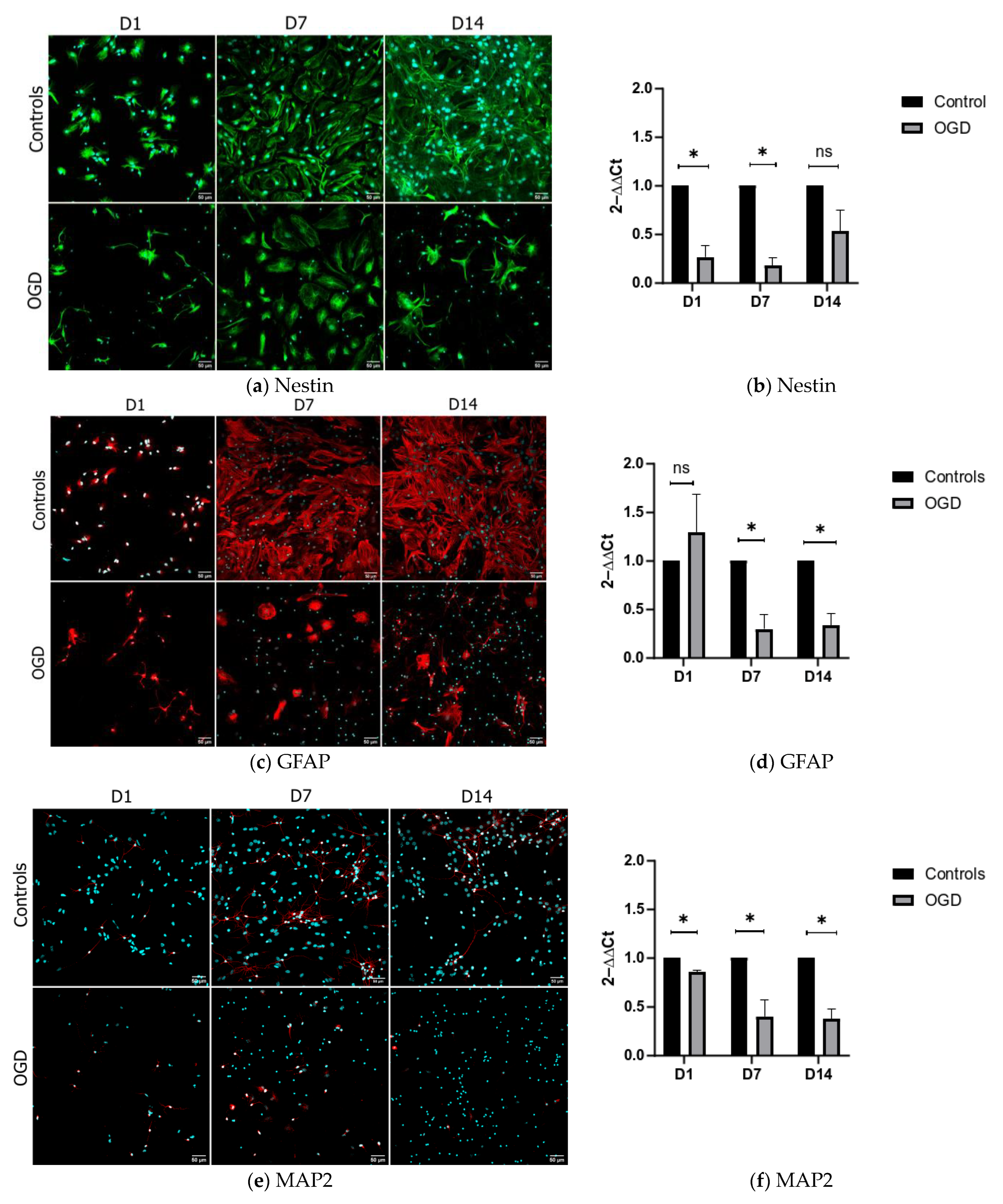
3.1. One and Two Weeks of In Vitro Differentiation of Neural Stem Cells Yields Various Precursors of the Nervous System
3.2. The Influence of Oxygen and Glucose Deprivation on Cytotoxicity and Differentiation of Neural Precursors Does Not Depend on the Level of Their Maturity
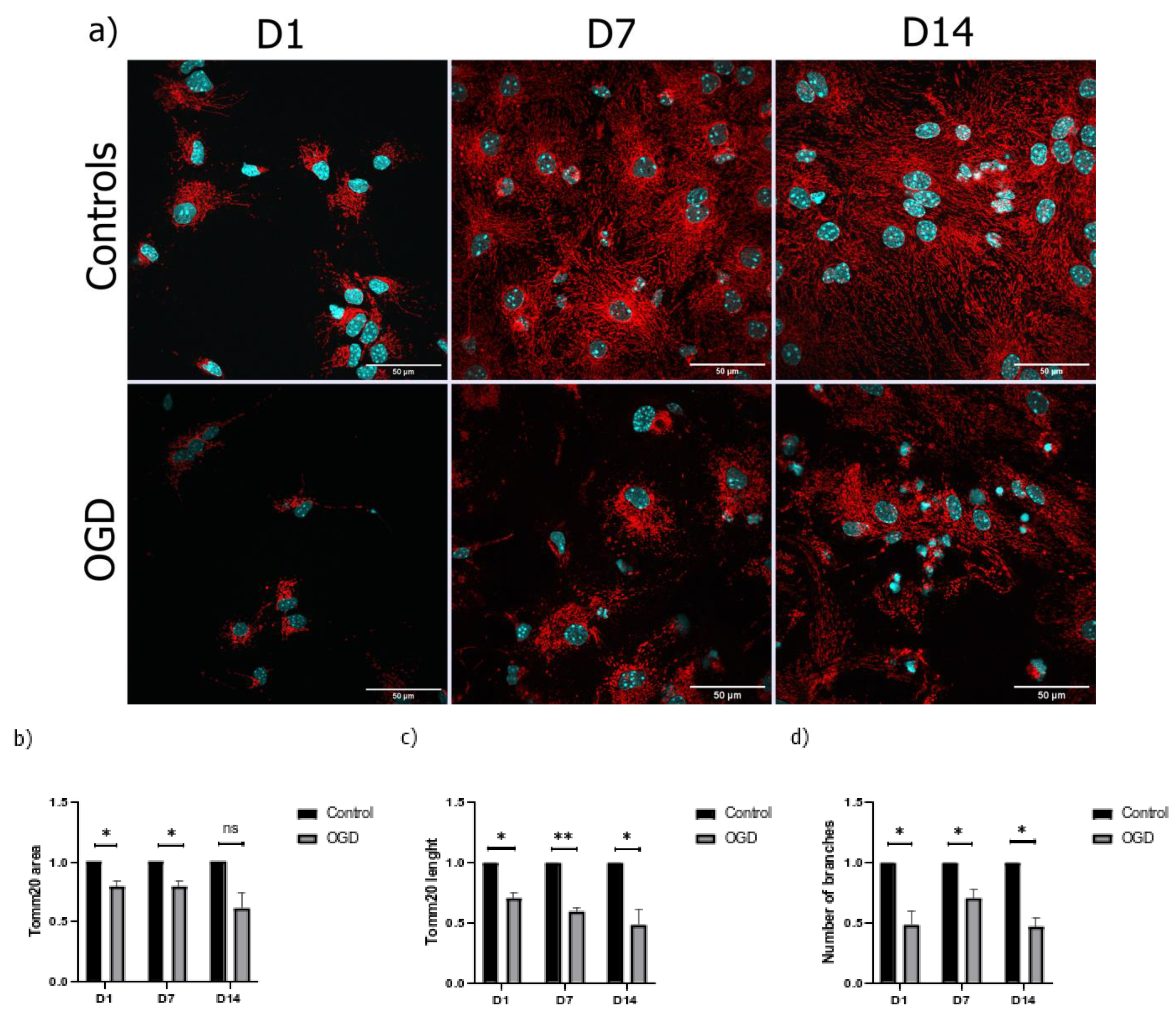
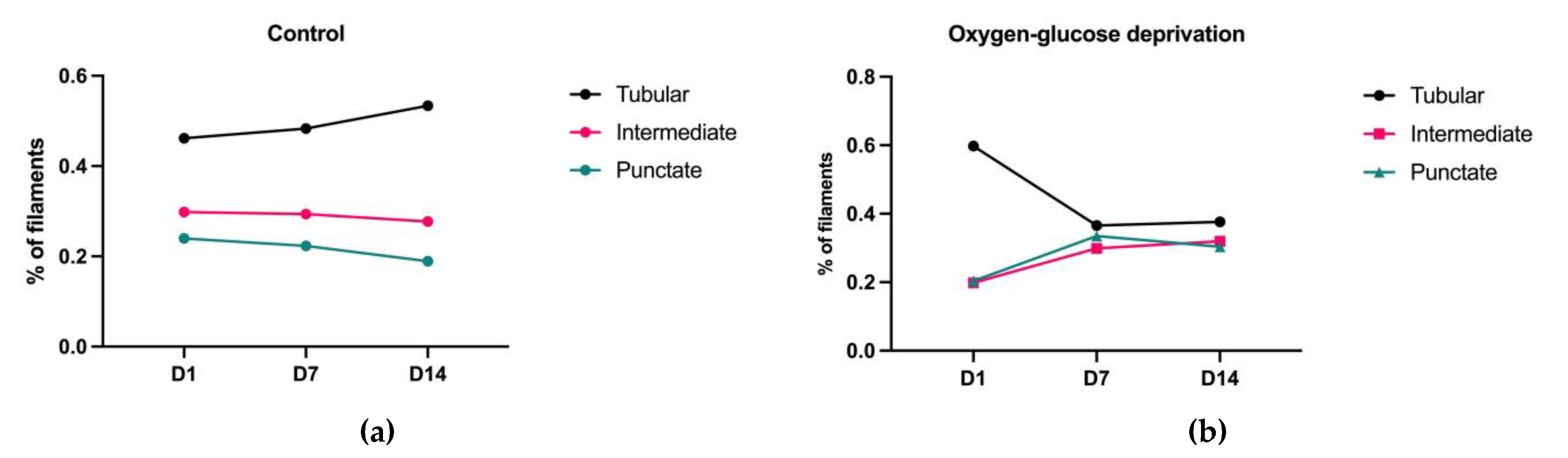
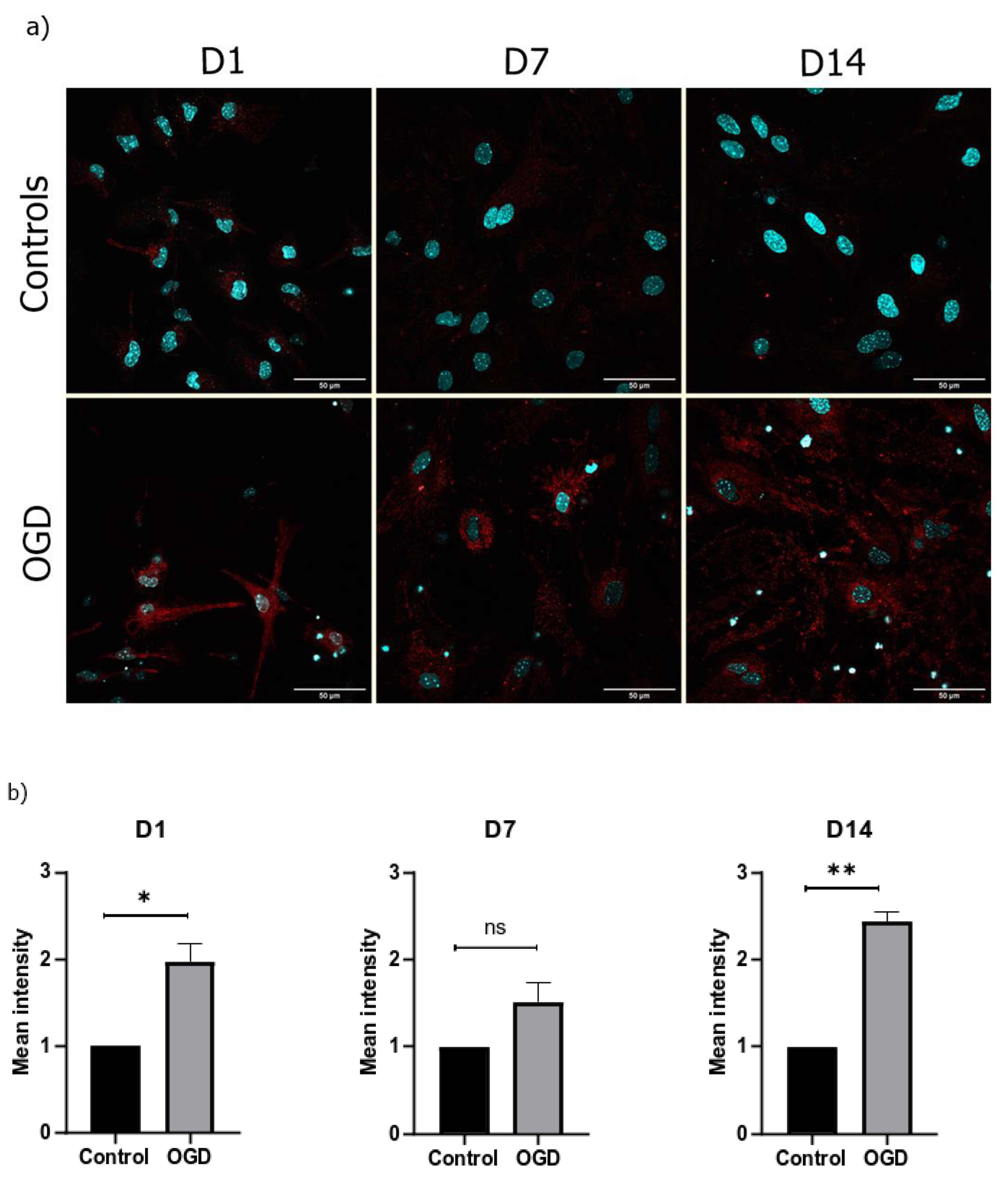
3.3. The Morphological Parameters of Mitochondria Change during Cell Differentiation Are Influenced by the Lack of Oxygen and Glucose
3.4. The Lack of Oxygen and Glucose Increases the Number of Oxygen Radicals in the Mitochondria and Causes Their Hyperpolarization
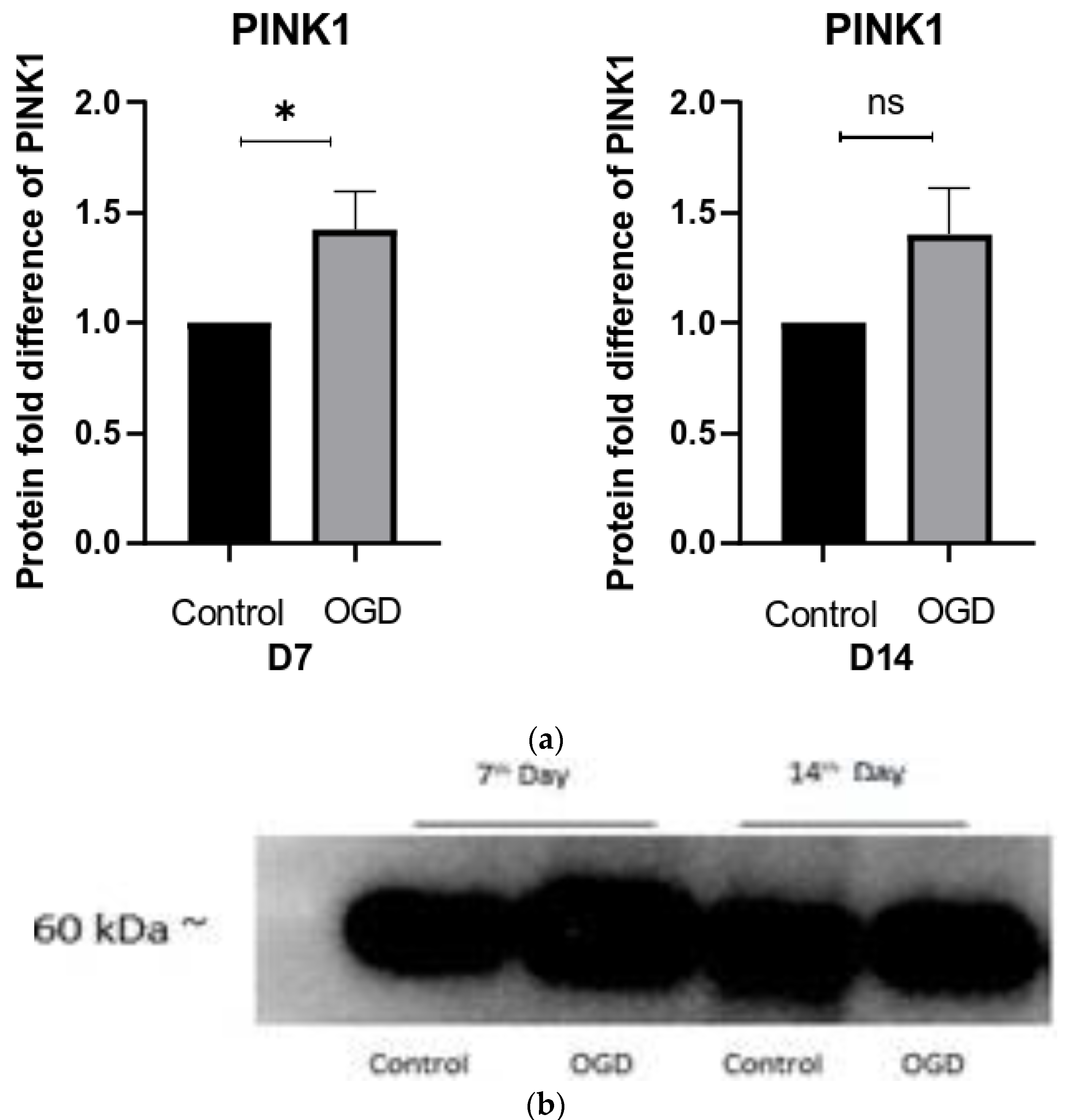
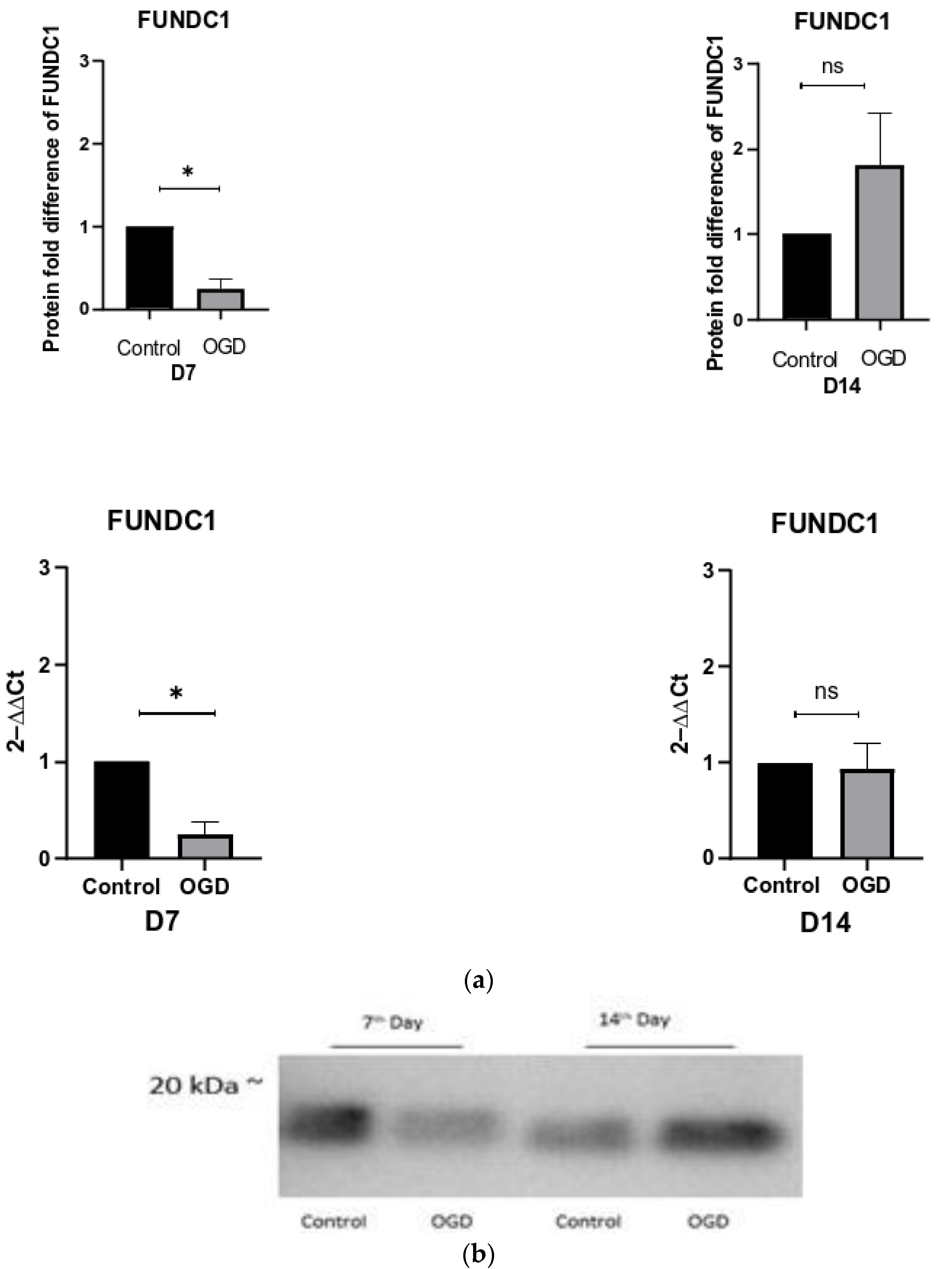
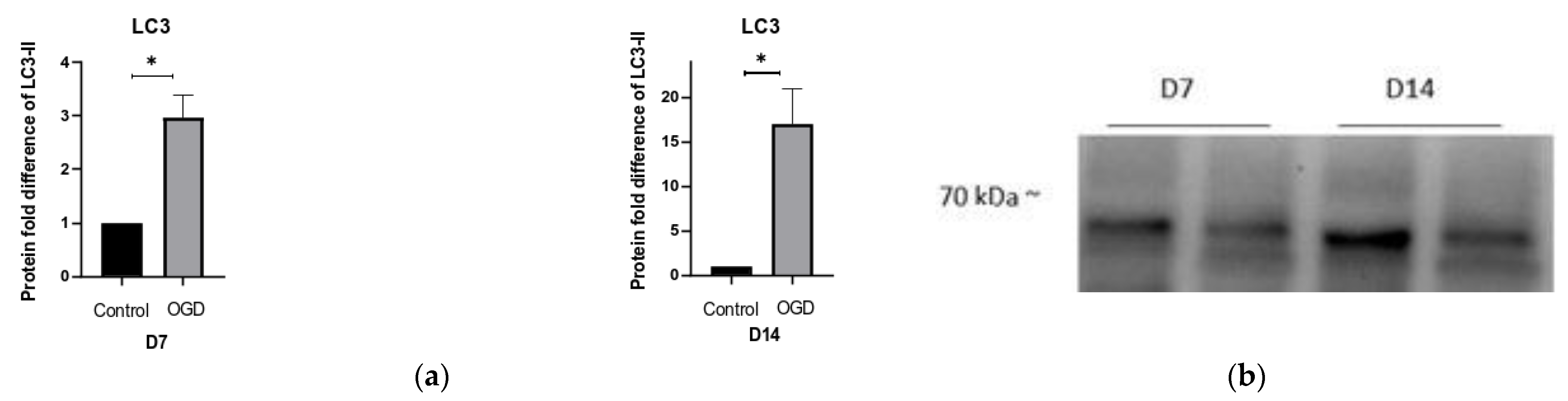
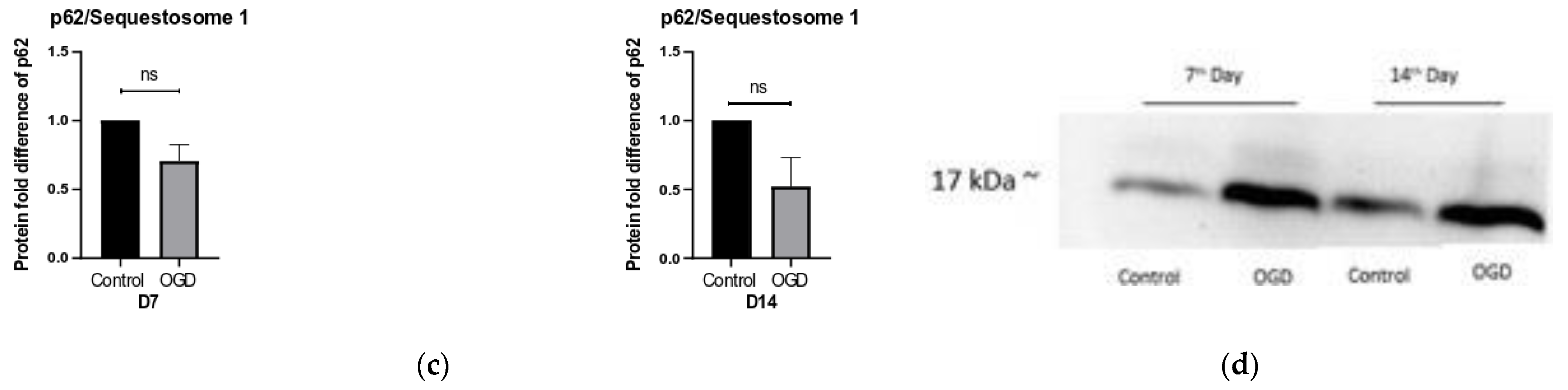
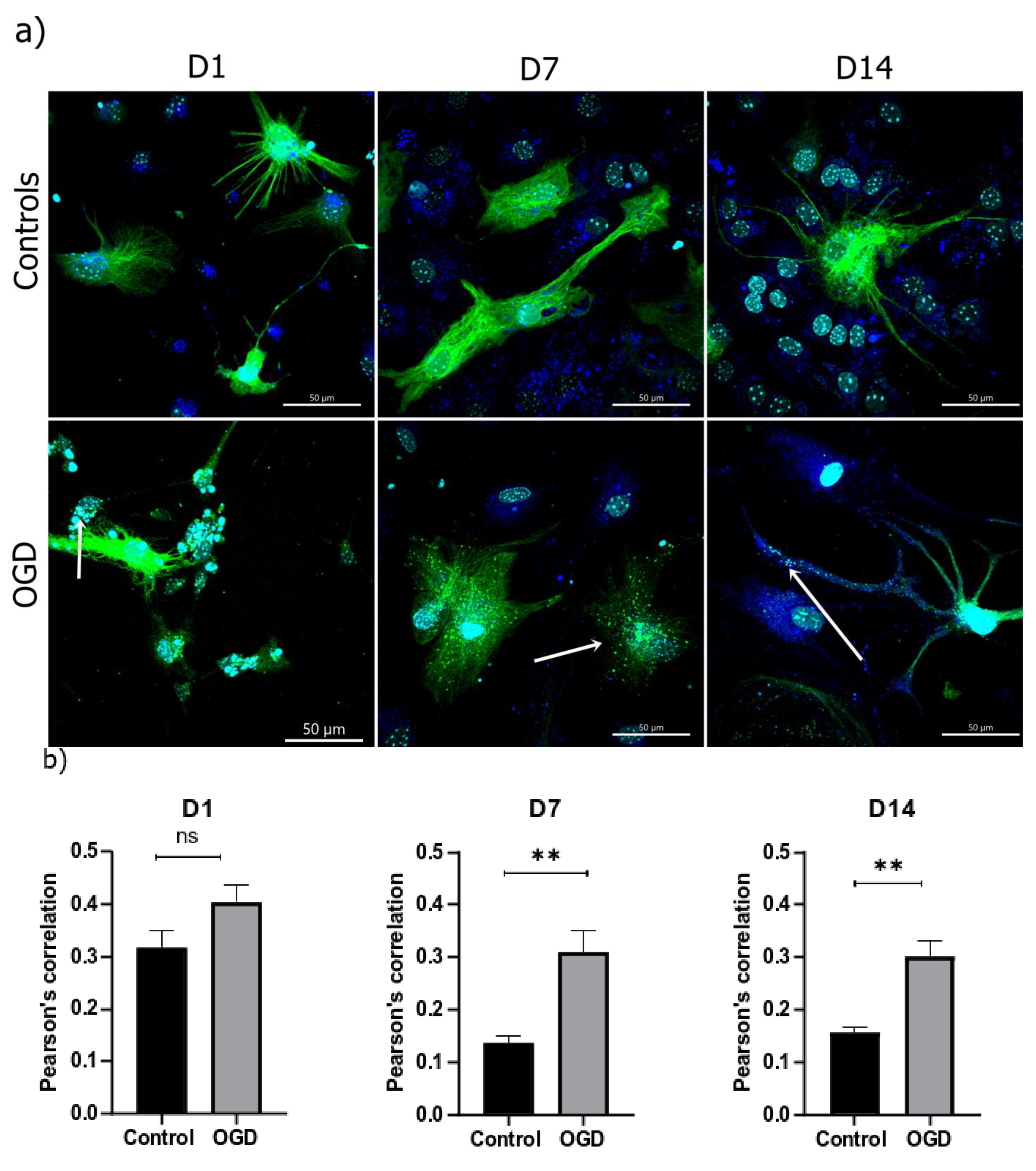
3.5. The Intensity of Autophagy and Mitophagy Depends on the Cells’ Differentiation Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bergström, T.; Forsberg-Nilsson, K. Neural Stem Cells: Brain Building Blocks and Beyond. Upsala J. Med. Sci. 2012, 117, 132–142. [Google Scholar] [CrossRef]
- Malik, S.; Vinukonda, G.; Vose, L.R.; Diamond, D.; Bhimavarapu, B.B.R.; Hu, F.; Zia, M.T.; Hevner, R.; Zecevic, N.; Ballabh, P. Neurogenesis Continues in the Third Trimester of Pregnancy and Is Suppressed by Premature Birth. J. Neurosci. 2013, 33, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Kostović, I.; Radoš, M.; Kostović-Srzentić, M.; Krsnik, Ž. Fundamentals of the Development of Connectivity in the Human Fetal Brain in Late Gestation: From 24 Weeks Gestational Age to Term. J. Neuropathol. Exp. Neurol. 2021, 80, 393–414. [Google Scholar] [CrossRef]
- Kelsch, W.; Mosley, C.P.; Lin, C.W.; Lois, C. Distinct Mammalian Precursors Are Committed to Generate Neurons with Defined Dendritic Projection Patterns. PLoS Biol. 2007, 5, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Shubhakaran, K.P. The Global Burden of Neurologic Diseases. Neurology 2015, 84, 758. [Google Scholar] [CrossRef]
- Weilinger, N.L.; Maslieieva, V.; Bialecki, J.; Sridharan, S.S.; Tang, P.L.; Thompson, R.J. Ionotropic Receptors and Ion Channels in Ischemic Neuronal Death and Dysfunction. Acta Pharmacol. Sin. 2013, 34, 39–48. [Google Scholar] [CrossRef]
- Liang, J.; Han, R.; Zhou, B. Metabolic Reprogramming: Strategy for Ischemic Stroke Treatment by Ischemic Preconditioning. Biology 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Chappell, M.; Park, C.S.; Grau, V.; Payne, S. Modelling of PH Dynamics in Brain Cells after Stroke. Interface Focus 2011, 1, 408–416. [Google Scholar] [CrossRef]
- Descloux, C.; Ginet, V.; Clarke, P.G.H.; Puyal, J.; Truttmann, A.C. Neuronal Death after Perinatal Cerebral Hypoxia-Ischemia: Focus on Autophagy-Mediated Cell Death. Int. J. Dev. Neurosci. 2015, 45, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.O.; Dean, J.M.; Fraser, M.; Wassink, G.; Andelius, T.C.; Dhillon, S.K.; Bennet, L.; Gunn, A.J. Perinatal Brain Injury: Mechanisms and Therapeutic Approaches. Front. Biosci. Landmark 2018, 23, 2204–2226. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Bai, Y.; Xiao, J.; Jiao, H.; He, R. Ginaton Improves Neurological Function in Ischemic Stroke Rats via Inducing Autophagy and Maintaining Mitochondrial Homeostasis. Neuropsychiatr. Dis. Treat. 2019, 15, 1813–1822. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Q.; Peng, Y.; Zhang, X.; Wang, Y.; Shi, L. A Natural Diarylheptanoid Protects Cortical Neurons against Oxygen–Glucose Deprivation-Induced Autophagy and Apoptosis. J. Pharm. Pharmacol. 2019, 71, 1110–1118. [Google Scholar] [CrossRef]
- Puka-Sundvall, M.; Gajkowska, B.; Cholewinski, M.; Blomgren, K.; Lazarewicz, J.W.; Hagberg, H. Subcellular Distribution of Calcium and Ultrastructural Changes after Cerebral Hypoxia-Ischemia in Immature Rats. Dev. Brain Res. 2000, 125, 31–41. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The Mitochondrial Permeability Transition in Cell Death: A Common Mechanism in Necrosis, Apoptosis and Autophagy. Biochim. Biophys. Acta Bioenerg. 1998, 1366, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Leaw, B.; Nair, S.; Lim, R.; Thornton, C.; Mallard, C.; Hagberg, H. Mitochondria, Bioenergetics and Excitotoxicity: New Therapeutic Targets in Perinatal Brain Injury. Front. Cell. Neurosci. 2017, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Demarest, T.G.; Waite, E.L.; Kristian, T.; Puche, A.C.; Waddell, J.; McKenna, M.C.; Fiskum, G. Sex-Dependent Mitophagy and Neuronal Death Following Rat Neonatal Hypoxia-Ischemia. Neuroscience 2016, 335, 103–113. [Google Scholar] [CrossRef]
- Nair, S.; Leverin, A.L.; Rocha-Ferreira, E.; Sobotka, K.S.; Thornton, C.; Mallard, C.; Hagberg, H. Induction of Mitochondrial Fragmentation and Mitophagy after Neonatal Hypoxia–Ischemia. Cells 2022, 11, 1193. [Google Scholar] [CrossRef]
- Ahmad, T.; Aggarwal, K.; Pattnaik, B.; Mukherjee, S.; Sethi, T.; Tiwari, B.K.; Kumar, M.; Micheal, A.; Mabalirajan, U.; Ghosh, B.; et al. Computational Classification of Mitochondrial Shapes Reflects Stress and Redox State. Cell Death Dis. 2013, 4, e461. [Google Scholar] [CrossRef] [PubMed]
- Strizek, B. Perinatal Brain Damage—What the Obstetrician Needs to Know. J. Perinat. Med. 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/36853861/ (accessed on 13 April 2023).
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Wappler, E.A.; Institoris, A.; Dutta, S.; Katakam, P.V.G.; Busija, D.W. Mitochondrial Dynamics Associated with Oxygen-Glucose Deprivation in Rat Primary Neuronal Cultures. PLoS ONE 2013, 8, e63206. [Google Scholar] [CrossRef]
- Laaper, M.; Jahani-Asl, A. Regulation of Neural Stem Cell Fate Decisions by Mitochondrial Dynamics. Neural Regen. Res. 2018, 13, 1548–1549. [Google Scholar] [CrossRef]
- Dubal, D.; Moghe, P.; Verma, R.K.; Uttekar, B.; Rikhy, R. Mitochondrial Fusion Regulates Proliferation and Differentiation in the Type II Neuroblast Lineage in Drosophila. PLoS Genet. 2022, 18, e1010055. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.D.; Pavlik, L.L.; Kirova, Y.I.; Belosludtseva, N.V.; Mosentsov, A.A.; Khmil, N.V.; Germanova, E.L.; Lukyanova, L.D. Effect of Hypoxia on Mitochondrial Enzymes and Ultrastructure in the Brain Cortex of Rats with Different Tolerance to Oxygen Shortage. J. Bioenerg. Biomembr. 2019, 51, 329–340. [Google Scholar] [CrossRef]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Adamson, D.C. Neuronal-Astrocyte Metabolic Interactions: Understanding the Transition into Abnormal Astrocytoma Metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Ganesh, S. Perinuclear Mitochondrial Clustering, Increased ROS Levels, and HIF1 Are Required for the Activation of HSF1 by Heat Stress. J. Cell Sci. 2020, 133, jcs245589. [Google Scholar] [CrossRef]
- Ward, M.W.; Huber, H.J.; Weisová, P.; Düssmann, H.; Nicholls, D.G.; Prehn, J.H.M. Mitochondrial and Plasma Membrane Potential of Cultured Cerebellar Neurons during Glutamate-Induced Necrosis, Apoptosis, and Tolerance. J. Neurosci. 2007, 27, 8238–8249. [Google Scholar] [CrossRef]
- Korenic, A.; Boltze, J.; Deten, A.; Peters, M.; Andjus, P.; Radenovic, L. Astrocytic Mitochondrial Membrane Hyperpolarization Following Extended Oxygen and Glucose Deprivation. PLoS ONE 2014, 9, e90697. [Google Scholar] [CrossRef]
- Katakam, P.V.G.; Dutta, S.; Sure, V.N.; Grovenburg, S.M.; Gordon, A.O.; Peterson, N.R.; Rutkai, I.; Busija, D.W. Depolarization of Mitochondria in Neurons Promotes Activation of Nitric Oxide Synthase and Generation of Nitric Oxide. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1097–H1106. [Google Scholar] [CrossRef]
- Sun, X.; Duan, Y.; Qin, C.; Li, J.C.; Duan, G.; Deng, X.; Ni, J.; Cao, X.; Xiang, K.; Tian, K.; et al. Distinct Multilevel Misregulations of Parkin and PINK1 Revealed in Cell and Animal Models of TDP-43 Proteinopathy. Cell Death Dis. 2018, 9, 953. [Google Scholar] [CrossRef]
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W.; et al. Mitochondrial Outer-Membrane Protein FUNDC1 Mediates Hypoxia-Induced Mitophagy in Mammalian Cells. Nat. Cell Biol. 2012, 14, 177–185. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagečić, D.; Petrović, D.J.; Šimunić, I.; Isaković, J.; Mitrečić, D. The Oxygen and Glucose Deprivation of Immature Cells of the Nervous System Exerts Distinct Effects on Mitochondria, Mitophagy, and Autophagy, Depending on the Cells’ Differentiation Stage. Brain Sci. 2023, 13, 910. https://doi.org/10.3390/brainsci13060910
Jagečić D, Petrović DJ, Šimunić I, Isaković J, Mitrečić D. The Oxygen and Glucose Deprivation of Immature Cells of the Nervous System Exerts Distinct Effects on Mitochondria, Mitophagy, and Autophagy, Depending on the Cells’ Differentiation Stage. Brain Sciences. 2023; 13(6):910. https://doi.org/10.3390/brainsci13060910
Chicago/Turabian StyleJagečić, Denis, Dražen Juraj Petrović, Iva Šimunić, Jasmina Isaković, and Dinko Mitrečić. 2023. "The Oxygen and Glucose Deprivation of Immature Cells of the Nervous System Exerts Distinct Effects on Mitochondria, Mitophagy, and Autophagy, Depending on the Cells’ Differentiation Stage" Brain Sciences 13, no. 6: 910. https://doi.org/10.3390/brainsci13060910
APA StyleJagečić, D., Petrović, D. J., Šimunić, I., Isaković, J., & Mitrečić, D. (2023). The Oxygen and Glucose Deprivation of Immature Cells of the Nervous System Exerts Distinct Effects on Mitochondria, Mitophagy, and Autophagy, Depending on the Cells’ Differentiation Stage. Brain Sciences, 13(6), 910. https://doi.org/10.3390/brainsci13060910






