Real-Time MRI Monitoring of Liquid Embolic Agent (Onyx) Injection in a Swine Arteriovenous Malformation Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animal
2.2. Procedure Description
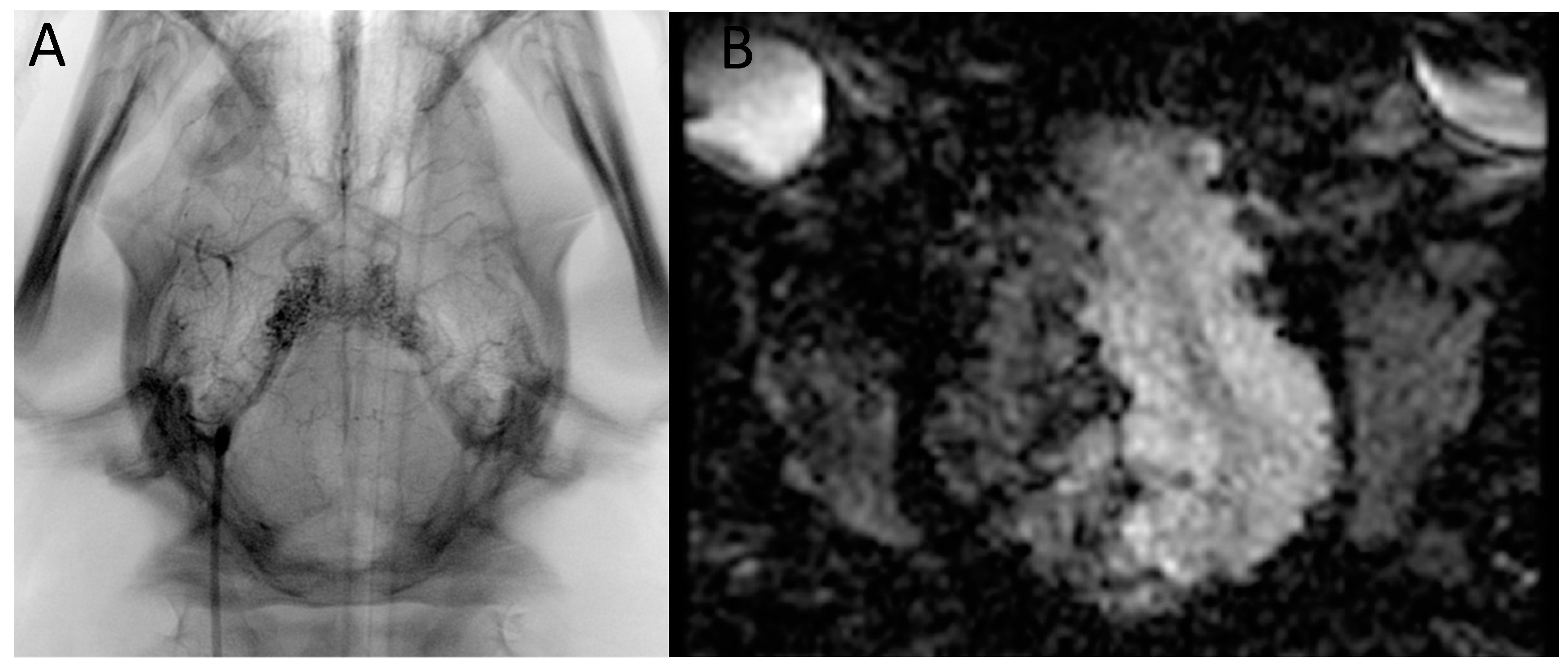
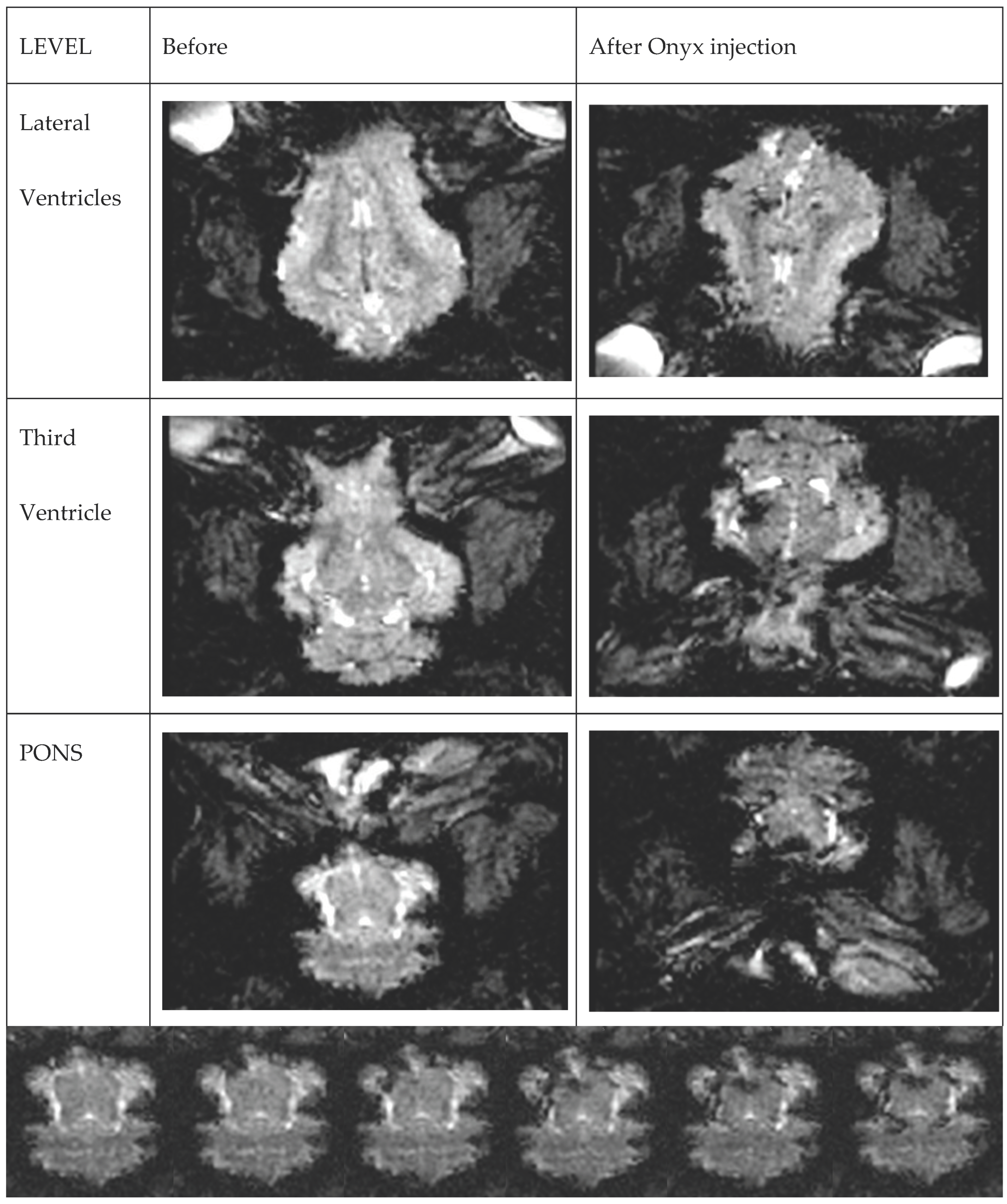
3. Results
4. Discussion
4.1. Presentation of the Image Data
4.2. Animal Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Rooij, W.; Jacobs, S.; Sluzewski, M.; Beute, G.; van der Pol, B. Endovascular Treatment of Ruptured Brain AVMs in the Acute Phase of Hemorrhage. Am. J. Neuroradiol. 2012, 33, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Pierot, L.; Cognard, C.; Herbreteau, D.; Fransen, H.; van Rooij, W.J.; Boccardi, E.; Beltramello, A.; Sourour, N.; Kupcs, K.; Biondi, A.; et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: Results of a prospective, multicentre study (BRAVO). Eur. Radiol. 2013, 23, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Iosif, C.; de Lucena, A.F.; Abreu-Mattos, L.G.; Ala, V.H.E.; El-Ghanam, A.; Saleme, S.; Caire, F.; Mounayer, C. Curative endovascular treatment for low-grade Spetzler-Martin brain arteriovenous malformations: A single-center prospective study. J. NeuroInterv. Surg. 2019, 11, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zawadzki, M. State of the Art in the Role of Endovascular Embolization in the Management of Brain Arteriovenous Malformations—A Systematic Review. J. Clin. Med. 2022, 11, 7208. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Mosimann, P.J.; Nordmeyer, H.; Heddier, M.; Krause, J.; Narata, A.-P.; El Serwi, A.; Stracke, C.P.; Chapot, R. The transvenous retrograde pressure cooker technique for the curative embolization of high-grade brain arteriovenous malformations. J. NeuroInterv. Surg. 2021, 13, 637–641. [Google Scholar] [CrossRef]
- Iosif, C.; Mendes, G.A.C.; Saleme, S.; Ponomarjova, S.; Silveira, E.P.; Caire, F.; Mounayer, C. Endovascular transvenous cure for ruptured brain arteriovenous malformations in complex cases with high Spetzler-Martin grades. J. Neurosurg. 2015, 122, 1229–1238. [Google Scholar] [CrossRef]
- Mendes, G.A.C.; Kalani, M.Y.S.; Iosif, C.; Lucena, A.F.; Carvalho, R.; Saleme, S.; Mounayer, C. Transvenous Curative Embolization of Cerebral Arteriovenous Malformations: A Prospective Cohort Study. Neurosurgery 2018, 83, 957–964. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Saeed, O.; Sahito, S.; Lobanova, I.; Liaqat, J.; Siddiq, F.; Gomez, C.R. Treatment Outcomes of Endovascular Embolization Only in Patients with Unruptured Brain Arteriovenous Malformations: A Subgroup Analysis of ARUBA (A Randomized Trial of Unruptured Brain Arteriovenous Malformations). AJNR Am. J. Neuroradiol. 2020, 41, 676–680. [Google Scholar] [CrossRef]
- Wu, E.M.; El Ahmadieh, T.Y.; McDougall, C.M.; Aoun, S.G.; Mehta, N.; Neeley, O.J.; Plitt, A.; Ban, V.S.; Sillero, R.; White, J.A.; et al. Embolization of brain arteriovenous malformations with intent to cure: A systematic review. J. Neurosurg. 2019, 132, 388–399. [Google Scholar] [CrossRef]
- Sato, K.; Matsumoto, Y.; Tominaga, T.; Satow, T.; Iihara, K.; Sakai, N. Complications of Endovascular Treatments for Brain Arteriovenous Malformations: A Nationwide Surveillance. Am. J. Neuroradiol. 2020, 41, 669–675. [Google Scholar] [CrossRef]
- Moftakhar, P.; Hauptman, J.S.; Malkasian, D.; Martin, N.A. Cerebral arteriovenous malformations. Part 2: Physiology. Neurosurg. Focus 2009, 26, E11. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E.; Tavallaei, M.A.; Pop, M.; Grant, E.K.; Chubb, H.; Rhode, K.; Wright, G.A. Real-time MRI guidance of cardiac interventions. J. Magn. Reson. Imaging 2017, 46, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, H.L.; Schneider, J.; Brockmann, C.; Brockmann, M.A.; Overhoff, D.; Becker, B.V.; Waldeck, S. Radiation exposure during angiographic interventions in interventional radiology—Risk and fate of advanced procedures. Int. J. Radiat. Biol. 2022, 98, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Segal, J.; Borenstein, D.; Jenkins, E.; Cho, S. Prevalence of Spinal Disc Disease Among Interventional Cardiologists. Am. J. Cardiol. 1997, 79, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Stahl, C.M.; Meisinger, Q.C.; Andre, M.P.; Kinney, T.B.; Newton, I.G. Radiation Risk to the Fluoroscopy Operator and Staff. Am. J. Roentgenol. 2016, 207, 737–744. [Google Scholar] [CrossRef]
- Guest, W.; Krings, T. Brain Arteriovenous Malformations. Neuroimaging Clin. N. Am. 2021, 31, 205–222. [Google Scholar] [CrossRef]
- Uecker, M.; Zhang, S.; Voit, D.; Karaus, A.; Merboldt, K.-D.; Frahm, J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010, 23, 986–994. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; O’shea, A.; Weissleder, R. Advances in clinical MRI technology. Sci. Transl. Med. 2019, 11, eaba2591. [Google Scholar] [CrossRef]
- Kholmovski, E.G.; Coulombe, N.; Silvernagel, J.; Angel, N.; Parker, D.; Macleod, R.; Marrouche, N.; Ranjan, R. Real-Time MRI-Guided Cardiac Cryo-Ablation: A Feasibility Study. J. Cardiovasc. Electrophysiol. 2016, 27, 602–608. [Google Scholar] [CrossRef]
- Eitel, C.; Hindricks, G.; Grothoff, M.; Gutberlet, M.; Sommer, P. Catheter ablation guided by real-time MRI. Curr. Cardiol. Rep. 2014, 16, 511. [Google Scholar] [CrossRef]
- Kaneko, N.; Ullman, H.; Ali, F.; Berg, P.; Ooi, Y.C.; Tateshima, S.; Colby, G.P.; Komuro, Y.; Hu, P.; Khatibi, K.; et al. In Vitro Modeling of Human Brain Arteriovenous Malformation for Endovascular Simulation and Flow Analysis. World Neurosurg. 2020, 141, e873–e879. [Google Scholar] [CrossRef]
- Raj, J.A.; Stoodley, M. Experimental Animal Models of Arteriovenous Malformation: A Review. Veter. Sci. 2015, 2, 97–110. [Google Scholar] [CrossRef]
- Chaloupka, J.C.; Viñuela, F.; Robert, J.; Duckwiler, G.R. An in vivo arteriovenous malformation model in swine: Preliminary feasibility and natural history study. Am. J. Neuroradiol. 1994, 15, 945–950. [Google Scholar]
- Haussen, D.C.; Ashour, R.; Johnson, J.N.; Elhammady, M.S.; Peterson, E.C.; Cesar, L.; Bowie, C.; Aziz-Sultan, M.A. Direct continuous measurement of draining vein pressure during Onyx embolization in a swine arteriovenous malformation model. J. NeuroInterv. Surg. 2015, 7, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, R.; Wakhloo, A.K.; Lieber, B.B.; Gounis, M.J.; Divani, A.A.; Hopkins, L.N. Modification of a Previously Described Arteriovenous Malformation Model in the Swine: Endovascular and Combined Surgical/Endovascular Construction and Hemodynamics. Am. J. Neuroradiol. 2000, 21, 1722–1725. [Google Scholar] [PubMed]
- Murayama, Y.; Massoud, T.F.; Viñuela, F. Hemodynamic Changes in Arterial Feeders and Draining Veins during Embolotherapy of Arteriovenous Malformations: An Experimental Study in a Swine Model. Neurosurgery 1998, 43, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.D.; Perkins, E.; Ross, I.B. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with N-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J. Neurosurg. 2003, 98, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Wakhloo, A.K.; Lieber, B.B.; Siekmann, R.; Eber, D.J.; Gounis, M.J. Acute and Chronic Swine Rete Arteriovenous Malformation Models: Hemodynamics and Vascular Remodeling. Am. J. Neuroradiol. 2005, 26, 1702–1706. [Google Scholar]
- Arakawa, H.; Murayama, Y.; Davis, C.; Howard, D.; Baumgardner, W.; Marks, M.; Do, H. Endovascular Embolization of the Swine Rete Mirabile with Eudragit-E 100 Polymer. Am. J. Neuroradiol. 2007, 28, 1191–1196. [Google Scholar] [CrossRef]
- Moftakhar, P.; Lillaney, P.; Losey, A.D.; Cooke, D.L.; Martin, A.J.; Thorne, B.R.H.; Arenson, R.L.; Saeed, M.; Wilson, M.W.; Hetts, S.W. New-Generation Laser-lithographed Dual-Axis Magnetically Assisted Remote-controlled Endovascular Catheter for Interventional MR Imaging: In Vitro Multiplanar Navigation at 1.5 T and 3 T versus X-ray Fluoroscopy. Radiology 2015, 277, 842–852. [Google Scholar] [CrossRef]
- Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M.S.; Gailloud, P.; et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J. Cereb. Blood Flow Metab. 2017, 37, 2346–2358. [Google Scholar] [CrossRef] [PubMed]
- Golubczyk, D.; Kalkowski, L.; Kwiatkowska, J.; Zawadzki, M.; Holak, P.; Glodek, J.; Milewska, K.; Pomianowski, A.; Janowski, M.; Adamiak, Z.; et al. Endovascular model of ischemic stroke in swine guided by real-time MRI. Sci. Rep. 2020, 10, 17318. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, M.; Walecki, J.; Kostkiewicz, B.; Kostyra, K.; Pearl, M.S.; Solaiyappan, M.; Walczak, P.; Janowski, M. Real-time MRI guidance for intra-arterial drug delivery in a patient with a brain tumor: Technical note. BMJ Case Rep. 2019, 12, bcr-2018. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, M.; Walecki, J.; Kostkiewicz, B.; Kostyra, K.; Walczak, P.; Janowski, M. Follow-up of intra-arterial delivery of bevacizumab for treatment of butterfly glioblastoma in patient with first-in-human, real-time MRI-guided intra-arterial neurointervention. J. NeuroInterv. Surg. 2021, 13, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Fomenko, A.; Lozano, A.M. Magnetic Resonance-Guided Focused Ultrasound: Current Status and Future Perspectives in Thermal Ablation and Blood-Brain Barrier Opening. J. Korean Neurosurg. Soc. 2019, 62, 10–26. [Google Scholar] [CrossRef]
- Miller, W.K.; Becker, K.N.; Caras, A.J.; Mansour, T.R.; Mays, M.T.; Rashid, M.; Schwalb, J. Magnetic resonance-guided focused ultrasound treatment for essential tremor shows sustained efficacy: A meta-analysis. Neurosurg. Rev. 2022, 45, 533–544. [Google Scholar] [CrossRef]
- Dallapiazza, R.F.; Timbie, K.F.; Holmberg, S.; Gatesman, J.; Lopes, M.B.; Price, R.J.; Miller, G.W.; Elias, W.J. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J. Neurosurg. 2018, 128, 875–884. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzki, M.; Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Walczak, P.; Gołubczyk, D.; Sady, M.; Gajewski, Z. Real-Time MRI Monitoring of Liquid Embolic Agent (Onyx) Injection in a Swine Arteriovenous Malformation Model. Brain Sci. 2023, 13, 915. https://doi.org/10.3390/brainsci13060915
Zawadzki M, Pinkiewicz M, Pinkiewicz M, Walecki J, Walczak P, Gołubczyk D, Sady M, Gajewski Z. Real-Time MRI Monitoring of Liquid Embolic Agent (Onyx) Injection in a Swine Arteriovenous Malformation Model. Brain Sciences. 2023; 13(6):915. https://doi.org/10.3390/brainsci13060915
Chicago/Turabian StyleZawadzki, Michał, Miłosz Pinkiewicz, Mateusz Pinkiewicz, Jerzy Walecki, Piotr Walczak, Dominika Gołubczyk, Maria Sady, and Zdzisław Gajewski. 2023. "Real-Time MRI Monitoring of Liquid Embolic Agent (Onyx) Injection in a Swine Arteriovenous Malformation Model" Brain Sciences 13, no. 6: 915. https://doi.org/10.3390/brainsci13060915




