Electroacupuncture Relieves Visceral Hypersensitivity via Balancing PAR2 and PAR4 in the Descending Pain Modulatory System of Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Grouping
2.2. Induction of Visceral Hypersensitivity
2.3. Electroacupuncture
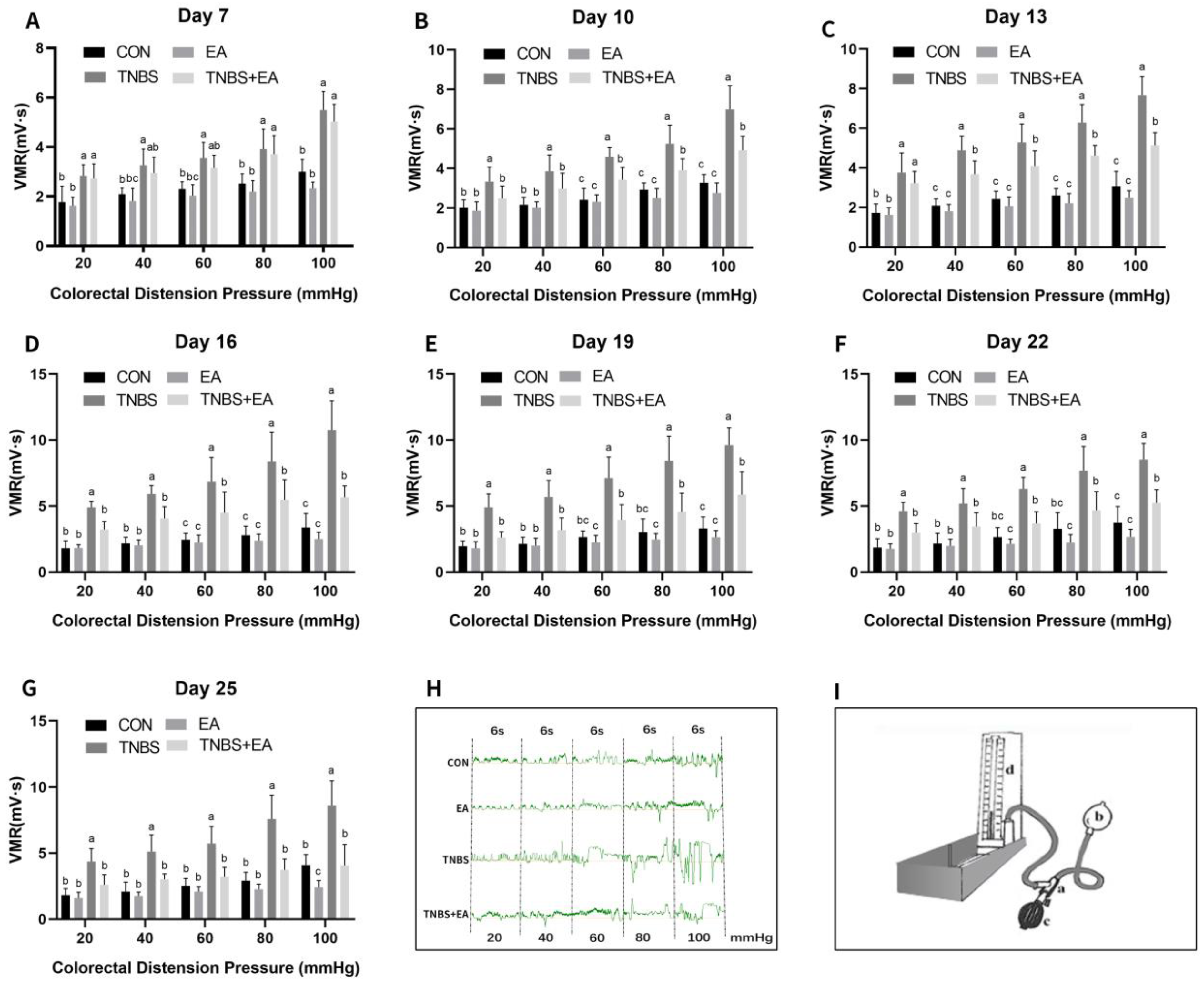
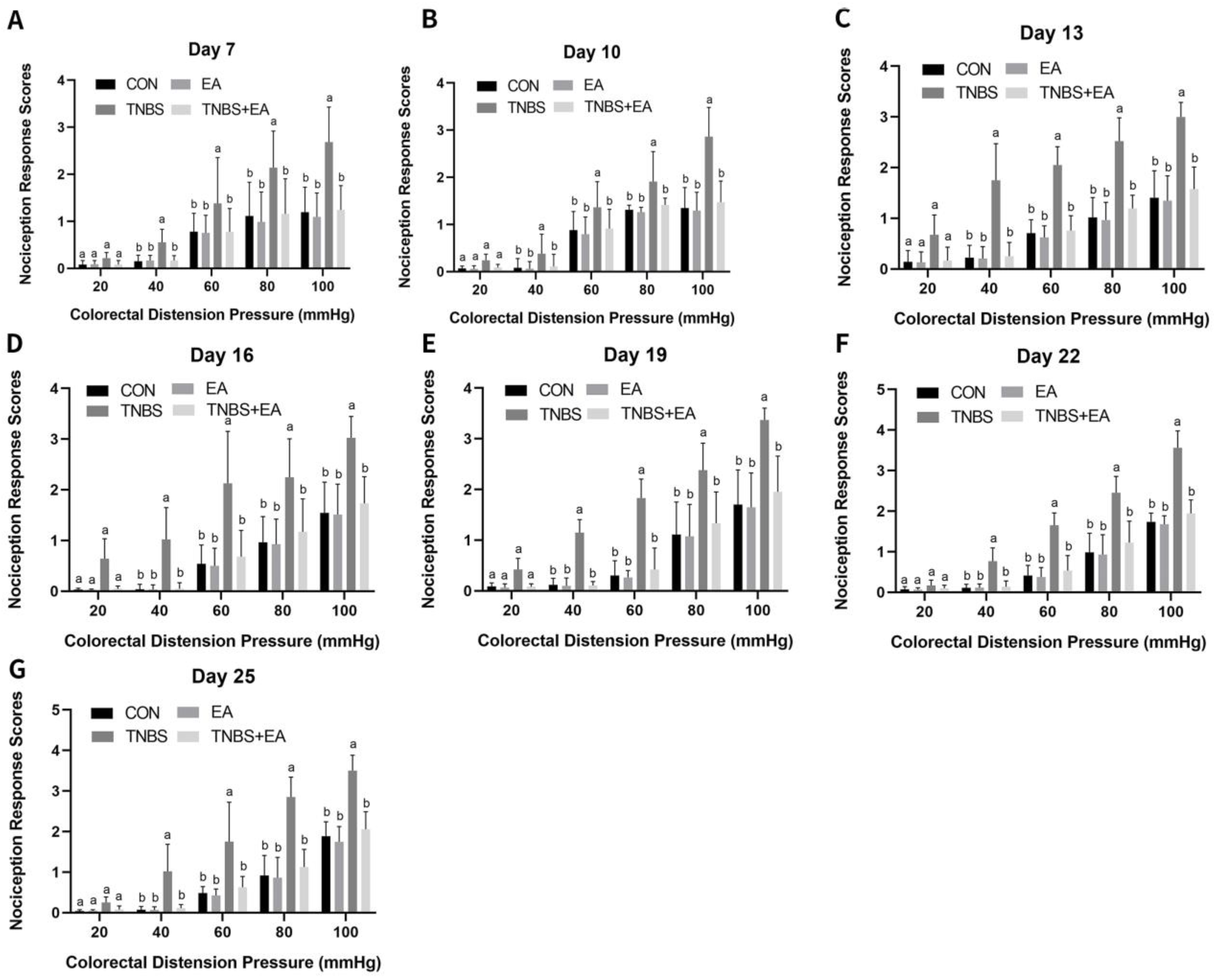
2.4. Visceromotor Response to Colorectal Distension
2.5. Sample Collection
2.6. Assessment of Ileal Inflammation
2.7. Western Blot Analysis
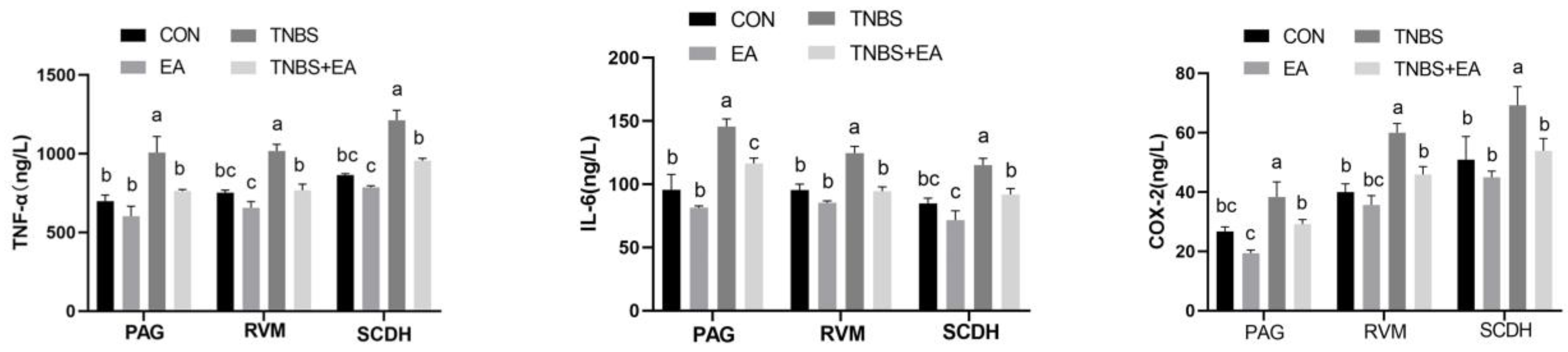
2.8. Measurement of Inflammatory Mediators in the Descending Pain Modulatory System
2.9. Immunohistochemistry and Immunofluorescence
2.10. Real-Time Quantitative PCR
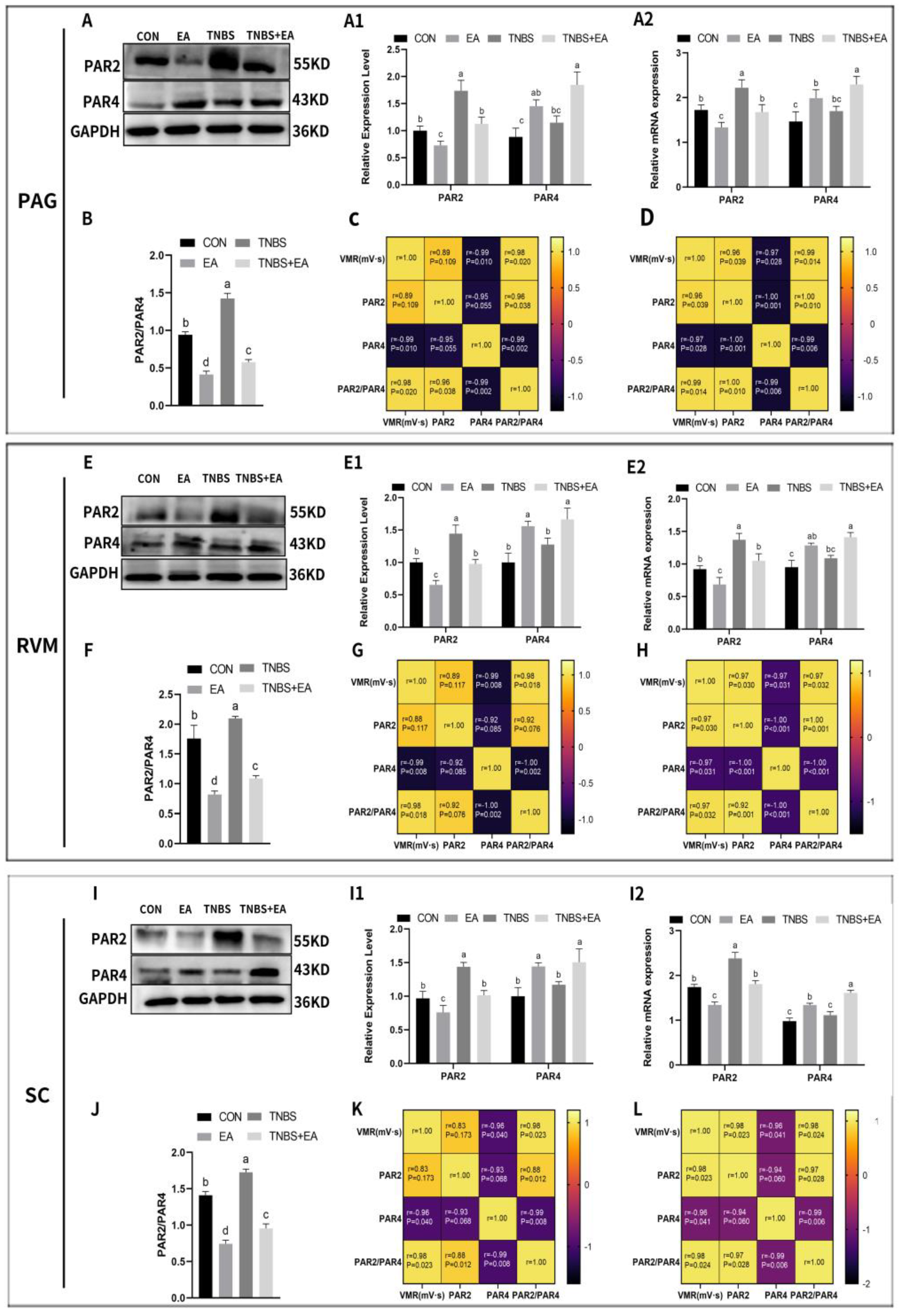
2.11. Correlation Analysis
2.12. Statistical Analysis
3. Results
3.1. TNBS-Induced Ileal Inflammation
3.2. Effect of Repeated Electroacupuncture Treatment on Response to Colorectal Distension
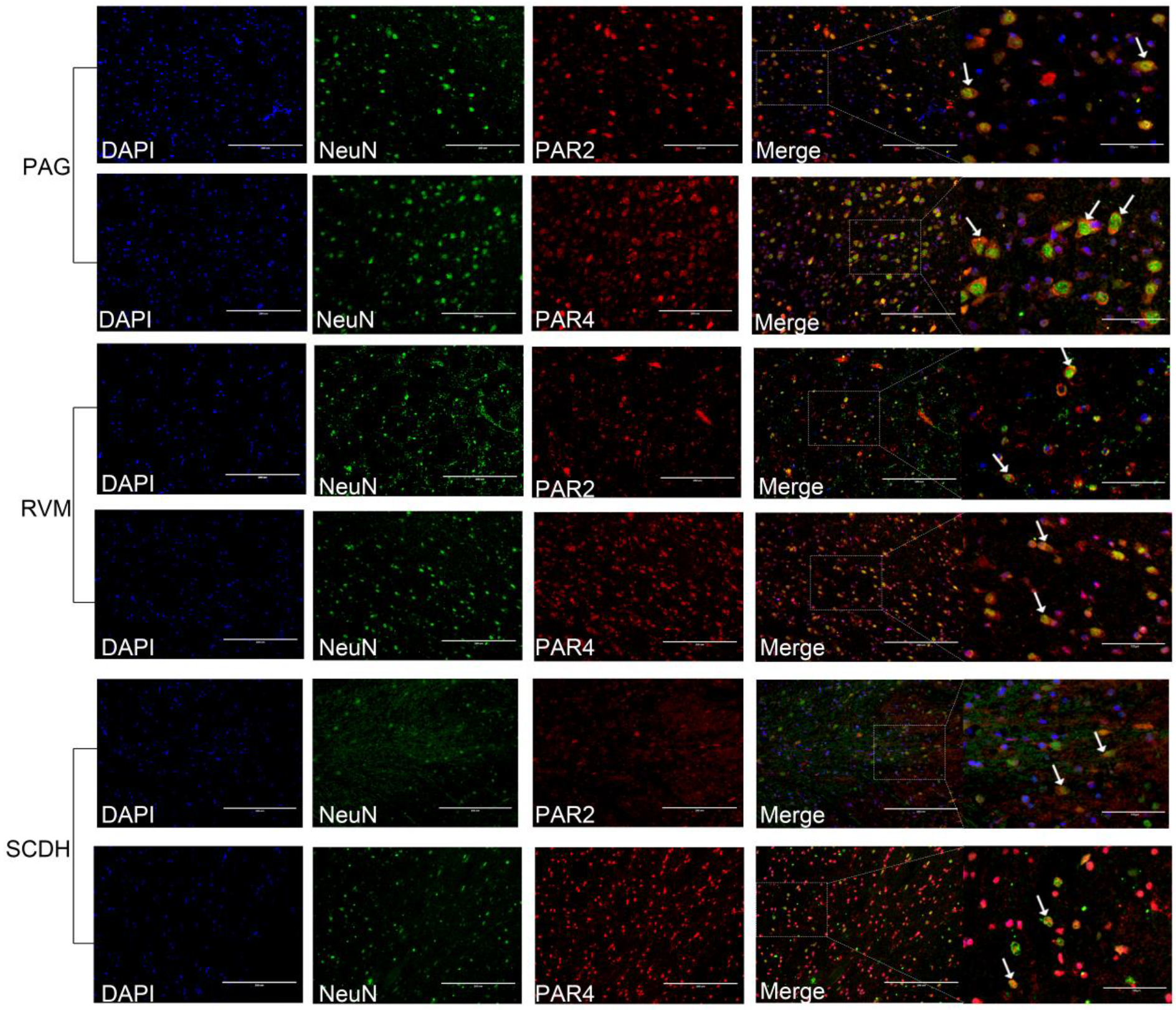
3.3. Effect of Electroacupuncture on PAR2 and PAR4 Expression in the Descending Pain Modulatory System
3.4. Effect of Electroacupuncture on c-Fos, CGRP, and SP Expression in the Descending Pain Modulatory System
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; He, P.; Lu, X.; Guo, Y.; Liu, M.; Li, G.; Ding, J. A Resting-state Functional Magnetic Resonance Imaging Study of Whole-brain Functional Connectivity of Voxel Levels in Patients with Irritable Bowel Syndrome with Depressive Symptoms. J. Neurogastroenterol. Motil. 2021, 27, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiang, W.; Li, C.Y.; Li, S.C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 2016, 22, 10450–10460. [Google Scholar] [CrossRef] [PubMed]
- Aczel, T.; Kecskes, A.; Kun, J.; Szenthe, K.; Banati, F.; Szathmary, S.; Herczeg, R.; Urban, P.; Gyenesei, A.; Gaszner, B.; et al. Hemokinin-1 Gene Expression Is Upregulated in Trigeminal Ganglia in an Inflammatory Orofacial Pain Model: Potential Role in Peripheral Sensitization. Int. J. Mol. Sci. 2020, 21, 2938. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, C.; Wang, Z.; Yang, Z.; Wei, J.; Ren, L.; Deng, Y.; Chen, S.; Ye, Z.; Zang, N.; et al. C-Fiber Degeneration Enhances Alveolar Macrophage-Mediated IFN-α/β Response to Respiratory Syncytial Virus. Microbiol. Spectr. 2022, 10, e0241022. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Materazzi, S.; De Logu, F.; Degl’Innocenti, D.R.; Fusi, C.; Li Puma, S.; Marone, I.M.; Coppi, E.; Holzer, P.; Geppetti, P.; et al. Transient receptor potential ankyrin 1 contributes to somatic pain hypersensitivity in experimental colitis. Sci. Rep. 2020, 10, 8632. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Lu, C.-C.; Huang, C.-L.; Tsai, H.-P.; Wang, W.-T.; Zhang, Z.-H.; Wu, C.-H. Linalyl Acetate Ameliorates Mechanical Hyperalgesia Through Suppressing Inflammation by TSLP/IL-33 Signaling. Neurochem. Res. 2022, 47, 3805–3816. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, J.T.; Santos, B.L.R.; Dias, F.C.; Martinez, A.M.B.; Lima, C.K.F.; Miranda, A.L.P. Long-Chain Omega-3 Fatty Acids Supplementation Accelerates Nerve Regeneration and Prevents Neuropathic Pain Behavior in Mice. Front. Pharmacol. 2017, 8, 723. [Google Scholar] [CrossRef]
- Wang, F.; Xie, N.; Zhou, J.; Dai, M.; Zhang, Q.; Hardiman, P.J.; Qu, F. Molecular mechanisms underlying altered neurobehavioural development of female offspring of mothers with polycystic ovary syndrome: FOS-mediated regulation of neurotrophins in placenta. Ebiomedicine 2020, 60, 102993. [Google Scholar] [CrossRef]
- Ramachandran, R.; Noorbakhsh, F.; DeFea, K.; Hollenberg, M.D. Targeting proteinase-activated receptors: Therapeutic potential and challenges. Nat. Rev. Drug Discov. 2012, 11, 69–86. [Google Scholar] [CrossRef]
- Hanning, N.; De Bruyn, M.; Ceuleers, H.; Boogaerts, T.; Berg, M.; Smet, A.; De Schepper, H.U.; Joossens, J.; van Nuijs, A.L.N.; De Man, J.G.; et al. Local Colonic Administration of a Serine Protease Inhibitor Improves Post-Inflammatory Visceral Hypersensitivity in Rats. Pharmaceutics 2021, 13, 811. [Google Scholar] [CrossRef]
- Schneider, M.A.; Richtmann, S.; Grunding, A.R.; Wrenger, S.; Welte, T.; Meister, M.; Kriegsmann, M.; Winter, H.; Muley, T.; Janciauskiene, S. Transmembrane serine protease 2 is a prognostic factor for lung adenocarcinoma. Int. J. Oncol. 2022, 60, 39. [Google Scholar] [CrossRef]
- Lieu, T.; Savage, E.; Zhao, P.; Edgington-Mitchell, L.; Barlow, N.; Bron, R.; Poole, D.P.; McLean, P.; Lohman, R.J.; Fairlie, D.P.; et al. Antagonism of the proinflammatory and pronociceptive actions of canonical and biased agonists of protease-activated receptor-2. Br. J. Pharmacol. 2016, 173, 2752–2765. [Google Scholar] [CrossRef]
- Shah, M.K.; Ding, Y.; Wan, J.; Janyaro, H.; Tahir, A.H.; Vodyanoy, V.; Ding, M.X. Electroacupuncture intervention of visceral hypersensitivity is involved in PAR-2-activation and CGRP-release in the spinal cord. Sci. Rep. 2020, 10, 11188. [Google Scholar] [CrossRef]
- Kawabata, A.; Kawao, N.; Kitano, T.; Matsunami, M.; Satoh, R.; Ishiki, T.; Masuko, T.; Kanke, T.; Saito, N. Colonic hyperalgesia triggered by proteinase-activated receptor-2 in mice: Involvement of endogenous bradykinin. Neurosci. Lett. 2006, 402, 167–172. [Google Scholar] [CrossRef]
- Vergnolle, N.; Bunnett, N.W.; Sharkey, K.A.; Brussee, V.; Compton, S.J.; Grady, E.F.; Cirino, G.; Gerard, N.; Basbaum, A.I.; Andrade-Gordon, P.; et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat. Med. 2001, 7, 821–826. [Google Scholar] [CrossRef]
- Coelho, A.M.; Vergnolle, N.; Guiard, B.; Fioramonti, J.; Bueno, L. Proteinases and proteinase-activated receptor 2: A possible role to promote visceral hyperalgesia in rats. Gastroenterology 2002, 122, 1035–1047. [Google Scholar] [CrossRef]
- Hao, Y.; Niu, H.; An, S.; Wang, M.; Wang, Z. Downregulation of iNOS, IL-1beta, and P2X7 Expression in Mast Cells via Activation of PAR4 Contributes to the Inhibition of Visceral Hyperalgesia in Rats. J. Immunol. Res. 2018, 2018, 3256908. [Google Scholar] [CrossRef]
- Auge, C.; Balz-Hara, D.; Steinhoff, M.; Vergnolle, N.; Cenac, N. Protease-activated receptor-4 (PAR(4)): A role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol. Motil. 2009, 21, 1189-e107. [Google Scholar] [CrossRef]
- Annahazi, A.; Gecse, K.; Dabek, M.; Ait-Belgnaoui, A.; Rosztoczy, A.; Roka, R.; Molnar, T.; Theodorou, V.; Wittmann, T.; Bueno, L.; et al. Fecal proteases from diarrheic-IBS and ulcerative colitis patients exert opposite effect on visceral sensitivity in mice. Pain 2009, 144, 209–217. [Google Scholar] [CrossRef]
- Annahazi, A.; Dabek, M.; Gecse, K.; Salvador-Cartier, C.; Polizzi, A.; Rosztoczy, A.; Roka, R.; Theodorou, V.; Wittmann, T.; Bueno, L.; et al. Proteinase-activated receptor-4 evoked colorectal analgesia in mice: An endogenously activated feed-back loop in visceral inflammatory pain. Neurogastroenterol. Motility 2012, 24, 76-e13. [Google Scholar] [CrossRef]
- Lv, P.-R.; Su, Y.-S.; He, W.; Wang, X.-Y.; Shi, H.; Zhang, X.-N.; Zhu, B.; Kan, Y.; Chen, L.-Z.; Wu, Q.-F.; et al. Electroacupuncture Alleviated Referral Hindpaw Hyperalgesia via Suppressing Spinal Long-Term Potentiation (LTP) in TNBS-Induced Colitis Rats. Neural Plast. 2019, 2019, 2098083. [Google Scholar] [CrossRef]
- Wan, J.; Qiu, Z.; Ding, Y.; Nan, S.; Ding, M. The Expressing Patterns of Opioid Peptides, Anti-opioid Peptides and Their Receptors in the Central Nervous System Are Involved in Electroacupuncture Tolerance in Goats. Front. Neurosci. 2018, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Ding, Y.; Tahir, A.H.; Shah, M.K.; Janyaro, H.; Li, X.; Zhong, J.; Vodyanoy, V.; Ding, M. Electroacupuncture Attenuates Visceral Hypersensitivity by Inhibiting JAK2/STAT3 Signaling Pathway in the Descending Pain Modulation System. Front. Neurosci. 2017, 11, 644. [Google Scholar] [CrossRef]
- Tahir, A.H.; Wan, J.; Shah, M.K.; Janyaro, H.; Li, X.J.; Ding, M.X. A novel model for studying ileitis-induced visceral hypersensitivity in goats. Acta Vet. Scand. 2016, 58, 72. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Qiu, Z.Y.; Hu, K.; Ding, M.X. Analgesic Neural Circuits Are Activated by Electroacupuncture at Two Sets of Acupoints. Evid. Based Complement. Altern. Med. 2016, 2016, 3840202. [Google Scholar] [CrossRef] [PubMed]
- Janyaro, H.; Wan, J.; Tahir, A.H.; Shah, M.K.; Li, X.J.; Ding, M.X. Visceral pain triggered by traction on the ileocecal ligament with ileitis. J. Pain Res. 2016, 9, 745–755. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Ding, Y.; Cui, L.Y.; Hu, M.L.; Ding, M.X. The Expression Patterns of c-Fos and c-Jun Induced by Different Frequencies of Electroacupuncture in the Brain. Evid. Based Complement. Altern. Med. 2015, 2015, 343682. [Google Scholar] [CrossRef]
- Dean, C.; Geiger, L.K.; Sprtel, B.M.; Ohtake, P.J.; Forster, H.V. An anatomic atlas of the medulla oblongata of the adult goat. J. Appl. Physiol. 1999, 87, 1220–1229. [Google Scholar] [CrossRef]
- West, C.; McVey Neufeld, K.-A. Animal models of visceral pain and the role of the microbiome. Neurobiol. Pain 2021, 10, 100064. [Google Scholar] [CrossRef]
- Ranger, M.; Tremblay, S.; Chau, C.M.Y.; Holsti, L.; Grunau, R.E.; Goldowitz, D. Adverse Behavioral Changes in Adult Mice Following Neonatal Repeated Exposure to Pain and Sucrose. Front. Psychol. 2019, 9, 2394. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, N.; Kim, Y.S.; Nam, R.H.; Ham, M.H.; Lee, H.S.; Jo, W.; Shim, Y.; Choi, Y.J.; Yoon, H.; et al. Repeated Water Avoidance Stress Alters Mucosal Mast Cell Counts, Interleukin-1 beta Levels with Sex Differences in the Distal Colon of Wistar Rats. J. Neurogastroenterol. Motil. 2016, 22, 694–704. [Google Scholar] [CrossRef]
- Lopez-Estevez, S.; Lopez-Torrellardona, J.M.; Parera, M.; Martinez, V. Long-lasting visceral hypersensitivity in a model of DSS-induced colitis in rats. Neurogastroenterol. Motil. 2022, 34, e14441. [Google Scholar] [CrossRef]
- Shah, M.K.; Wan, J.; Janyaro, H.; Tahir, A.H.; Cui, L.Y.; Ding, M.X. Visceral Hypersensitivity Is Provoked by 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Ileitis in Rats. Front. Pharmacol. 2016, 7, 214. [Google Scholar] [CrossRef]
- Merritt, A.M.; Buergelt, C.D.; Sanchez, L.C. Porcine ileitis model induced by TNBS-ethanol instillation. Dig. Dis. Sci. 2002, 47, 879–885. [Google Scholar] [CrossRef]
- Zhou, Q.; Price, D.D.; Caudle, R.M.; Verne, G.N. Visceral and somatic hypersensitivity following TNBS-induced in a subset of rats colitis. Pain 2008, 134, 9–15. [Google Scholar] [CrossRef]
- Weng, Z.-J.; Hu, S.-X.; Zhang, F.; Zhang, Z.-Y.; Zhou, Y.; Zhao, M.; Huang, Y.; Xin, Y.-H.; Wu, H.-G.; Liu, H.-R. Spinal cord astrocyte P2X7Rs mediate the inhibitory effect of electroacupuncture on visceral hypersensitivity of rat with irritable bowel syndrome. Purinergic Signal. 2023, 19, 43–53. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Ding, G.; Chen, Y.; Wu, H.; Li, N.; Zhou, E.; Qin, X.; Yuan, L. Effects of electroacupuncture on c-Fos expression in the spinal cord and brain of rats with chronic visceral hypersensitivity. Neural Regen. Res. 2009, 4, 339–343. [Google Scholar] [CrossRef]
- Weng, Z.; Wu, L.; Lu, Y.; Wang, L.; Tan, L.; Dong, M.; Xin, Y. Electroacupuncture diminishes P2X(2) and P2X(3) purinergic receptor expression in dorsal root ganglia of rats with visceral hypersensitivity. Neural Regen. Res. 2013, 8, 802–808. [Google Scholar] [CrossRef]
- Siwiec, R.M.; Babaei, A.; Kern, M.; Samuel, E.A.; Li, S.J.; Shaker, R. Esophageal acid stimulation alters insular cortex functional connectivity in gastroesophageal reflux disease. Neurogastroenterol. Motil. 2015, 27, 201–211. [Google Scholar] [CrossRef]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef]
- Nie, L.; Sun, K.; Gong, Z.; Li, H.; Quinn, J.P.; Wang, M. Src Family Kinases Facilitate the Crosstalk between CGRP and Cytokines in Sensitizing Trigeminal Ganglion via Transmitting CGRP Receptor/PKA Pathway. Cells 2022, 11, 3498. [Google Scholar] [CrossRef] [PubMed]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [PubMed]
- Do, J.; Woo, J. From Gut to Brain: Alteration in Inflammation Markers in the Brain of Dextran Sodium Sulfate-induced Colitis Model Mice. Clin. Psychopharmacol. Neurosci. 2018, 16, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Zonis, S.; Pechnick, R.N.; Ljubimov, V.A.; Mahgerefteh, M.; Wawrowsky, K.; Michelsen, K.S.; Chesnokova, V. Chronic intestinal inflammation alters hippocampal neurogenesis. J. Neuroinflammation 2015, 12, 65. [Google Scholar] [CrossRef]
- Cenac, N.; Garcia-Villar, R.; Ferrier, L.; Larauche, M.; Vergnolle, N.; Bunnett, N.W.; Coelho, A.M.; Fioramonti, J.; Bueno, L. Proteinase-activated receptor-2-induced colonic inflammation in mice: Possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J. Immunol. 2003, 170, 4296–4300. [Google Scholar] [CrossRef]
- Mrozkova, P.; Spicarova, D.; Palecek, J. Spinal PAR2 Activation Contributes to Hypersensitivity Induced by Peripheral Inflammation in Rats. Int. J. Mol. Sci. 2021, 22, 991. [Google Scholar] [CrossRef]
- Shah, M. Intervention of PAR-2 Mediated CGRP in Animal Model of Visceral Hyperalgesia. In Animal Models and Experimental Research in Medicine; Mahmut, K., Volkan, G., Abdulsamed, K., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Cui, L.Y.; Guo, N.N.; Li, Y.L.; Li, M.; Ding, M.X. Analgesic and physiological effect of electroacupuncture combined with epidural lidocaine in goats. Vet. Anaesth. Analg. 2017, 44, 959–967. [Google Scholar] [CrossRef]
- Sun, J.H.; Wu, X.L.; Meng, Y.F.; Cheng, J.; Ning, H.X.; Peng, Y.J.; Pei, L.X.; Zhang, W. Electro-acupuncture decreases 5-HT, CGRP and increases NPY in the brain-gut axis in two rat models of Diarrhea-predominant irritable bowel syndrome(D-IBS). BMC Complement. Altern. Med. 2015, 15, 340. [Google Scholar] [CrossRef]
- Chen, S.P.; Wang, S.B.; Rong, P.J.; Wang, J.Y.; Qiao, L.N.; Feng, X.M.; Liu, J.L.; Zhang, J.L. Acupuncture for Visceral Pain: Neural Substrates and Potential Mechanisms. Evid. Based Complement. Altern. Med. 2014, 2014, 609594. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, Z.K.; Liu, R.; Wang, H.B.; Gu, C.Y.; Luo, H.M.; Wang, H.; Du, M.H.; Lv, Y.; Shi, X. Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J. Gastroenterol. 2015, 21, 1468–1478. [Google Scholar] [CrossRef]
- Kim, D.-H.; Ryu, Y.; Hahm, D.H.; Sohn, B.Y.; Shim, I.; Kwon, O.S.; Chang, S.; Gwak, Y.S.; Kim, M.S.; Kim, J.H.; et al. Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci. Rep. 2017, 7, 15214. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, F.; Gan, X.; Yang, X.; Zhou, L.; Chen, J.; He, Y.; Zhang, R.; Zhu, B.; Liu, L. The Involvement of Descending Pain Inhibitory System in Electroacupuncture-Induced Analgesia. Front. Integr. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef]
- Farrell, K.E.; Rank, M.M.; Keely, S.; Brichta, A.M.; Graham, B.A.; Callister, R.J. In Vivo characterization of colorectal and cutaneous inputs to lumbosacral dorsal horn neurons in the mouse spinal cord. Neuroscience 2016, 316, 13–25. [Google Scholar] [CrossRef]
- Lyubashina, O.A.; Sivachenko, I.B.; Mikhalkin, A.A. Impaired visceral pain-related functions of the midbrain periaqueductal gray in rats with colitis. Bull. B.R. 2022, 182, 12–25. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Xin, J.; Lao, L.; Ren, K.; Berman, B.M.; Zhang, R.X. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007, 1186, 171–179. [Google Scholar] [CrossRef]
- Chen, T.; Taniguchi, W.; Chen, Q.-Y.; Tozaki-Saitoh, H.; Song, Q.; Liu, R.-H.; Koga, K.; Matsuda, T.; Kaito-Sugimura, Y.; Wang, J.; et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 2018, 9, 1886. [Google Scholar] [CrossRef]
- Qi, D.B.; Wu, S.Q.; Zhang, Y.H.; Li, W.M. Electroacupuncture analgesia with different frequencies is mediated via different opioid pathways in acute visceral hyperalgesia rats. Life Sci. 2016, 160, 64–71. [Google Scholar] [CrossRef]
- Song, S.; An, J.; Li, Y.; Liu, S. Electroacupuncture at ST-36 ameliorates DSS-induced acute colitis via regulating macrophage polarization induced by suppressing NLRP3/IL-1 beta and promoting Nrf2/HO-1. Mol. Immunol. 2019, 106, 143–152. [Google Scholar] [CrossRef]
- Zhang, H.; He, W.; Hu, X.-F.; Li, Y.-Z.; Liu, Y.-M.; Ge, W.-Q.; Zhanmu, O.-Y.; Chen, C.; Lan, Y.-Y.; Su, Y.-S.; et al. Electroacupuncture Reduces Visceral Pain Via Cannabinoid CB2 Receptors in a Mouse Model of Inflammatory Bowel Disease. Front. Pharmacol. 2022, 13, 861799. [Google Scholar] [CrossRef]
- Russell, F.A.; Zhan, S.; Dumas, A.; Lagarde, S.; Pouliot, M.; McDougall, J.J. The pronociceptive effect of proteinase-activated receptor-4 stimulation in rat knee joints is dependent on mast cell activation. Pain 2011, 152, 354–360. [Google Scholar] [CrossRef]
- Han, Y.L.; Peng, X.M.; Zhang, H.X.; Chen, S.; Zhang, L.Y. Electroacupuncture Regulates TRPV1 through PAR2/PKC Pathway to Alleviate Visceral Hypersensitivity in FD Rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 1975228. [Google Scholar] [CrossRef] [PubMed]





| Name | Accession Number | Primer Sequence |
|---|---|---|
| PAR-2 | XM_018053694.1 | F:5′- GATCTGCTTCACGCCCAGTA-3′ |
| R:5′-CCGGAAGTCCTGTGAAACGA-3′ | ||
| PAR-4 | XM_018051355.1 | F:5′- CTGCTGCTGCACTTCTCAAAC-3′ |
| R:5′-ATAGATGAAGGGGTCCACGC-3′ | ||
| SP | XM_005686308.3 | F:5′- CAGCGACCAGATCAAGGAGG-3′ |
| R:5′-CATGTCCAGCATCCCGTTTG-3′ | ||
| CGRP | XM_005697758.3 | F:5′- GATATGGAAGTGAAGGATGCC-3′A |
| R:5′-ACAATCTCAGGACTCTGGTGC-3′ | ||
| c-Fos | XM_018063375.1 | F:5′- CTTCAACGCCGACTACGAGG-3′ |
| R:5′-TCTGCCGGTGAGTGGTAGTA-3′ | ||
| GAPDH | XM_005680968.3 | F:5′- CTGCTGCTGCACTTCTCAAAC-3′ |
| R:5′-ATAGATGAAGGGGTCCACGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Zhang, Q.; Nan, S.; Wang, H.; Ma, N.; Kiani, F.A.; Ding, M.; Chen, J. Electroacupuncture Relieves Visceral Hypersensitivity via Balancing PAR2 and PAR4 in the Descending Pain Modulatory System of Goats. Brain Sci. 2023, 13, 922. https://doi.org/10.3390/brainsci13060922
Guo P, Zhang Q, Nan S, Wang H, Ma N, Kiani FA, Ding M, Chen J. Electroacupuncture Relieves Visceral Hypersensitivity via Balancing PAR2 and PAR4 in the Descending Pain Modulatory System of Goats. Brain Sciences. 2023; 13(6):922. https://doi.org/10.3390/brainsci13060922
Chicago/Turabian StyleGuo, Panpan, Qiulin Zhang, Sha Nan, Haolong Wang, Ning Ma, Faisal Ayub Kiani, Mingxing Ding, and Jianguo Chen. 2023. "Electroacupuncture Relieves Visceral Hypersensitivity via Balancing PAR2 and PAR4 in the Descending Pain Modulatory System of Goats" Brain Sciences 13, no. 6: 922. https://doi.org/10.3390/brainsci13060922
APA StyleGuo, P., Zhang, Q., Nan, S., Wang, H., Ma, N., Kiani, F. A., Ding, M., & Chen, J. (2023). Electroacupuncture Relieves Visceral Hypersensitivity via Balancing PAR2 and PAR4 in the Descending Pain Modulatory System of Goats. Brain Sciences, 13(6), 922. https://doi.org/10.3390/brainsci13060922





