The Effect of Transcranial Electrical Stimulation on the Recovery of Sleep Quality after Sleep Deprivation Based on an EEG Analysis
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Participants
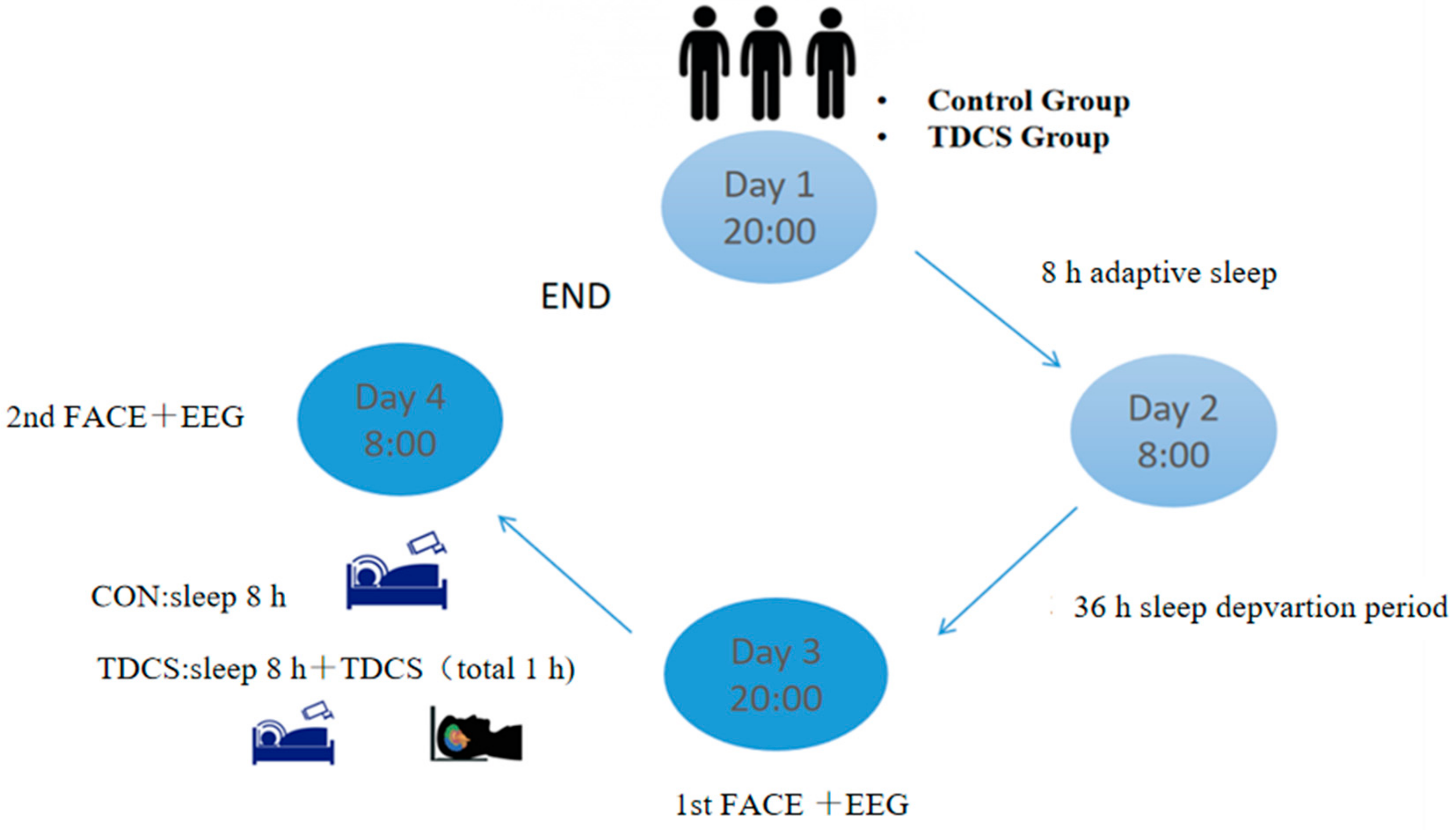
2.3. Experimental Procedures
2.4. Electrical Stimulation
2.5. Data Recording and Preprocessing
2.6. Statistical Analysis
3. Results
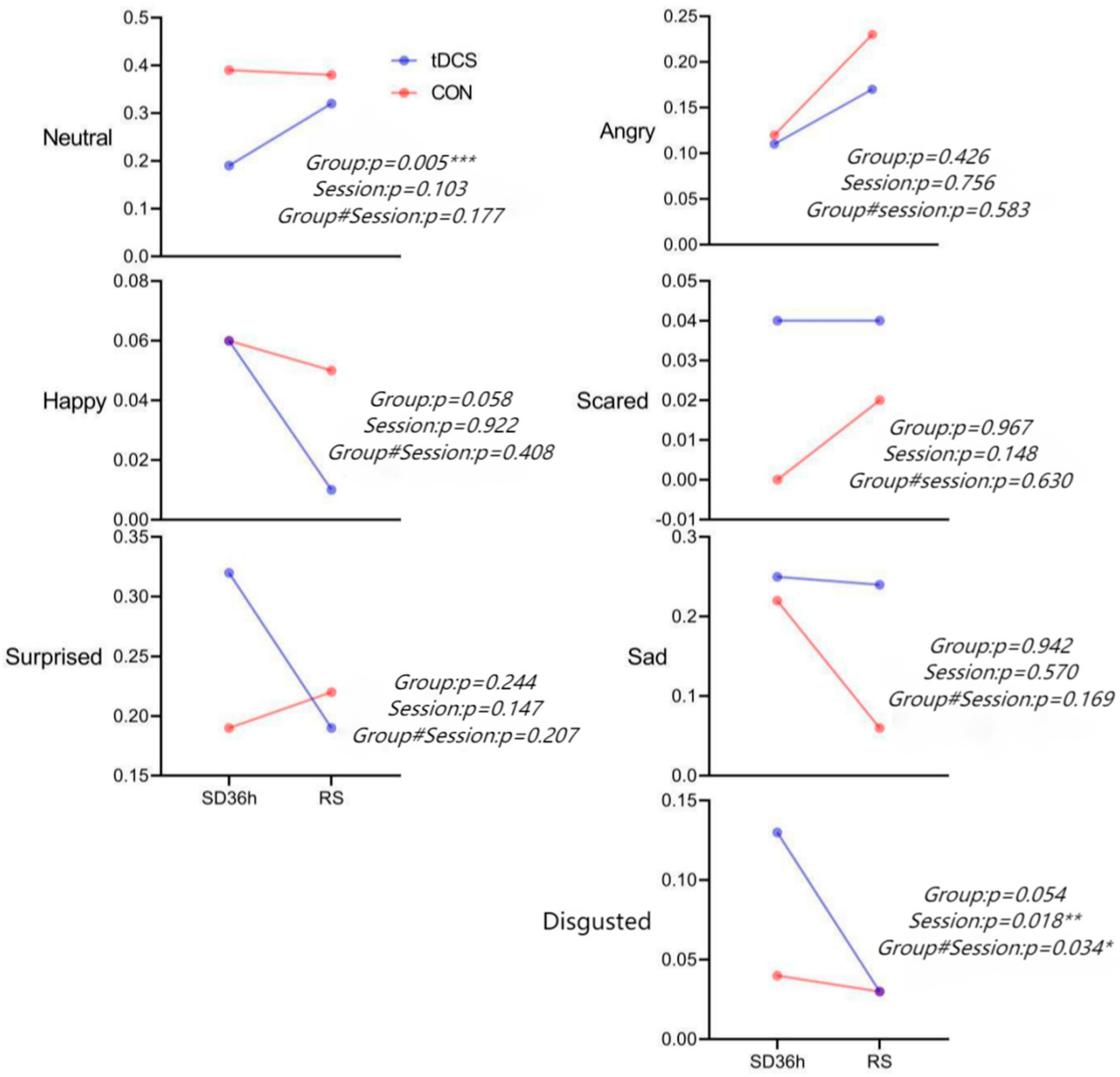
3.1. Facial Expression Results
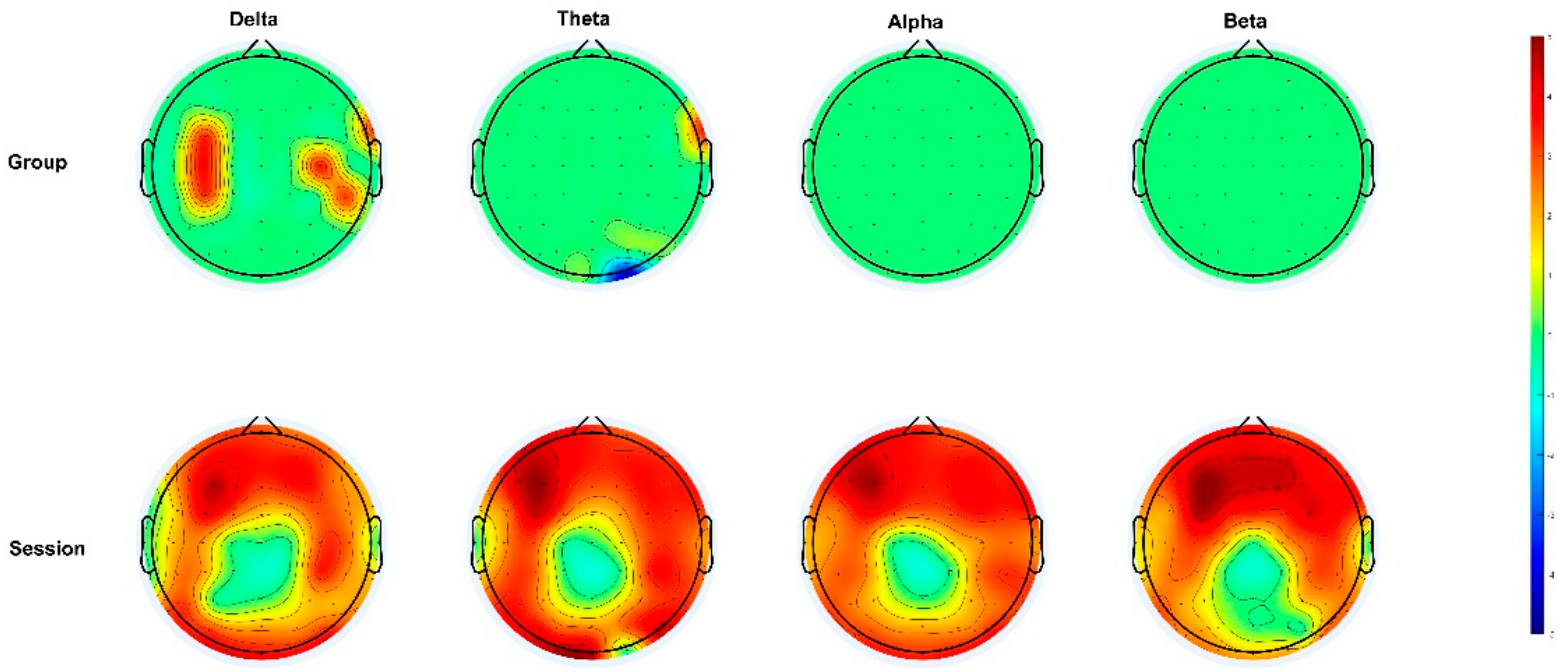
3.2. Power Spectrum Analysis
3.3. Correlation Analysis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SD | Sleep deprivation |
| RS | Restorative sleep |
| TDCS | Transcranial direct current stimulation |
| BC | Blank control |
| DLPFC | Dorsolateral prefrontal cortex |
| TMS | Transcranial magnetic stimulation |
References
- Antza, C.; Kostopoulos, G.A.-O.X.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125. [Google Scholar] [CrossRef]
- Liu, H.; Chen, A. Roles of sleep deprivation in cardiovascular dysfunctions. Life Sci. 2019, 219, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Korostovtseva, L.; Bochkarev, M.; Sviryaev, Y. Sleep and Cardiovascular Risk. Sleep Med. Clin. 2021, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.A.-O.; Krone, L.B.; Wulff, K.; Nissen, C.A.-O. Sleep, insomnia, and depression. Neuropsychopharmacology 2020, 45, 74–85. [Google Scholar] [CrossRef]
- Siccoli, M.M.; Valko, P.O.; Hermann, D.M.; Bassetti, C.L. Central periodic breathing during sleep in 74 patients with acute ischemic stroke—Neurogenic and cardiogenic factors. J. Neurol 2008, 255, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.L.; Burr, R.L.; Buchanan, D.T.; Pike, K.C.; Kamp, K.J.; Heitkemper, M.M. Indirect effect of sleep on abdominal pain through daytime dysfunction in adults with irritable bowel syndrome. J. Clin. Sleep Med. 2020, 16, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Hudson, A.N.; Van Dongen, H.A.-O.; Honn, K.A. Sleep deprivation, vigilant attention, and brain function: A review. Neuropsychopharmacology 2020, 45, 21–30. [Google Scholar] [CrossRef]
- Gibbings, A.; Ray, L.B.; Berberian, N.; Nguyen, T.; Shahidi Zandi, A.; Owen, A.M.; Comeau, F.J.E.; Fogel, S.M. EEG and behavioural correlates of mild sleep deprivation and vigilance. Clin. Neurophysiol. 2021, 132, 45–55. [Google Scholar] [CrossRef]
- Zeng, H.R.; Xu, F.; Zhang, J.; Cao, Q.F.; Wang, Y.H.; Zhang, P.; Shao, Y.C.; Wu, S.P.; Weng, X.A.-O. Vigilant Attention, Cerebral Blood Flow and Grey Matter Volume Change after 36 h of Acute Sleep Deprivation in Healthy Male Adults: A Pilot Study. Brain Sci. 2022, 12, 1534. [Google Scholar] [CrossRef]
- Elmenhorst, E.M.; Elmenhorst, D.A.-O.X.; Benderoth, S.; Kroll, T.; Bauer, A.; Aeschbach, D. Cognitive impairments by alcohol and sleep deprivation indicate trait characteristics and a potential role for adenosine A(1) receptors. Proc. Natl. Acad. Sci. USA 2018, 115, 8009–8014. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Dinges, D.F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010, 136, 375–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trockel, M.T.; Menon, N.K.; Rowe, S.G.; Stewart, M.T.; Smith, R.; Lu, M.; Kim, P.K.; Quinn, M.A.; Lawrence, E.; Marchalik, D.; et al. Assessment of Physician Sleep and Wellness, Burnout, and Clinically Significant Medical Errors. Jama Netw. Open 2020, 3, e2028111. [Google Scholar] [CrossRef]
- Lunde, L.K.; Skare, Ø.; Mamen, A.; Sirnes, P.A.; Aass, H.C.D.; Øvstebø, R.; Goffeng, E.; Matre, D.; Nielsen, P.; Heglum, H.S.A.; et al. Cardiovascular Health Effects of Shift Work with Long Working Hours and Night Shifts: Study Protocol for a Three-Year Prospective Follow-Up Study on Industrial Workers. Int. J. Environ. Res. Public Health 2020, 17, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, D.A.; Satterfield, B.C.; Layton, M.E.; Van Dongen, H.P.A. Sleep Deprivation and Sleep-Onset Insomnia are Associated with Blunted Physiological Reactivity to Stressors. Mil. Med. 2021, 186, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Gast, H.; Schindler, K.; Rummel, C.; Herrmann, U.S.; Roth, C.; Hess, C.W.; Mathis, J. EEG correlation and power during maintenance of wakefulness test after sleep-deprivation. Clin. Neurophysiol. 2011, 122, 2025–2031. [Google Scholar] [CrossRef]
- Canali, P.; Casarotto, S.; Rosanova, M.; Sferrazza-Papa, G.; Casali, A.G.; Gosseries, O.; Massimini, M.; Smeraldi, E.; Colombo, C.; Benedetti, F. Abnormal brain oscillations persist after recovery from bipolar depression. Eur. Psychiatry 2017, 41, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Bersagliere, A.; Pascual-Marqui, R.D.; Tarokh, L.; Achermann, P.A.-O. Mapping Slow Waves by EEG Topography and Source Localization: Effects of Sleep Deprivation. Brain Topogr. 2018, 31, 257–269. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, C.; Yu, H.; Zhang, Y.; Liu, Z.; Hu, Z.; Yuan, T.F.; Zhou, D. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. 2020, 70, 17–26. [Google Scholar] [CrossRef]
- Doerig, N.; Seinsche, R.J.; Moisa, M.; Seifritz, E.; Ruff, C.C.; Kleim, B. Enhancing reappraisal of negative emotional memories with transcranial direct current stimulation. Sci. Rep. 2021, 11, 14760. [Google Scholar] [CrossRef]
- Hone-Blanchet, A.; Edden, R.A.; Fecteau, S. Online Effects of Transcranial Direct Current Stimulation in Real Time on Human Prefrontal and Striatal Metabolites. Biol. Psychiatry 2016, 80, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.; da Silva, K.M.; Leathlean, C.; Jiang, H.; Ang, C.S.; Searle, R. Investigation of physical activity, sleep, and mental health recovery in treatment resistant depression (TRD) patients receiving repetitive transcranial magnetic stimulation (rTMS) treatment. J. Affect Disord. Rep. 2022, 8, 100337. [Google Scholar] [CrossRef]
- Nardone, R.; Sebastianelli, L.; Versace, V.; Brigo, F.; Golaszewski, S.; Pucks-Faes, E.; Saltuari, L.; Trinka, E. Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 2020, 71, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. tDCS for the treatment of depression: A comprehensive review. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Nalven, M.; Ault, A.; Eskenazi, M.A. tDCS as a treatment for anxiety and related cognitive deficits. Int. J. Psychophysiol. 2020, 158, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.J.; Brand, B.A.-O.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Skiendziel, T.; Rösch, A.G.; Schultheiss, O.A.-O. Assessing the convergent validity between the automated emotion recognition software Noldus FaceReader 7 and Facial Action Coding System Scoring. PLoS ONE 2019, 14, e0223905. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.; Klumpp, H. Sleep quality and emotion recognition in individuals with and without internalizing psychopathologies. J. Behav. Ther. Exp. Psychiatry 2022, 75, 101719. [Google Scholar] [CrossRef]
- Lee, H.J.; Stein, M.B. Update on treatments for anxiety-related disorders. Curr. Opin. Psychiatry 2023, 36, 140–145. [Google Scholar] [CrossRef]
- Rao, V.R.; Sellers, K.K.; Wallace, D.L.; Lee, M.B.; Bijanzadeh, M.; Sani, O.G.; Yang, Y.; Shanechi, M.M.; Dawes, H.E.; Chang, E.F. Direct Electrical Stimulation of Lateral Orbitofrontal Cortex Acutely Improves Mood in Individuals with Symptoms of Depression. Curr. Biol. 2018, 28, 3893–3902.e4. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhai, S.; Guo, D.; Chen, S.; He, Y.; Ke, Y.; Ming, D. Transcranial Direct Current Stimulation Over the Left Dorsolateral Prefrontal Cortex Reduced Attention Bias Toward Negative Facial Expression: A Pilot Study in Healthy Subjects. Front. Neurosci. 2022, 419, 34–45. [Google Scholar] [CrossRef]
- Pellegrino, G.A.-O.; Maran, M.A.-O.; Turco, C.; Weis, L.A.-O.; Di Pino, G.; Piccione, F.A.-O.X.; Arcara, G. Bilateral Transcranial Direct Current Stimulation Reshapes Resting-State Brain Networks: A Magnetoencephalography Assessment. Neural. Plast. 2018, 2018, 2782804. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Jin, J.N.; Wang, X.; Qi, H.Z.; Liu, Z.P.; Yin, T. Theta and Alpha Oscillations during the Retention Period of Working Memory by rTMS Stimulating the Parietal Lobe. Front. Behav. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef]
- Gorgoni, M.; Ferlazzo, F.; Moroni, F.; D’Atri, A.; Donarelli, S.; Fanelli, S.; Gizzi Torriglia, I.; Lauri, G.; Ferrara, M.; Marzano, C.; et al. Sleep deprivation affects somatosensory cortex excitability as tested through median nerve stimulation. Brain Stimul. 2014, 7, 732–739. [Google Scholar] [CrossRef]
- Badawy, R.A.; Loetscher, T.; Macdonell, R.A.L.; Brodtmann, A. Cortical excitability and neurology: Insights into the pathophysiology. Funct. Neurol. 2012, 27, 131. [Google Scholar]
- Chellappa, S.L.; Gaggioni, G.; Ly, J.Q.; Papachilleos, S.; Borsu, C.; Brzozowski, A.; Rosanova, M.; Sarasso, S.; Luxen, A.; Middleton, B.; et al. Circadian dynamics in measures of cortical excitation and inhibition balance. Sci. Rep. 2016, 6, 33661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, C.A.-O.; Huang, Y.A.-O.X.; Herrero, J.L.; Fini, M.A.-O.; Du, V.; Lado, F.A.-O.; Honey, C.A.-O.; Mehta, A.A.-O. Induction and Quantification of Excitability Changes in Human Cortical Networks. J. Neurosci. 2018, 38, 5384–5398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehinejad, M.A.-O.; Ghanavati, E.A.-O.; Reinders, J.; Hengstler, J.G.; Kuo, M.A.-O.; Nitsche, M.A.-O. Sleep-dependent upscaled excitability, saturated neuroplasticity, and modulated cognition in the human brain. Elife 2022, 11, e69308. [Google Scholar] [CrossRef]
- Xie, J.; Wang, L.; Xiao, C.; Ying, S.; Ren, J.; Chen, Z.; Yu, Y.; Xu, D.; Yao, D.; Wu, B.; et al. Low Frequency Transcranial Alternating Current Stimulation Accelerates Sleep Onset Process. IEEE Trans. Neural. Syst. Rehabil. Eng. 2021, 29, 2540–2549. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Jakoubkova, M.; Thirugnanasambandam, N.; Schmalfuss, L.; Hullemann, S.; Sonka, K.; Paulus, W.; Trenkwalder, C.; Happe, S. Contribution of the premotor cortex to consolidation of motor sequence learning in humans during sleep. J. Neurophysiol. 2010, 104, 2603–2614. [Google Scholar] [CrossRef]
- Barbieri, M.; Negrini, M.; Nitsche, M.A.; Rivolta, D. Anodal-tDCS over the human right occipital cortex enhances the perception and memory of both faces and objects. Neuropsychologia 2016, 81, 238–244. [Google Scholar] [CrossRef]
- Li, B.Z.; Cao, Y.; Zhang, Y.; Chen, Y.; Gao, Y.H.; Peng, J.X.; Shao, Y.C.; Zhang, X. Relation of Decreased Functional Connectivity Between Left Thalamus and Left Inferior Frontal Gyrus to Emotion Changes Following Acute Sleep Deprivation. Front. Neurol. 2021, 12, 642411. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Vallar, G. The history of the neurophysiology and neurology of the parietal lobe. Handb. Clin. Neurol. 2018, 151, 3–30. [Google Scholar] [PubMed]
- Yuvaraj, R.; Murugappan, M.; Ibrahim, N.M.; Sundaraj, K.; Omar, M.I.; Mohamad, K.; Palaniappan, R. Optimal set of EEG features for emotional state classification and trajectory visualization in Parkinson’s disease. Int. J. Psychophysiol. 2014, 94, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, L.A.-O.; Besting, L.; Roebruck, F.; Jarczok, T.A.; Bender, S. Fearful facial expressions reduce inhibition levels in the dorsolateral prefrontal cortex in subjects with specific phobia. Depress. Anxiety 2022, 39, 26–36. [Google Scholar] [CrossRef]

| Facial Expression | Scared | |
|---|---|---|
| Channels | Encephalic Region | |
| theta | P6 | parietal |
| alpha | F7 | anterior temporal |
| Facial Expression | Happy | Disgusted | ||
|---|---|---|---|---|
| Channels | Encephalic Region | Channels | Encephalic Region | |
| Delta | AF4 | frontal pole | FC1, CP5, P5 | frontal pole |
| Theta | FP2, AF3, AF4, AF8,F1,P8 | frontal pole frontal lobe | CP5, P5 | central |
| Alpha | F1 | frontal lobe | -- | -- |
| Beta | AF3, AF4 | frontal pole | --- | -- |
| Facial Expression | Sad | Surprised | Scared | |||
|---|---|---|---|---|---|---|
| Channels | Encephalic Region | Channels | Encephalic Region | Channels | Encephalic Region | |
| Delta | C3, C5 | central | -- | -- | F3 | frontal |
| Theta | -- | -- | CP4 | central | -- | -- |
| Facial Expression | Happy | Sad | Surprised | Disgusted | ||||
|---|---|---|---|---|---|---|---|---|
| Channels | Encephalic Region | Channels | Encephalic Region | Channels | Encephalic Region | Channels | Encephalic Region | |
| Delta | AF4, F1, FC1 | frontal pole | CP4 | central | C3, C5 | central | -- | -- |
| Theta | F2, F4, CP4 | frontal pole | -- | -- | -- | -- | CP6 | central |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, Q.; Wei, C.; Xu, F.; Zhang, P.; Zeng, H.; Shao, Y.; Weng, X.; Meng, R. The Effect of Transcranial Electrical Stimulation on the Recovery of Sleep Quality after Sleep Deprivation Based on an EEG Analysis. Brain Sci. 2023, 13, 933. https://doi.org/10.3390/brainsci13060933
Wang Y, Cao Q, Wei C, Xu F, Zhang P, Zeng H, Shao Y, Weng X, Meng R. The Effect of Transcranial Electrical Stimulation on the Recovery of Sleep Quality after Sleep Deprivation Based on an EEG Analysis. Brain Sciences. 2023; 13(6):933. https://doi.org/10.3390/brainsci13060933
Chicago/Turabian StyleWang, Yuhan, Qiongfang Cao, Changyou Wei, Fan Xu, Peng Zhang, Hanrui Zeng, Yongcong Shao, Xiechuan Weng, and Rong Meng. 2023. "The Effect of Transcranial Electrical Stimulation on the Recovery of Sleep Quality after Sleep Deprivation Based on an EEG Analysis" Brain Sciences 13, no. 6: 933. https://doi.org/10.3390/brainsci13060933






