miRNA Expression Analysis of the Hippocampus in a Vervet Monkey Model of Fetal Alcohol Spectrum Disorder Reveals a Potential Role in Global mRNA Downregulation
Abstract
:1. Introduction
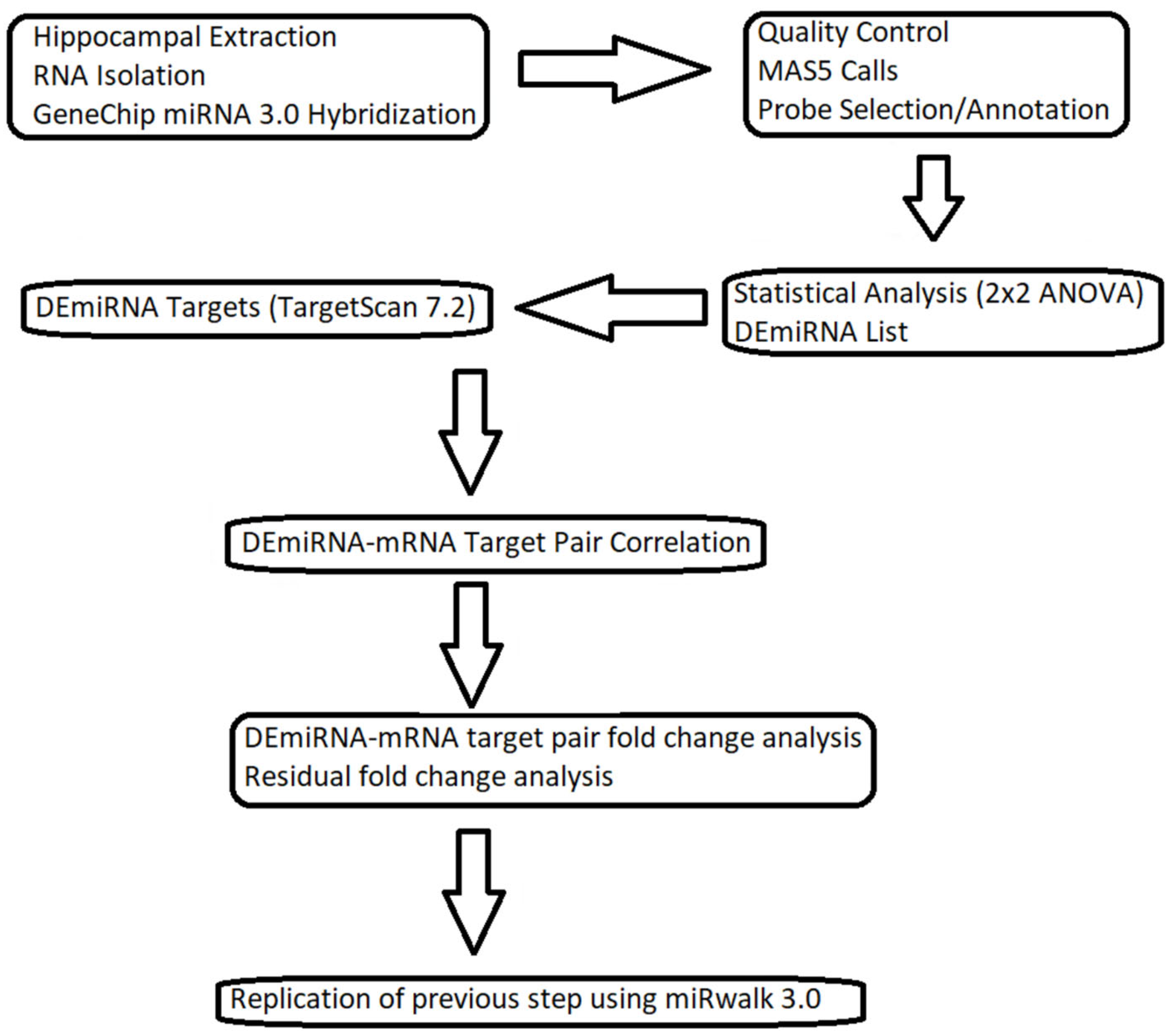
2. Materials and Methods
3. Results
3.1. ANOVA
3.1.1. Main Effect: Alcohol
3.1.2. Main Effect: Age
3.1.3. Interaction of Alcohol × Age
3.2. Integrated Analysis miRNA–mRNA
3.2.1. miRNA–mRNA Target Correlations
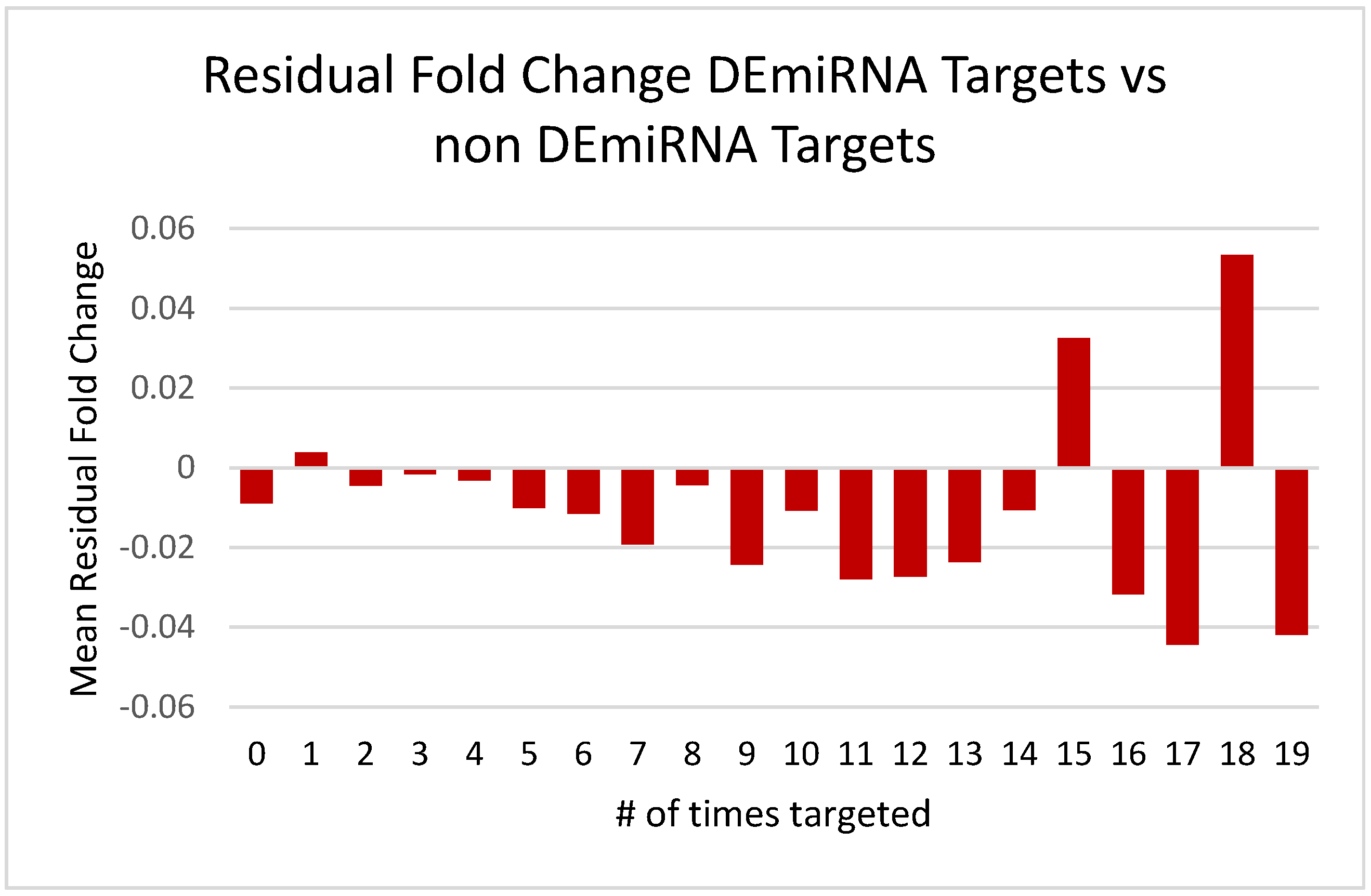
3.2.2. Differentially Expressed miRNAs and mRNA Fold Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burd, L.; Klug, M.G.; Li, Q.; Kerbeshian, J.; Martsolf, J.T. Diagnosis of fetal alcohol spectrum disorders: A validity study of the fetal alcohol syndrome checklist. Alcohol 2010, 44, 605–614. [Google Scholar] [CrossRef]
- Jones, K.L.; Smith, D.W.; Ulleland, C.N.; Streissguth, P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973, 1, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Harousseau, H.; Borteyru, J.P.; Menuet, J.C. Les enfants des parents alcoholiques; anomalies observées a propos de 127 cas. Quest Med. 1968, 25, 476–482. [Google Scholar]
- Loser, H.; Pfefferkorn, J.R.; Themann, H. Alcohol in pregnancy and fetal heart damage. Klin. Padiatr. 1992, 204, 335–339. [Google Scholar] [CrossRef] [PubMed]
- De Cornulier, M.; de Lacour, F.; Avet-Loiseau, H.; Passuti, N.; Branger, B.; Lemoine, P.; Picherot, G. Vertebral involvement and fetal alcohol syndrome. Pediatrie 1991, 46, 685–689. [Google Scholar]
- Boggan, W.O.; Randall, C.M.; DeBeukelaer, M.; Smith, R. Renal anomalies in mice prenatally exposed to ethanol. Res. Commun. Chem. Pathol. Pharmacol. 1979, 23, 127–142. [Google Scholar]
- Ribeiro, I.M.; Vale, P.J.; Tenedorio, P.A.; Rodrigues, P.A.; Bilhoto, M.A.; Pereira, H.C. Ocular manifestations in fetal alcohol syndrome. Eur. J. Ophthalmol. 2007, 17, 104–109. [Google Scholar] [CrossRef]
- Pettigrew, A.G.; Hutchinson, I. Effects of alcohol on functional development of the auditory pathway in the brainstem of infants and chick embryos. Ciba Found. Symp. 1984, 105, 26–46. [Google Scholar]
- Church, M.W.; Abel, E.L. Fetal alcohol syndrome. Hearing, speech, language, and vestibular disorders. Obstet. Gynecol. Clin. N. Am. 1998, 25, 85–97. [Google Scholar] [CrossRef]
- Rasmussen, C.; Becker, M.; McLennan, J.; Urichuk, L.; Andrew, G. An evaluation of social skills in children with and without prenatal alcohol exposure. Child Care Health Dev. 2011, 37, 711–718. [Google Scholar] [CrossRef]
- Kodituwakku, P.W. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neurosci. Biobehav. Rev. 2007, 31, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Sulik, K.K. Genesis of alcohol-induced craniofacial dysmorphism. Exp. Biol. Med. 2005, 230, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, D.L.; Gossage, J.P.; Brooke, L.; Adnams, C.M.; Jones, K.L.; Robinson, L.K.; Hoyme, H.E.; Snell, C.; Khaole, N.C.; Kodituwakku, P.; et al. Fetal alcohol syndrome epidemiology in a South African community: A second study of a very high prevalence area. J. Stud. Alcohol 2005, 66, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Streissguth, A.P.; Dehaene, P. Fetal alcohol syndrome in twins of alcoholic mothers: Concordance of diagnosis and IQ. Am. J. Med. Genet. 1993, 47, 857–861. [Google Scholar] [CrossRef]
- Warren, K.R.; Li, T.K. Genetic polymorphisms: Impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Goodlett, C.R.; Gilliam, D.M.; Nichols, J.M.; West, J.R. Genetic influences on brain growth restriction induced by development exposure to alcohol. Neurotoxicology 1989, 10, 321–334. [Google Scholar]
- Gilliam, D.M.; Kotch, L.E. Dose-related growth deficits in LS but not SS mice prenatally exposed to alcohol. Alcohol 1996, 13, 47–51. [Google Scholar] [CrossRef]
- Abel, E.L.; Sokol, R.J. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet 1986, 2, 1222. [Google Scholar] [CrossRef]
- Abel, E.L.; Sokol, R.J. Incidence of fetal alcohol syndrome and economic impact of FAS-related anomalies. Drug Alcohol Depend. 1987, 19, 51–70. [Google Scholar] [CrossRef]
- Abel, E.L.; Sokol, R.J. A revised conservative estimate of the incidence of FAS and its economic impact. Alcohol. Clin. Exp. Res. 1991, 15, 514–524. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Hard, M.L.; Abdolell, M.; Robinson, B.H.; Koren, G. Gene-expression analysis after alcohol exposure in the developing mouse. J. Lab. Clin. Med. 2005, 145, 47–54. [Google Scholar] [CrossRef]
- Zhang, C.; Frazier, J.M.; Chen, H.; Liu, Y.; Lee, J.A.; Cole, G.J. Molecular and morphological changes in zebrafish following transient ethanol exposure during defined developmental stages. Neurotoxicol. Teratol. 2014, 44, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Shawa, H.; Abbott, C.W., 3rd; Huffman, K.J. Prenatal ethanol exposure disrupts intraneocortical circuitry, cortical gene expression, and behavior in a mouse model of FASD. J. Neurosci. 2013, 33, 18893–18905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringer, R.L.; Laufer, B.I.; Kleiber, M.L.; Singh, S.M. Reduced expression of brain cannabinoid receptor 1 (Cnr1) is coupled with an increased complementary micro-RNA (miR-26b) in a mouse model of fetal alcohol spectrum disorders. Clin. Epigenetics 2013, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiber, M.L.; Laufer, B.I.; Wright, E.; Diehl, E.J.; Singh, S.M. Long-term alterations to the brain transcriptome in a maternal voluntary consumption model of fetal alcohol spectrum disorders. Brain Res. 2012, 1458, 18–33. [Google Scholar] [CrossRef]
- Downing, C.; Flink, S.; Florez-McClure, M.L.; Johnson, T.E.; Tabakoff, B.; Kechris, K.J. Gene expression changes in C57BL/6J and DBA/2J mice following prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2012, 36, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Green, M.L.; Singh, A.V.; Zhang, Y.; Nemeth, K.A.; Sulik, K.K.; Knudsen, T.B. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev. Dyn. 2007, 236, 613–631. [Google Scholar] [CrossRef]
- Hashimoto-Torii, K.; Kawasawa, Y.I.; Kuhn, A.; Rakic, P. Combined transcriptome analysis of fetal human and mouse cerebral cortex exposed to alcohol. Proc. Natl. Acad. Sci. USA 2011, 108, 4212–4217. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.A.; Chiu, J.; Reynolds, J.N.; Brien, J.F. Effect of chronic prenatal ethanol exposure on nitric oxide synthase I and III proteins in the hippocampus of the near-term fetal guinea pig. Neurotoxicol. Teratol. 1999, 21, 251–259. [Google Scholar] [CrossRef]
- Mandal, C.; Park, K.S.; Jung, K.H.; Chai, Y.G. Ethanol-related alterations in gene expression patterns in the developing murine hippocampus. Acta Biochim. Biophys. Sin. 2015, 47, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Alberry, B.L.J.; Castellani, C.A.; Singh, S.M. Hippocampal transcriptome analysis following maternal separation implicates altered RNA processing in a mouse model of fetal alcohol spectrum disorder. J. Neurodev. Disord. 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Laufer, B.I.; Mantha, K.; Kleiber, M.L.; Diehl, E.J.; Addison, S.M.; Singh, S.M. Long-lasting alterations to DNA methylation and ncRNAs could underlie the effects of fetal alcohol exposure in mice. Dis. Model. Mech. 2013, 6, 977–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portales-Casamar, E.; Lussier, A.A.; Jones, M.J.; MacIsaac, J.L.; Edgar, R.D.; Mah, S.M.; Barhdadi, A.; Provost, S.; Lemieux-Perreault, L.P.; Cynader, M.S.; et al. DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin 2016, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Balaraman, Y.; Wang, G.; Nephew, K.P.; Zhou, F.C. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics Off. J. DNA Methylation Soc. 2009, 4, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Marjonen, H.; Sierra, A.; Nyman, A.; Rogojin, V.; Grohn, O.; Linden, A.-M.; Hautaniemi, S.; Kaminen-Ahola, N. Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model. PLoS ONE 2015, 10, e0124931. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S. Epigenetics in fetal alcohol spectrum disorder. Prog. Mol. Biol. Trans. Sci. 2023, 197, 211–239. [Google Scholar] [CrossRef]
- Kiefer, J.C. microRNAs under the microscope. Dev. Dyn. 2006, 235, 846–853. [Google Scholar] [CrossRef]
- Vreugdenhil, E.; Berezikov, E. Fine-tuning the brain: MicroRNAs. Front. Neuroendocrinol. 2010, 31, 128–133. [Google Scholar] [CrossRef]
- Smalheiser, N.R.; Lugli, G. microRNA regulation of synaptic plasticity. Neuromolecular Med. 2009, 11, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Schratt, G. Fine-tuning neural gene expression with microRNAs. Curr. Opin. Neurobiol. 2009, 19, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, P. Roles of small regulatory RNAs in determining neuronal identity. Nat. Rev. Neurosci. 2010, 11, 329–338. [Google Scholar] [CrossRef]
- Balaraman, S.; Tingling, J.D.; Tsai, P.C.; Miranda, R.C. Dysregulation of microRNA expression and function contributes to the etiology of fetal alcohol spectrum disorders. Alcohol Res. Curr. Rev. 2013, 35, 18–24. [Google Scholar]
- Sathyan, P.; Golden, H.B.; Miranda, R.C. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: Evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J. Neurosci. 2007, 27, 8546–8557. [Google Scholar] [CrossRef] [Green Version]
- Tal, T.L.; Franzosa, J.A.; Tilton, S.C.; Philbrick, K.A.; Iwaniec, U.T.; Turner, R.T.; Waters, K.M.; Tanguay, R.L. MicroRNAs control neurobehavioral development and function in zebrafish. FASEB J. 2012, 26, 1452–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignacio, C.; Mooney, S.M.; Middleton, F.A. Effects of Acute Prenatal Exposure to Ethanol on microRNA Expression are Ameliorated by Social Enrichment. Front. Pediatr. 2014, 2, 103. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Chen, Y.; Carreon, S.; Qiang, M. Chronic intermittent ethanol exposure and its removal induce a different miRNA expression pattern in primary cortical neuronal cultures. Alcohol Clin. Exp. Res. 2012, 36, 1058–1066. [Google Scholar] [CrossRef]
- Mahnke, A.H.; Sideridis, G.D.; Salem, N.A.; Tseng, A.M.; Carter, R.C.; Dodge, N.C.; Rathod, A.B.; Molteno, C.D.; Meintjes, E.M.; Jacobson, S.W.; et al. Infant circulating MicroRNAs as biomarkers of effect in fetal alcohol spectrum disorders. Sci. Rep. 2021, 11, 1429. [Google Scholar] [CrossRef]
- Lewohl, J.M.; Nunez, Y.O.; Dodd, P.R.; Tiwari, G.R.; Harris, R.A.; Mayfield, R.D. Up-regulation of microRNAs in brain of human alcoholics. Alcohol. Clin. Exp. Res. 2011, 35, 1928–1937. [Google Scholar] [CrossRef] [Green Version]
- Nunez, Y.O.; Truitt, J.M.; Gorini, G.; Ponomareva, O.N.; Blednov, Y.A.; Harris, R.A.; Mayfield, R.D. Positively correlated miRNA-mRNA regulatory networks in mouse frontal cortex during early stages of alcohol dependence. BMC Genom. 2013, 14, 725. [Google Scholar] [CrossRef] [Green Version]
- Soares, A.R.; Pereira, P.M.; Ferreira, V.; Reverendo, M.; Simoes, J.; Bezerra, A.R.; Moura, G.R.; Santos, M.A. Ethanol exposure induces upregulation of specific microRNAs in zebrafish embryos. Toxicol. Sci. 2012, 127, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livy, D.J.; Miller, E.K.; Maier, S.E.; West, J.R. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol. Teratol. 2003, 25, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Gil-Mohapel, J.; Boehme, F.; Kainer, L.; Christie, B.R. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Res. Rev. 2010, 64, 283–303. [Google Scholar] [CrossRef] [PubMed]
- McGooey, T.N.; Reynolds, J.N.; Brien, J.F. Chronic prenatal ethanol exposure-induced decrease of guinea pig hippocampal CA1 pyramidal cell and cerebellar Purkinje cell density. Can. J. Physiol. Pharmacol. 2003, 81, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.W.; Ptito, M.; Ervin, F.R.; Palmour, R.M. Hippocampal neuron populations are reduced in vervet monkeys with fetal alcohol exposure. Dev. Psychobiol. 2015, 57, 470–485. [Google Scholar] [CrossRef] [Green Version]
- Gillis, R.F.; Palmour, R.M. mRNA expression analysis of the hippocampus in a vervet monkey model of fetal alcohol spectrum disorder. J. Neurodev. Disord. 2022, 14, 21. [Google Scholar] [CrossRef]
- American Veterinary Medical Association (AVMA). AVMA Guidelines for the Euthanasia of Animals. Retrieved March 2020, 2013, 2020–2021. [Google Scholar]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Woody, O.Z.; Nadon, R. The ShivPlot: A graphical display for trend elucidation and exploratory analysis of microarray data. Source Code Biol. Med. 2006, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- McCreight, J.C.; Schneider, S.E.; Wilburn, D.B.; Swanson, W.J. Evolution of microRNA in primates. PLoS ONE 2017, 12, e0176596. [Google Scholar] [CrossRef] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Storey, J. The Positive False Discovery Rate: A Bayesian Interpretation and the q-Value. Ann. Stat. 2001, 31, 2013–2035. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.com (accessed on 22 November 2021).
- Davis, J.A.; Saunders, S.J.; Mann, M.; Backofen, R. Combinatorial ensemble miRNA target prediction of co-regulation networks with non-prediction data. Nucleic Acids Res. 2017, 45, 8745–8757. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, W. microRNA Target Prediction. Methods Mol. Biol. 2017, 1513, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Gretz, N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat. Methods 2015, 12, 697. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.S.; Tam, W.L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Zhang, Z.; Li, Q.; Yang, R.; Pei, X.; Xu, Y.; Wang, J.; Zhou, S.F.; Li, Y. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum. Reprod. 2009, 24, 562–579. [Google Scholar] [CrossRef] [Green Version]
- Tapocik, J.D.; Solomon, M.; Flanigan, M.; Meinhardt, M.; Barbier, E.; Schank, J.R.; Schwandt, M.; Sommer, W.H.; Heilig, M. Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. Pharm. J. 2013, 13, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Mahnke, A.H.; Salem, N.A.; Tseng, A.M.; Chung, D.D.; Miranda, R.C. Nonprotein-coding RNAs in Fetal Alcohol Spectrum Disorders. Prog. Mol. Biol. Transl. Sci. 2018, 157, 299–342. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Coolen, M.; Thieffry, D.; Drivenes, O.; Becker, T.S.; Bally-Cuif, L. miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev. Cell 2012, 22, 1052–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leucht, C.; Stigloher, C.; Wizenmann, A.; Klafke, R.; Folchert, A.; Bally-Cuif, L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat. Neurosci. 2008, 11, 641–648. [Google Scholar] [CrossRef]
- Rogic, S.; Wong, A.; Pavlidis, P. Meta-Analysis of Gene Expression Patterns in Animal Models of Prenatal Alcohol Exposure Suggests Role for Protein Synthesis Inhibition and Chromatin Remodeling. Alcohol Clin. Exp. Res. 2016, 40, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, D.D.; Pinson, M.R.; Bhenderu, L.S.; Lai, M.S.; Patel, R.A.; Miranda, R.C. Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood. Int. J. Mol. Sci. 2021, 22, 8785. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal Alcohol Spectrum Disorders. Nat. Rev. Dis. Prim. 2023, 9, 11. [Google Scholar] [CrossRef]
- Gomez, D.A.; Abdul-Rahman, O.A. Fetal Alcohol Spectrum Disorders: Current State of Diagnosis and Treatment. Curr. Opin. Pediatr. 2021, 33, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Ritfeld, G.; Kable, J.A.; Holton, J.E.; Coles, C.D. Psychopharmacological Treatments in Children with Fetal Alcohol Spectrum Disorders: A Review. Child Psychiatry Hum. Dev. 2022, 53, 268–277. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillis, R.F.; Palmour, R.M. miRNA Expression Analysis of the Hippocampus in a Vervet Monkey Model of Fetal Alcohol Spectrum Disorder Reveals a Potential Role in Global mRNA Downregulation. Brain Sci. 2023, 13, 934. https://doi.org/10.3390/brainsci13060934
Gillis RF, Palmour RM. miRNA Expression Analysis of the Hippocampus in a Vervet Monkey Model of Fetal Alcohol Spectrum Disorder Reveals a Potential Role in Global mRNA Downregulation. Brain Sciences. 2023; 13(6):934. https://doi.org/10.3390/brainsci13060934
Chicago/Turabian StyleGillis, Rob F., and Roberta M. Palmour. 2023. "miRNA Expression Analysis of the Hippocampus in a Vervet Monkey Model of Fetal Alcohol Spectrum Disorder Reveals a Potential Role in Global mRNA Downregulation" Brain Sciences 13, no. 6: 934. https://doi.org/10.3390/brainsci13060934





