Sensitivity and Specificity of the Brentano Illusion Test in the Detection of Visual Hemi-Field Deficits in Patients with Unilateral Spatial Neglect
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Standard Neuropsychological Assessment of Neglect
2.3. Standard Visual Field Assessment
2.4. The Brentano Illusion Test (BRIT)
2.5. Data Analysis
2.5.1. Group Comparisons
2.5.2. BRIT Scoring and Analysis
2.5.3. Sensitivity and Specificity of the BRIT
2.5.4. Subsidiary Goals
3. Results
3.1. Standard Neuropsychological Assessment of Neglect
3.2. Standard Visual Field Assessment
3.3. N+H+ and N+H– Groups
3.4. The Brentano Illusion Test (BRIT)
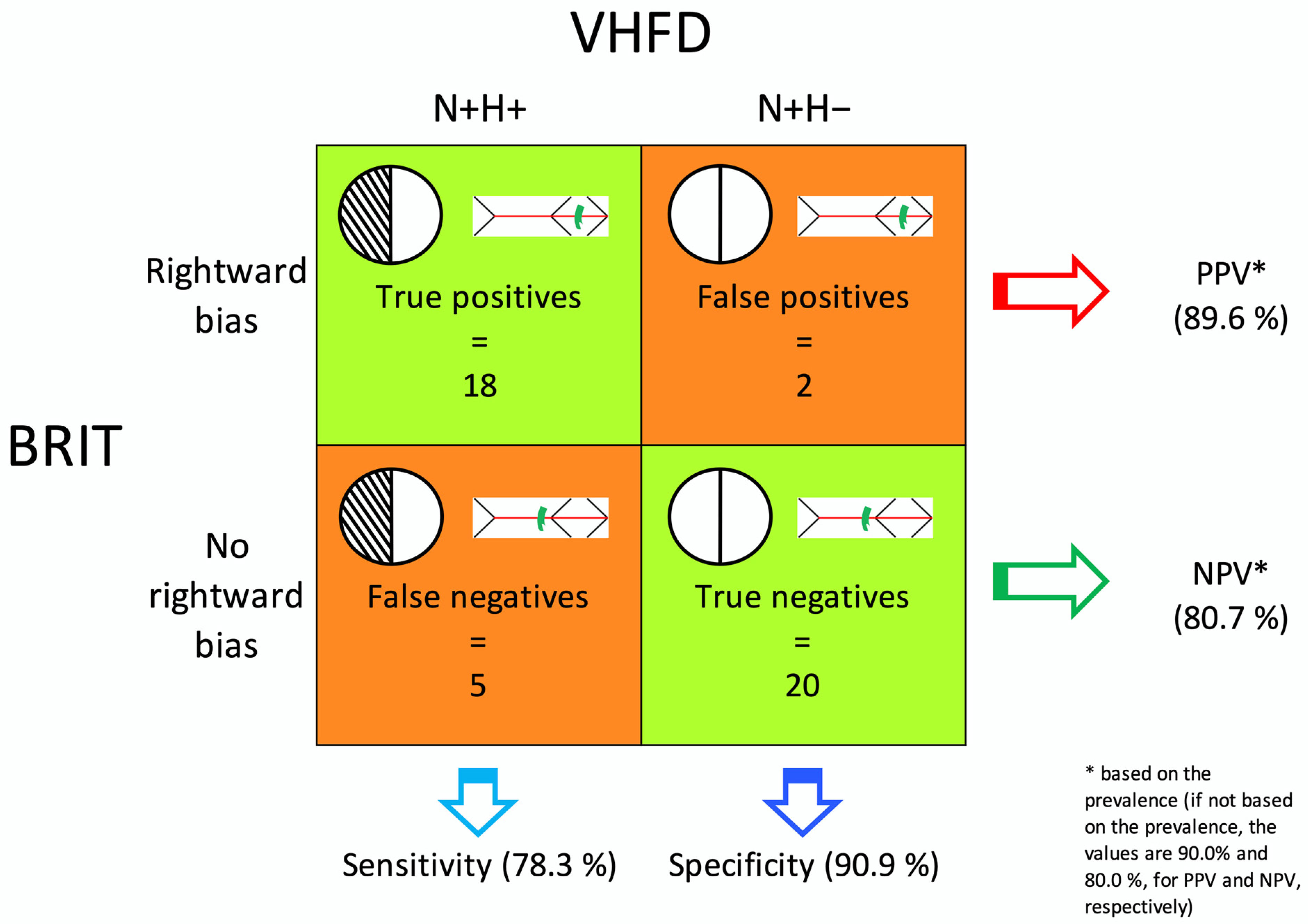
3.5. Sensitivity and Specificity of the BRIT
3.6. Subsidiary Goals
3.6.1. Outlying Responses
3.6.2. One vs. Two Lines
3.6.3. Length Effect
3.6.4. Leftward Bias in the SIE Score
4. Discussion
4.1. Sensitivity and Specificity of the BRIT
4.1.1. Big Data
4.1.2. Implementation Costs and Complexity
4.2. Subsidiary Goals
4.2.1. Outlying Responses
4.2.2. One vs. Two Lines
4.2.3. Length Effect
4.2.4. Leftward Bias in the SIE Score
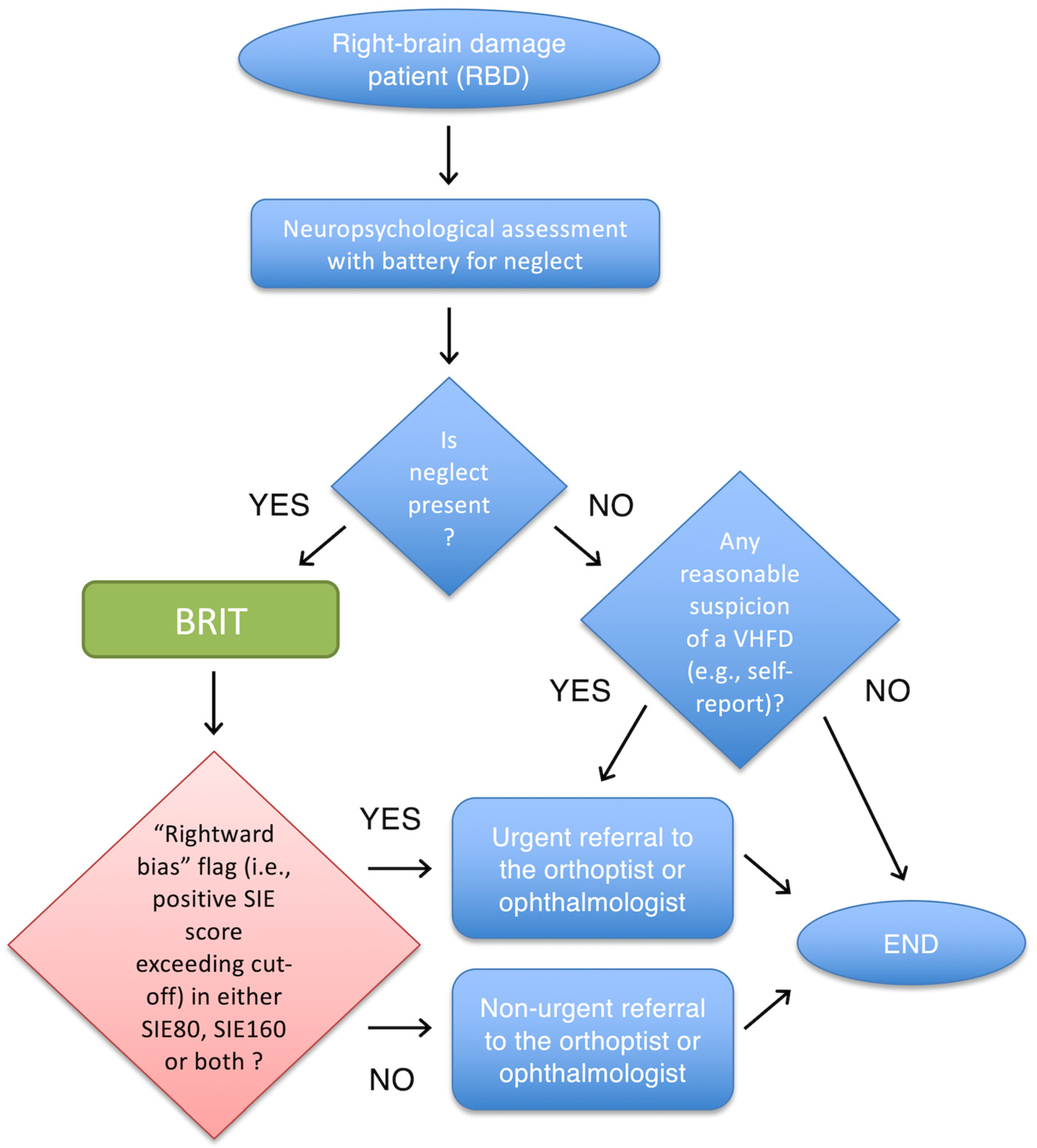
4.3. Practical Suggestions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRIT | Brentano Illusion Test |
| CI | confidence interval |
| CoV | coefficient of variation |
| HFA | Humphrey Field Analyzer |
| LB | line bisection |
| LE | length effect |
| N+ | patients with neglect |
| N– | patients without neglect |
| N+H+ | neglect patients with a left visual hemi-field deficit |
| N+H– | neglect patients without a left visual hemi-field deficit |
| NPV | negative predictive value |
| PPV | positive predictive value |
| RBD | right-brain damage |
| SIE | Symmetry of the Illusory Effect |
| VFD | visual field deficits |
| VHFD | visual hemi-field deficits |
References
- Esposito, E.; Shekhtman, G.; Chen, P. Prevalence of spatial neglect post-stroke: A systematic review. Ann. Phys. Rehabil. Med. 2021, 64, 101459. [Google Scholar]
- Williams, L.J.; Kernot, J.; Hillier, S.L.; Loetscher, T. Spatial neglect subtypes, definitions and assessment tools: A scoping review. Front. Neurol. 2021, 12, 742365. [Google Scholar]
- Hammerbeck, U.; Gittins, M.; Vail, A.; Paley, L.; Tyson, S.F.; Bowen, A. Spatial neglect in stroke: Identification, disease process and association with outcome during inpatient rehabilitation. Brain Sci. 2019, 9, 374. [Google Scholar]
- Parton, A.; Malhotra, P.; Husain, M. Hemispatial neglect. J. Neurol. Neurosurg. Psychiatry 2004, 75, 13–21. [Google Scholar]
- Halligan, P.W. Hemianopia and visual neglect: A question of balance? J. Neurol. Neurosurg. Psychiatry 1999, 67, 565–566. [Google Scholar] [CrossRef]
- Karnath, H.O.; Rennig, J.; Johannsen, L.; Rorden, C. The anatomy underlying acute versus chronic spatial neglect: A longitudinal study. Brain 2011, 134, 903–912. [Google Scholar] [CrossRef]
- Buxbaum, L.J.; Ferraro, M.K.; Veramonti, T.; Farne, A.; Whyte, J.; Ladavas, E.; Frassinetti, F.; Coslett, H.B. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology 2004, 62, 749–756. [Google Scholar] [CrossRef]
- Cassidy, T.P.; Bruce, D.W.; Lewis, S.; Gray, C.S. The association of visual field deficits and visuo-spatial neglect in acute right-hemisphere stroke patients. Age Ageing 1999, 28, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Vallar, G.; Perani, D. The anatomy of spatial neglect in humans. Adv. Psychol. 1987, 45, 235–258. [Google Scholar]
- Nardo, D.; De Luca, M.; Rotondaro, F.; Spanò, B.; Bozzali, M.; Doricchi, F.; Paolucci, S.; Macaluso, E. Left hemispatial neglect and overt orienting in naturalistic conditions: Role of high-level and stimulus-driven signals. Cortex 2019, 113, 329–346. [Google Scholar] [PubMed]
- Suchoff, I.B.; Gianutsos, R. Rehabilitative optometric interventions for the adult with acquired brain injury. In Physical Medicine and Rehabilitation; Grabois, M., Garrison, S.J., Hart, K.A., Lemkuhl, L.D., Eds.; Blackwell Scientific Publication: Malden, MA, USA, 2000; pp. 606–621. [Google Scholar]
- Rowe, F.J.; Hepworth, L.R.; Howard, C.; Hanna, K.L.; Cheyne, C.P.; Currie, J. High incidence and prevalence of visual problems after acute stroke: An epidemiology study with implications for service delivery. PLoS ONE 2019, 14, e0213035. [Google Scholar] [CrossRef]
- Cassidy, T.P.; Bruce, D.W.; Gray, C.S. Visual field loss after stroke: Confrontation and perimetry in the assessment of recovery. J. Stroke Cereb. Dis. 2001, 10, 113–117. [Google Scholar] [CrossRef]
- Jariyakosol, S.; Jaru-Ampornpan, P.; Manassakorn, A.; Itthipanichpong, R.; Hirunwiwatkul, P.; Tantisevi, V.; Somkijrungroj, T.; Rojanapongpun, P. Sensitivity and specificity of new visual field screening software for diagnosing hemianopia. Eye Brain 2021, 13, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.J.; Wright, D.; Brand, D.; Jackson, C.; Harrison, S.; Maan, T.; Scott, C.; Vogwell, L.; Peel, S.; Akerman, N.; et al. A prospective profile of visual field loss following stroke: Prevalence, type, rehabilitation, and outcome. Biomed. Res. Int. 2013, 2013, 719096. [Google Scholar]
- Rowe, F.J.; VIS Writing Group. Vision in stroke cohort: Profile overview of visual impairment. Brain Behav. 2017, 7, e00771. [Google Scholar]
- Suchoff, I.B.; Kapoor, N.; Ciuffreda, K.J.; Rutner, D.; Han, E.; Craig, S. The frequency of occurrence, types, and characteristics of visual field defects in acquired brain injury: A retrospective analysis. Optometry 2008, 79, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, C.A.; Heilman, K.M. Hemispatial visual inattention masquerading as hemianopia. Neurology 1989, 39, 1125–1127. [Google Scholar] [CrossRef]
- Ferber, S.; Karnath, H.O. How to assess spatial neglect—Line bisection or cancellation tasks? J. Clin. Exp. Neuropsychol. 2001, 23, 599–607. [Google Scholar] [CrossRef]
- Liu, G.T.; Volpe, N.J.; Galetta, S.L. The neuro-ophthalmic examination. In Neuro-Ophthalmology: Diagnosis and Management; Liu, G.T., Volpe, N.J., Galetta, S.L., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 7–36. [Google Scholar]
- Kerr, N.M.; Chew, S.S.; Eady, E.K.; Gamble, G.D.; Danesh-Meyer, H.V. Diagnostic accuracy of confrontation visual field tests. Neurology 2010, 74, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.J.S.; Benatar, M. Field of Vision: A Manual and Atlas of Perimetry; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar]
- Facchin, A.; Vallar, G.; Daini, R. The Brentano Illusion Test (BRIT): An implicit task of perceptual processing for the assessment of visual field defects in neglect patients. Neuropsychol. Rehabil. 2021, 31, 39–56. [Google Scholar] [CrossRef]
- Porac, C. Comparison of the wings-in, wings-out, and Brentano variants of the Mueller-Lyer illusion. Am. J. Psychol. 1994, 107, 69–83. [Google Scholar] [CrossRef]
- Daini, R.; Angelelli, P.; Antonucci, G.; Cappa, S.F.; Vallar, G. Exploring the syndrome of spatial unilateral neglect through an illusion of length. Exp. Brain Res. 2002, 144, 224–237. [Google Scholar] [CrossRef]
- Daini, R.; Angelelli, P.; Antonucci, G.; Cappa, S.F.; Vallar, G. Illusions of length in spatial unilateral neglect. Cortex 2001, 37, 710–714. [Google Scholar] [CrossRef]
- Vallar, G.; Daini, R.; Antonucci, G. Processing of illusion of length in spatial hemineglect: A study of line bisection. Neuropsychologia 2000, 38, 1087–1097. [Google Scholar] [CrossRef]
- Bujang, M.A.; Adnan, T.H. Requirements for minimum sample size for sensitivity and specificity analysis. J. Clin. Diagn. Res. 2016, 10, YE01–YE06. [Google Scholar] [CrossRef]
- Diller, L.; Ben-Yishay, Y.; Gerstamm, L.J. Studies in Cognition and Rehabilitation; New York Medical Center Institute of Rehabilitation Medicine: New York, NY, USA, 1974. [Google Scholar]
- Pizzamiglio, L.; Judica, A.; Razzano, C.; Zoccolotti, P. Toward a comprehensive diagnosis of visual-spatial disorders in unilateral brain damaged patients. Evaluación Psicológica 1989, 5, 199–218. [Google Scholar]
- Wilson, B.; Cockburn, J.; Halligan, P. Behavioral Inattention Test Manual; Tames Valley Test Company: London, UK, 1987. [Google Scholar]
- Spinazzola, L.; Pagliari, C.; Beschin, N. BIT—Behavioural Inattention Test; Italian Adaptation Manual; Giunti O.S.: Firenze, Italy, 2010. [Google Scholar]
- Albert, M.L. A simple test of visual neglect. Neurology 1973, 23, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Bickerton, W.L.; Samson, D.; Williamson, J.; Humphreys, G.W. Separating forms of neglect using the Apples Test: Validation and functional prediction in chronic acute stroke. Neuropsychology 2011, 25, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Rosadoni, S.; Capitani, D.; Bickerton, W.L.; Humphreys, G.W.; De Tanti, A.; Zampolini, M.; Galardi, G.; Caputo, M.; De Pellegrin, S.; et al. Italian standardization of the Apples Cancellation Test. Neurol. Sci. 2015, 36, 1233–1240. [Google Scholar] [CrossRef]
- Heilman, K.M.; Valenstein, E. Mechanisms underlying hemispatial neglect. Ann. Neurol. 1979, 5, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Guariglia, P.; Matano, A.; Piccardi, L. Bisecting or not bisecting: This is the neglect question. Line bisection performance in the diagnosis of neglect in right brain-damaged patients. PLoS ONE 2014, 9, e99700. [Google Scholar] [CrossRef]
- Massironi, M.; Antonucci, G.; Pizzamiglio, L.; Vitale, M.V.; Zoccolotti, P. The Wundt-Jastrow illusion in the study of spatial hemi-inattention. Neuropsychologia 1988, 26, 161–166. [Google Scholar] [CrossRef]
- Zoccolotti, P.; Antonucci, G.; Judica, A. Psychometric characteristics of two semi-structured scales for the functional evaluation of heminattention in extrapersonal and personal space. Neuropsychol. Rehabil. 1992, 2, 179–191. [Google Scholar] [CrossRef]
- Jehkonen, M.; Ahonen, J.P.; Dastidar, P.; Koivisto, A.M.; Laippala, P.; Vilkki, J. How to detect visual neglect in acute stroke. Lancet 1998, 351, 727–728. [Google Scholar] [PubMed]
- Azouvi, P.; Samuel, C.; Louis-Dreyfus, A.; Bernati, T.; Bartolomeo, P.; Beis, J.M.; Chokron, S.; Leclercq, M.; Marchal, F.; Martin, Y.; et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J. Neurol. Neurosurg. Psychiatry 2002, 73, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Azouvi, P.; Bartolomeo, P.; Beis, J.M.; Perennou, D.; Pradat-Diehl, P.; Rousseaux, M. A battery of tests for the quantitative assessment of unilateral neglect. Restor. Neurol. Neurosci. 2006, 24, 273–285. [Google Scholar] [PubMed]
- Trevethan, R. Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Front. Public Health 2017, 5, 307. [Google Scholar]
- Alberg, A.J.; Park, J.W.; Hager, B.W.; Brock, M.V.; Diener-West, M. The use of “overall accuracy” to evaluate the validity of screening or diagnostic tests. J. Gen. Intern. Med. 2004, 19, 460–465. [Google Scholar] [CrossRef]
- Thai, M.T.; Wu, W.; Xiong, H. (Eds.) Big Data in Complex and Social Networks; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Liew, S.L.; Zavaliangos-Petropulu, A.; Jahanshad, N.; Lang, C.E.; Hayward, K.S.; Lohse, K.R.; Juliano, J.M.; Assogna, F.; Baugh, L.A.; Bhattacharya, A.K.; et al. The ENIGMA Stroke Recovery Working Group: Big data neuroimaging to study brain-behavior relationships after stroke. Hum. Brain Mapp. 2022, 43, 129–148. [Google Scholar]
- Mittelstadt, B.D.; Floridi, L. The ethics of Big Data: Current and foreseeable issues in biomedical contexts. Sci. Eng. Ethics 2016, 22, 303–341. [Google Scholar] [PubMed]
- Miller, J.B. Big data and biomedical informatics: Preparing for the modernization of clinical neuropsychology. Clin. Neuropsychol. 2019, 33, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Kulikowski, C.A.; Shortliffe, E.H.; Currie, L.M.; Elkin, P.L.; Hunter, L.E.; Johnson, T.R.; Kalet, I.J.; Lenert, L.A.; Musen, M.A.; Ozbolt, J.G.; et al. AMIA Board white paper: Definition of biomedical informatics and specification of core competencies for graduate education in the discipline. J. Am. Med. Inform. Assoc. 2012, 19, 931–938. [Google Scholar] [CrossRef] [PubMed]

| N+ | N– | Comparison | N+H+ | N+H– | Comparison | ||
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| N | 45 | 19 | 23 | 22 | |||
| Sex | 26 M; 19 F | 9 M; 10 F | n.s. | 14 M; 9 F | 12 M; 10 F | n.s. | |
| Age (years) | 60.9 (13.1) | 64.9 (14.1) | n.s. | 57.4 (13.5) | 64.5 (12.0) | n.s. | |
| Education (years) | 12.0 (4.7) | 11.9 (4.8) | n.s. | 13.1 (4.3) | 10.8 (5.0) | n.s. | |
| Time since stroke (days) | 80.0 (73.5) | 44.8 (28.7) | n.s. | 94.9 (81.3) | 64.5 (62.4) | n.s. | |
| N Ischaemic stroke | 28 | 16 | n.s. | 14 | 14 | n.s. | |
| N Haemorrhagic stroke | 17 | 3 | n.s. | 9 | 8 | n.s. | |
| Battery for neglect (at admittance) | Cut-offs | ||||||
| Letter cancellation (spatial bias) | ≥4 | 19.0 (14.4) | 1.0 (1.9) | <0.001 | 20.3 (12.1) | 17.8 (16.6) | n.s. |
| Star cancellation (spatial bias) | ≥3 | 10.3 (9.2) | 0.4 (0.9) | <0.001 | 10.7 (8.8) | 10.0 (9.9) | n.s. |
| Line cancellation (spatial bias) | ≥2 | 2.7 (3.5) | 0.0 (0.0) | <0.001 | 4.4 (3.8) | 1.0 (2.1) | <0.001 |
| Apples test accuracy (N targets) | ≤44 | 22.4 (14.8) | 46.0 (10.4) | <0.001 | 19.6 (14.2) | 25.2 (15.2) | n.s. |
| Apples test space-centered (asymmetry score) | ≥3 | 9.1 (6.6) | 0.1 (0.9) | <0.001 | 9.3 (6.1) | 8.8 (7.3) | n.s. |
| Apples test object-centered (asymmetry score) | ≥2 | 8.5 (10.8) | 0.4 (1.3) | <0.001 | 11.0 (11.3) | 6.1 (10.1) | n.s. |
| Line bisection 200 mm (% of shift from the midpoint) | >6.73 | 21.5 (24.3) | 2.3 (3.5) | <0.001 | 33.0 (28.2) | 9.4 (10.6) | <0.001 |
| Wundt-Jastrow illusion (N unexpected responses) | ≥2 | 8.8 (7.9) | 0.2 (0.5) | <0.001 | 11.5 (7.8) | 6.0 (7.2) | n.s. |
| Sentence reading (N correct sentences) | ≤5 * | 3.5 (2.6) | 6.0 (0.0) | <0.001 | 2.2 (2.6) | 4.8 (1.8) | <0.001 |
| Personal neglect (asymmetry score) | ≥2 | 1.8 (2.0) | 0.2 (0.7) | <0.001 | 1.5 (1.8) | 2.2 (2.2) | n.s. |
| Room description (asymmetry score) | ≥1 | 1.5 (1.2) | 0.1 (0.2) | <0.001 | 1.4 (1.2) | 1.5 (1.2) | n.s. |
| BRIT | Cut-offs | ||||||
| LB80 (%) | ≥8.50 | 5.4 (16.9) | 1.1 (2.4) | n.s. | 9.7 (21.2) | 1.0 (9.2) | n.s. |
| LB160 (%) | ≥12.25 | 15.0 (21.5) | 1.4 (3.8) | <0.001 | 24.5 (26.2) | 5.1 (7.2) | n.s. |
| LE (%) | ≥6.7 | 9.6 (11.3) | 0.4 (3.9) | <0.001 | 14.7 (13.1) | 4.3 (5.5) | n.s. |
| SIE80 (mm) | ≥7.8 | 5.5 (9.4) | −1.0 (2.1) | <0.001 | 11.5 (8.5) | −0.7 (5.6) | <0.001 |
| SIE80 (%) | ≥19.5 | 13.9 (23.5) | −2.5 (5.2) | 28.7 (21.2) | −1.6 (14.0) | ||
| SIE160 (mm) | ≥11.8 | 8.6 (19.9) | −0.1 (4.8) | n.s. | 17.2 (21.7) | −0.4 (12.8) | n.s. |
| SIE160 (%) | ≥14.75 | 10.8 (24.8) | −0.2 (6.0) | 21.6 (27.2) | −0.5 (16.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Baroncini, M.; Matano, A.; Di Lorenzo, C.; Magnotti, L.; Lucatello, S.; Mulas, M.; Pollarini, V.; Ciurli, M.P.; Nardo, D. Sensitivity and Specificity of the Brentano Illusion Test in the Detection of Visual Hemi-Field Deficits in Patients with Unilateral Spatial Neglect. Brain Sci. 2023, 13, 937. https://doi.org/10.3390/brainsci13060937
De Luca M, Baroncini M, Matano A, Di Lorenzo C, Magnotti L, Lucatello S, Mulas M, Pollarini V, Ciurli MP, Nardo D. Sensitivity and Specificity of the Brentano Illusion Test in the Detection of Visual Hemi-Field Deficits in Patients with Unilateral Spatial Neglect. Brain Sciences. 2023; 13(6):937. https://doi.org/10.3390/brainsci13060937
Chicago/Turabian StyleDe Luca, Maria, Matteo Baroncini, Alessandro Matano, Concetta Di Lorenzo, Luisa Magnotti, Susanna Lucatello, Martina Mulas, Virginia Pollarini, Maria Paola Ciurli, and Davide Nardo. 2023. "Sensitivity and Specificity of the Brentano Illusion Test in the Detection of Visual Hemi-Field Deficits in Patients with Unilateral Spatial Neglect" Brain Sciences 13, no. 6: 937. https://doi.org/10.3390/brainsci13060937
APA StyleDe Luca, M., Baroncini, M., Matano, A., Di Lorenzo, C., Magnotti, L., Lucatello, S., Mulas, M., Pollarini, V., Ciurli, M. P., & Nardo, D. (2023). Sensitivity and Specificity of the Brentano Illusion Test in the Detection of Visual Hemi-Field Deficits in Patients with Unilateral Spatial Neglect. Brain Sciences, 13(6), 937. https://doi.org/10.3390/brainsci13060937








