Abstract
Developmental coordination disorder (DCD) is a developmental disorder characterized by impaired motor coordination, often co-occurring with attention deficit disorder, autism spectrum disorders, and other psychological and behavioural conditions. The aetiology of DCD is believed to involve brain changes and environmental factors, with genetics also playing a role in its pathogenesis. Recent research has identified several candidate genes and genetic factors associated with motor impairment, including deletions, copy number variations, single nucleotide polymorphisms, and epigenetic modifications. This review provides an overview of the current knowledge in genetic research on DCD, highlighting the importance of continued research into the underlying genetic mechanisms. While evidence suggests a genetic contribution to DCD, the evidence is still in its early stages, and much of the current evidence is based on studies of co-occurring conditions. Further research to better understand the genetic basis of DCD could have important implications for diagnosis, treatment, and our understanding of the condition’s aetiology.
1. Introduction
Developmental coordination disorder (DCD) is a neurodevelopmental disorder characterized by difficulties in the execution and coordination of body movements which cannot be accounted for in terms of intellectual impairment or of identifiable physical or neurological disorder [1,2]. Children with DCD display difficulties with fine and/or gross body movements such as handwriting and riding a bicycle, and are often observed frequently tripping and bumping into things [1]. Such movement difficulties have a negative impact on everyday life [3,4], and the social life and well-being of parents of children with DCD [5]. Studies have shown that 5–6% of school-aged children are diagnosed with the condition depending on the selection criteria used [3], and subtypes of DCD may exist [6]. DCD is often accompanied by other neurodevelopmental disorders including attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). The co-occurrence rates vary between studies; it is estimated that children with DCD have a 30–50% chance of having ADHD [7,8], and a 14–50% chance of having ASD [1,9,10]. While certain risk factors for developmental coordination disorder (DCD), such as preterm birth and male sex, have been identified [11], it is worth noting that some children diagnosed with DCD do not exhibit these conventional risk factors. This observation raises the possibility that a genetic component may underlie their motor impairment [12,13,14,15].
The mechanisms involved in DCD are not fully understood and are thought to be multifactorial. To date, advances in understanding the mechanism of DCD have been relatively limited. Much of the literature focuses on the brain changes associated with DCD, including changes in brain function and brain structure (Figure 1). For example, research has demonstrated that children with DCD have significant brain differences in motor and sensorimotor white matter pathways and has suggested that axonal development in DCD may be disrupted in this neurodevelopmental disorder [16]. There are also preliminary findings that children with DCD have smaller brain volumes within the pallidum [17]. In terms of brain function, Van Dyck et al. observed the brain resting-state functional connectivity (rsFC) of children with DCD. Their findings suggest that children with DCD exhibited increased rsFC and that this was mostly found in the dorsal extrastriate visual brain system and the cerebellum [18]. Reynolds et al. demonstrated that children with DCD had deficits supportive of the mirror neuron system (MNS) dysfunction hypothesis at a behavioural level using fMRI. Their findings also suggest that decreased activation of the thalamus, caudate, and posterior cingulate regions is associated with motor planning and attentional processes [19]. Lust et al., using sensitive electroencephalography (EEG)-based measures of MNS activation during action observation, showed that MNS function is disrupted in children with DCD [20].
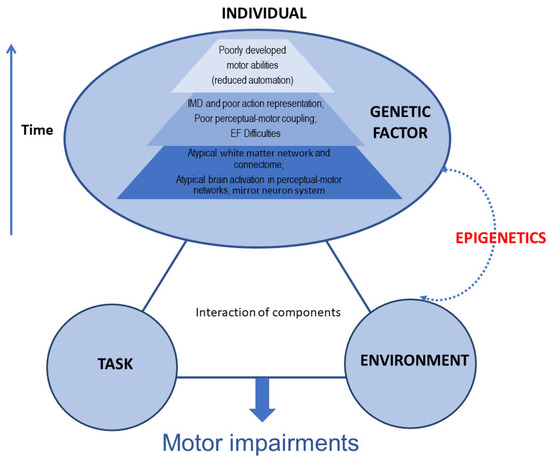
Figure 1.
A multi-component account of developmental coordination disorder (DCD) (adjusted from Blank et al. [2]). IMD: internal modelling deficit; EF: executive function.
In recent years, advances in gene detection technologies have led to the gradual discovery of the genome sequence and gene expression profiles associated with neurodevelopmental disorders. Although the number of studies on the genetics of DCD is limited, researchers have examined genetic factors associated with DCD, as well as genes linked with genetic susceptibility to DCD and related disorders. These studies have identified chromosome deletions, copy number variations, single nucleotide polymorphisms, and epigenetic factors that may be related to DCD. However, it should be noted that these studies are limited by relatively small patient numbers and also require function investigations to prove causation. Additionally, despite international diagnosis criteria for defining DCD [1,2], many published studies have not applied these definitions of DCD. Moreover, the evidence for a genetic contribution to DCD is largely based on studies of co-occurring conditions, and it is important to differentiate between the genetics of co-occurring conditions and the genetics of DCD itself, and that further research is needed to investigate the specific genetic mechanisms underlying DCD. Furthermore, as a complex disorder with multiple subtypes and aetiologies, it is unlikely that DCD has a single genetic mechanism that can account for its development. Nonetheless, investigating the genetic basis of DCD may provide valuable insights into the underlying neurobiological pathways that contribute to the disorder. Similarly, research on related conditions such as ASD and cerebral palsy (CP) has shown that studying individually rare mutations can illuminate the collective genetic factors underlying common disorders [21,22].
In this review, we mainly focus on the current genetic progress of DCD, as well as its co-occurrence with ADHD and ASD. Additionally, we discuss the potential environmental and epigenetic factors involved in DCD and their potential role in its development.
2. The Genetics of Motor Impairments
The mild-to-moderate motor impairments observed in DCD represent some of the earliest and most visible signs among affected developmental functions in neurodevelopmental disorders [23,24,25,26]. With recent technological advances, many gene-based syndromes have been identified that provide ways to reduce phenotypic heterogeneity [27,28]. For example, chromosome 22Q11.2 deletion syndrome (22q11.2 DS) is a genetic disorder caused by the deletion of a specific region on chromosome 22, with an estimated incidence of 1 in every 2000 to 4000 new-borns [29]. In addition to congenital heart disease and immunodeficiency [30], 22q11.2 DS is also associated with a high incidence of mental disorders, including ADHD, ASD, emotional disorders, and schizophrenia in adulthood [31]. Studies have found that children with 22q11.2 DS often have motor coordination impairments, including balance, hand–eye coordination, and visual motor ability. Specifically, a high percentage (81.4%) of children with 22q11.2 DS had indicative DCD (57/70). Furthermore, nine children with indicative DCD were assessed via the MABC-2 and eight of them met the DSM-5 criteria for DCD [1]. In addition, all children with 22q11.2 DS and ADHD had indicative DCD (20 of 20) [32]. 22q11.2 DS children also exhibit atypical sensorimotor control behaviours, which may contribute to the development of DCD [33]. Therefore, clinicians should be aware of the mental disorders and cognitive defects related to DCD in children with 22q11.2 DS. Moreover, this evidence suggests the implication of genetic elements within 22q11.2 in motor coordination.
Klinefelter (47, XXY) and (48, XXYY) syndromes are genetic disorders found in males, characterized by additional sex chromosomes compared with the typical male karyotype of 46, XY. Both conditions have been previously associated with delays in motor development and deficits in motor skills; indeed, motor coordination impairments are common in these syndromes [34,35]. Martin et al. found that 39% of males with XXY and 73% of males with XXYY had below average visual motor integration (VMI) scores and males with XXYY had lower average scores compared with males with XXY in the domains of manual coordination, body coordination, and strength/agility measured with the BOT-2 assessments. Furthermore, fine motor dexterity and coordination deficits are also common. In order to evaluate the contributors to VMI skills, this study also supplemented tests of visual perception and motor coordination. The results show that these individuals had strengths in visual perceptual skills, which suggested that motor coordination deficits may be the predominant contributor to VMI deficits. Therefore, such motor phenotypes in XXY and XXYY should be noted for clinical practice, in order to aid in diagnosis and to guide intervention [35].
Terminal deletion of the long arm of chromosome 6 (associated with developmental delay) may also impact motor coordination. Engwerda et al. found that the 6q14.2–q14.3 region had the most influential phenotype on neurodevelopment in rare 6q deletions in the region of 6q11 to 6q15. The majority of individuals over the age of 2 years with a deletion in this region had moderate-to-severe developmental delay [36]. Additionally, autism spectrum disorders are frequently related to 6q deletion and an increasing number of genes in 6q have been associated with this behavioural phenotype. A patient with a 6q11 to 6q15 deletion was found to have severe developmental delay and autism, indicating that autism may be part of the SYNCRIP-related phenotype [37]. Children with a 6q25 deletion spanning 11.1 Mb and 108 genes have also been found to suffer from motor impairment [38,39,40]. Their speech characterization showed elements of both childhood apraxia of speech and dysarthria due to deficits in motor coordination and low muscle tone, respectively. The overlapping deletion of the IGF2R–AIRN–SLC22A2–SLC22A3 gene cluster in both patients is possibly associated with motor discoordination and hypotonia across multiple motor systems as well as language delays, though other genes in the deletion region may also be associated with hypotonia [38]. The 6q25 microdeletion region should be considered in the differential clinical phenotype and chromosomal microarray analysis should be performed to confirm the phenotype.
In a longitudinal study that included 56 children aged between 6 months and 8 years with 16p11.2BP4-BP5 deletion or duplication (33 deletion and 23 duplication carriers), poor motor skills and nonverbal cognitive ability were observed in 22 children with 16p11.2 deletion who were diagnosed with DCD at 24 months. Language development, however, was affected only in carriers with a duplication. These findings underscore the importance of monitoring the motor developmental trajectory of children with 16p11.2 deletion to provide personalized treatment [41]. The Chd6 gene has been found to have high levels of expression in the brain of mice. Lathrop et al. have shown that the deletion of exon 12 of this gene led to motor coordination problems in mouse models, after excluding muscle weakness or bradykinesia and brain morphology issues [42].
To better understand the molecular basis of developmental disorders, studies have increasingly been using genomics and transcriptomics. For example, Werling et al. used RNA sequencing to identify genes related to the developmental trajectory of the human cortex, as well as common variations altering gene expression. They collected 176 dorsolateral prefrontal cortex samples from 6 gestational weeks to early adulthood (20 years old). Their analysis revealed that higher expression of RHEBL1 was associated with the genes related to increased educational attainment [43]. However, to obtain a more comprehensive account of the molecular mechanisms underlying normal and abnormal development, it is necessary to acquire higher-resolution datasets throughout development, such as single cells, additional brain regions, and larger sample sizes. Additionally, complementary analyses of the brains of individuals with neuropsychiatric and rare genetic disorders are required. By carefully evaluating clinical and dysmorphological features, and comparing cases with overlapping rearrangements, we can begin to ascribe specific symptoms to different copy number variations (CNVs). Ultimately, this research may lead to new insights and interventions for individuals with developmental disorders.
3. The Genetics of DCD Co-Occurring with ADHD
Given the high co-occurrence between DCD and ADHD and other neurodevelopmental disorders, it may be beneficial to assess the genetic reasons for different neurodevelopmental disorders in individuals who present with symptoms of either disorder. In the literature, 47, XXY—a chromosomal abnormality that occurs in 1 out of 650 male births—has received increased attention in recent years [44,45]. In a case report involving an 11-year-old boy diagnosed with ADHD, conventional G-banding karyotyping revealed a 47, XYY karyotype. Subsequent array comparative genomic hybridization (aCGH) analysis identified an additional duplication and two deletions, each of which were associated with speech and language delay and behavioural symptoms. Specifically, the 20q13.33 deletion was linked to autism and early onset schizophrenia, while the 11p15.5 microdeletion was associated with developmental delay, autism, and epilepsy [46]. These findings underscore the importance of considering other micro-deletions, duplications, and genetic syndromes that may present with similar symptoms and of making appropriate differential diagnoses.
Moreover, recent evidence suggests that attention and motor disorders may share a common neural substrate. To investigate this relationship, Albajara et al. conducted a study examining the relationship between Developmental Coordination Disorder Questionnaire (DCDQ) scores and regional brain volumes in six regions (pre-central gyrus, post-central gyrus, inferior parietal cortex, superior frontal gyrus, middle frontal gyrus, and medial frontal gyrus) in children with ASD, ADHD, and typically developing (TD) children. Their results show that, in children with ADHD, motor impairment was associated with increased grey matter volume in the right superior frontal gyrus [47]. This unique characteristic of concurrent DCD and ADHD has the potential to improve diagnostic definitions and offer insights into the shared and separate genetic and environmental causes of attention and motor disorders.
It is worth noting that children with ADHD frequently encounter issues with motor coordination. Fliers et al. conducted genetic association testing of children with ADHD from the International Multicentre ADHD Genetic (IMAGE) cohort and found that none of the findings reached genome-wide significance. However, further bioinformatics analysis of the top-ranked findings indicated that intergenic SNP rs11002745 on chromosome 10 and SNP rs2839083 located 18.7 kb downstream of the COL6A1 gene on chromosome 21, showed significant association after multi-test correction. The former SNP was correlated with gross motor problems, while the latter was correlated with fine motor problems and control in sports [48]. This study showed that eight of the top nine protein species played a role in the signal network of neural development. Enrichment analysis showed that the genes of motor neuropathy and amyotrophic lateral sclerosis were abundant, and the genes related to neurite growth and muscle function were also enriched. It has been speculated that the problem of motor coordination might be related to the genes expressed in nerve tissue and skeletal muscle. The findings of this study provide a genetic clue for the aetiology of ADHD and its associated motor coordination problems and point to a direction for the genetic research of DCD associated with ADHD. More recently, Mountford and colleagues conducted a population-based study examining the genetic association of quantitative motor coordination in children. Their findings indicate that no single nucleotide polymorphisms (SNPs) reached the level of genome-wide significance. However, they identified three chromosome regions (3p25.2, 6p12.1, and 14q24.2) that contained multiple SNPs that were suggestively associated with motor coordination. This study highlights the need for further research to better understand the genetic basis of motor coordination and related disorders [49]. To further investigate the potential shared genetic and environmental factors underlying DCD and ADHD, future studies could focus on analysing the specific genes and environmental factors that are implicated in both disorders. Additionally, studies could explore the potential interactions between these factors and how they may impact the development and manifestation of DCD and ADHD.
4. The Genetics of DCD Co-Occurring with ASD and Other Neurodevelopmental Disorders
Individuals with autism spectrum disorders (ASD) often experience motor problems [23,50,51,52]. One of the most well-investigated aetiologies of ASD is deletion or duplication in the 16p11.2 BP4 and BP5 region, a recurrent ~600kb copy number variant (CNV) with an incidence of approximately 1 in 2000 (deletion) and 1 in 1100 (duplication) [33]. The neurobehavioral profile of children with the 16p11.2 deletion is characterized by pervasive speech and language impairment (>70%) and motor coordination difficulties (~60%), with 20–25% also experiencing autistic and other behavioural/psychiatric issues. Future studies are underway to expand the cohort of 16p11.2 deletion and duplication carriers, following them longitudinally over time [53]. Comprehensive clinical examinations of individuals with the 1q21.1 deletion or duplication have shown that psychiatric, neurological, and medical disorders are common. While individuals with 1q21.1 deletions or duplications share several traits, such as borderline cognitive functioning, motor impairments, and articulation abnormalities, the duplication carriers have higher rates of ASD diagnoses and increased dimensional ASD symptom severity compared with deletion carriers. Additionally, the duplication cases had greater deficits in verbal cognitive abilities and fine motor functioning compared with familial controls with no carrier. A persistent decrease in motor abilities in carrier adults without other neurodevelopmental disorders suggests that children with 1q21.1 have a high frequency of motor impairment [54]. The phenotypic evaluation of seven new patients with interstitial microdeletions in the 8p23.2 region revealed language and speech delay and/or motor impairment, behavioural anomalies, ADHD, ASD, and dysmorphisms. However, the conventional cytogenetic analysis showed a low-level mosaicism for chromosome 8 monosomy that was not excluded by array-based comparative genomic hybridization (array-CGH) analysis, making it difficult to assess its contribution to the clinical phenotype [55].
The genetic component plays a significant role in ASD, and the most severe type of de novo mutation, likely gene disruptive (LGD), is closely linked with IQ, which is a phenotypic characteristic commonly associated with ASD but is not a core feature. To investigate the relationship between phenotypes and harmful de novo mutations in greater detail, Buja et al. conducted a study revealing that IQ and motor skills displayed distinct associations with detrimental mutations, with motor skills proving to be a more sensitive indicator of mutational severity in comparison with IQ, based on the type of mutation and target gene involved [56]. Mutations in chromodomain helicase DNA-binding protein 8 (Chd8), which encodes a chromatin remodeller, are a known risk factor for ASD. In a study by Kawamura et al. granule neuron progenitor (GNP)-specific deletion of Chd8 in the cerebellum of mice was found to cause cerebellar hypoplasia and motor coordination weakness, but not ASD-like behavioural defects. This suggests that Chd8 in cerebellar development may play a role in the pathogenesis of motor dysfunction in ASD [57]. Similarly, a study conducted by Xiao et al. focused on BTBR T+ Itprtf/J (BTBR) mice as a model to explore abnormal cerebellar development associated with motor impairment in autism spectrum disorder (ASD). Through their transcription analysis, they identified TRPC as a novel risk gene implicated in motor dysfunction in ASD [58]. Conversely, Emily et al. employed Neurexin 1α knock-out mice to investigate the behavioural outcomes and motor learning deficits. Interestingly, their findings revealed that juvenile and adult male Neurexin 1α knock-out mice exhibited social deficits and increased levels of aggression but did not display any deficits in motor learning [59]. These studies emphasize the significance of considering the developmental trajectory in mouse models used to study neurodevelopmental disorders.
Studies have also explored children with neurodevelopmental disorders with CNVs and found that coordination disorder was prevalent among them. It was also found that children’s motor coordination ability mediated ADHD symptoms, ASD clinical manifestations, and anxiety. Therefore, the abnormal development of motor coordination skills caused by CNV-induced neurodevelopmental disorders may have a cascade effect on the subsequent development of other skills, such as cognition and attention [60]. According to these findings, it is speculated that the occurrence of DCD and other neurodevelopmental disorders may be the result of the same underlying genetic causes, with different manifestations of symptoms occurring at different stages of development. This genetic pleiotropy may affect children’s brain development.
A study has examined the genetic evidence of motor impairment in neurodevelopmental disorders including ASD, ADHD, schizophrenia, and obsessive–compulsive disorder and revealed that children with motor impairment often exhibit deletions in brain-expressed genes commonly associated with other neurodevelopmental disorders [61]. Furthermore, Iman et al. conducted transcriptome profiling and found notable transcriptome similarities between ASD and schizophrenia [62]. In patients with schizophrenia, neurological soft signs (NSS), which refer to minor and subtle neurological abnormalities in sensory integration and motor performance, have consistently been observed [63].
The relationship between altered development and NSS has been reported in several studies. For instance, Peter et al. conducted a study on the British 1946 birth cohort and found that early-life motor milestones, particularly walking, may be linked to the origins of schizophrenia [64]. Birgitte et al. assessed domain-specific motor aberrations and disorder specificity among 7-year-old children with a familial risk of schizophrenia and found that motor abnormalities in children with a familial risk of schizophrenia are specific at 7 years of age with respect to fine motor function and balance [65]. Delayed motor developmental milestones, such as walking, sitting, and standing unsupported, have also been proposed as predictors of later schizophrenia [66]. Cox and Butler reviewed 200 individuals with the 15q11.2 BP1–BP2 microdeletion and identified delayed motor development, seizures/epilepsy, ASD, ADHD, and schizophrenia/paranoid psychosis as commonly observed features [67]. Another study identified CYFIP1 as the likely gene responsible for key phenotypes of ASD and schizophrenia within the 15q11.2 region. Deficiency in Cyfip1 leads to abnormalities in motor coordination, sensorimotor gating, and sensory perception [68]. Mohajer and Andrew investigated the offspring of parents with schizophrenia and found distinct motor and cognitive deficits, as well as abnormalities in social behaviour. Furthermore, a significant percentage of children at familial high risk for schizophrenia developed psychotic disorders in adulthood [69]. Martin et al. used a Tubb5 mouse model, which allows for the conditional expression of the pathogenic E401K mutation, and found that brain-specific knock-in of the pathogenic Tubb5 E401K allele causes defects in motor coordination and prepulse inhibition, a phenotype associated with sensorimotor gating and considered an endophenotype for schizophrenia [70]. Motor signs have also been identified as a cluster of signs sharing substantial genetic vulnerability with schizophrenia [71,72,73].
The genetic evidence supporting the co-occurrence of DCD and ASD has been further strengthened by recent research. A comprehensive study conducted on a Japanese population uncovered significant overlap in pathogenic CNVs and biological pathways between ASD and schizophrenia, highlighting their shared genetic underpinnings [74]. Importantly, investigations have consistently shown that children with DCD exhibit a higher prevalence of CNVs compared with other neurodevelopmental disorders, and rare CNVs have been identified in this population [75]. Notably, specific mutations and small deletions in SHANK3 (NM_033517.1) have been associated with approximately 1% of ASD cases in children with DCD but without additional complications [76]. These compelling findings provide robust support for the notion that common genetic factors contribute to the concurrent occurrence of DCD and ASD, reinforcing the need for further exploration of shared mechanisms and pathways underlying these disorders.
5. Advances in Epigenetics of DCD and Its Comorbidity
Recently, researchers have shown increased interest in studying environmental effects and their interaction with genetics, particularly on epigenetic mechanisms, including DNA methylation, histone modification, and non-coding RNA regulation. In mammals, DNA methylation is a main epigenetic marker regulating genome function. It has been found that developmental environment, including maternal effects, may result in permanent changes in DNA methylation and gene expression [77]. Nevertheless, neurodevelopmental disorders, such as DCD, have limited epigenetic evidence available for reference.
Previous studies have shown that DCD may result from a combination of antenatal and postnatal environmental exposures [78,79,80] and other risk factors including preterm birth and low birth weight [79,80,81]. The multifactorial aetiology of DCD may involve both genetic and environmental risk factors during the maternal and early life periods [82]. Prematurity, such as preterm birth [11,83,84,85] and low birth weight [11,86,87], has been associated with DCD when compared with full-term delivery. The motor development of children born at different gestational ages displays variation. Specifically, preterm children exhibit comparatively poorer fine motor skills compared with full-term children [88]. Previous research indicates that white matter alterations in brain microstructure are associated with motor impairment in preterm children [89]. Moreover, an epigenome-wide study reported that 12 single-CpG- and 46 sector-based DNA methylations were linked to white matter hyperintensity burden. This finding suggests that the utilization of effective epigenomic-based biomarkers could potentially enhance health outcomes for children who are at risk of preterm birth [90]. DNA methylation is a fundamental epigenetic mechanism that monitors gene expression and determines specific transcription profiles for distinct species and cell types [91]. The addition of methyl groups to guanine bases in CpG islands by methyltransferase enzymes can impact the expression of associated genes [92]. The establishment of epigenetic patterns during development may influence gene expression, thereby increasing susceptibility to chronic diseases and influencing an individual’s health over their lifetime [93].
Various prenatal exposures, such as environmental pollutants, smoking, and alcohol have been associated with an increased risk of DCD [94,95]. A national retrospective cohort study conducted in China revealed that ambient PM2.5 exposure during pregnancy and the first 3 years of life is a significant contributor to an elevated risk of DCD [96]. The potential long-term effects of air pollution on children’s neurodevelopment are not yet fully understood. In a previous study, our team demonstrated that exposure to PM2.5 during pregnancy can induce cognitive and motor impairment in offspring mice, primarily mediated by persistent brain damage of aseptic inflammatory response and cerebellar oxidative stress [97]. Furthermore, a parallel study provided additional insight into the underlying biological mechanism of PM2.5-induced brain impairment, which is mediated by an inflammatory reaction and regulated by DNA methylation [55]. Specifically, the methylation levels of CpG sites in the promoter region of interleukin-6 were significantly reduced in mice exposed daily to concentrated ambient PM2.5 compared with those exposed to filtered air [98]. This is a hypothesis and further study is required to confirm this as a direct cause of DCD in humans.
On the other hand, the impact of maternal smoking during pregnancy on DCD has been extensively studied. It has been found that maternal smoking during the first trimester of pregnancy is significantly associated with DCD in term-born children at the age of 7 [99]; while maternal smoking during the second or third trimester has been associated with an increased risk of DCD at 8 to 9 years but not at younger ages (5–7 years) [78]. Additionally, it is important to note that, while two studies did not find any association between maternal smoking and DCD at 7–8 years [100,101] or 10 years [102], these studies had less detailed assessments of smoking (maternal report of ever smoking during pregnancy) [103,104] compared with the other two studies [78,99]. In the field of epigenetic epidemiology, the effects of maternal smoking during pregnancy on DNA methylation have been widely investigated compared with miRNA and histone modifications [105]. These investigations have revealed important epigenetic effects of maternal smoking during pregnancy that might contribute to the development of DCD in children.
Prenatal exposure to metals has been linked to neurodevelopmental outcomes, with specific metals showing negative associations with motor development [106,107,108]. While there is evidence that prenatal exposure to aluminium is associated with lower fine motor developmental quotient (DQ) and that cadmium exposure is linked to lower gross motor DQ [109], it is important to note that the causal mechanisms underlying these associations are not yet fully understood. Similarly, while lead and mercury have been identified as being neurotoxic at low doses during early development [110], further research is needed to confirm their direct impact on motor development. Nonetheless, levels of lead in maternal blood have been negatively associated with motor development [111], and postnatal mercury exposure has been found to impair fine motor function in school-age children [112]. This study also documented a relationship between plasma concentrations of polychlorinated biphenyls in 11-year-old children and poorer manual dexterity and slower fine motor speed [112]. Long-term exposure to methylmercury has been shown to damage the motor cortex in adult animals, leading to oxidative stress and a decrease in the number of neurons and astrocytes, which can impact motor skills [113]. These findings suggest that epigenetic changes in gene expression, induced by exposure to heavy metals [114], may play a critical role in shaping neurodevelopment and risk for DCD, highlighting the importance of maternal environment and other non-genetic factors in this process.
In summary, the development of neurodevelopmental disorders is influenced by a multifaceted interplay between genetic susceptibility and environmental risk factors. Prenatal exposure to certain environmental factors can increase the risk of behavioural changes in offspring, as well as reciprocal deletions and duplications in DNA, gene expression, and chromatin structure, which can significantly impact neural development. Although limited research exists on the association between DNA methylation and DCD, leveraging existing research on DCD complications and the unique characteristics of brain structure can facilitate the analysis of genome-wide methylation levels using microarray technology. This approach can identify additional factors that contribute to coordination difficulties in children with DCD. It is important to note, however, that while there is evidence linking environmental factors to motor coordination difficulties and white matter changes, there is currently no direct link between these environmental factors and specific epigenetic changes in DCD.
6. Conclusions and Prospects
Compared with other neurodevelopmental conditions affecting children, research on the genetics of DCD is relatively limited in both scope and quantity. Motor impairment can result from complex interactions between environmental and genetic factors. Epigenetic mechanisms, such as DNA methylation and histone modifications, may play a key role in mediating these interactions. Environmental factors, such as exposure to toxins, can cause epigenetic modifications that alter the expression of genes involved in motor function. Similarly, genetic mutations or variations can affect epigenetic marks and modify gene expression patterns in response to environmental cues. These epigenetic changes can have long-lasting effects on neural development and function, leading to motor impairment in DCD. However, while there is evidence supporting a genetic contribution to DCD, the evidence is still in its early stages, and much of the current evidence is based on studies of co-occurring conditions. Given the possibility of a shared genetic susceptibility between DCD and other neurodevelopmental disorders, future research could involve large samples of DCD-affected children as well as healthy children. Case-control and cohort studies could be conducted using whole exome sequencing and whole genome sequencing methods while taking into account the clinical manifestations and epidemiological characteristics of the disorder in the population. Genome-wide association studies (GWAS) will allow for the exploration of complex genetics and susceptibility. Analogous to other neurodevelopmental disorders, such as ADHD and ASD, the genomic architecture of DCD is likely to be highly complex, and there are currently no genes or epigenetic mechanisms directly and robustly linked to DCD. It will also be important to control potential confounding factors, such as age, in order to identify the genetic basis of DCD onset and provide insights into early diagnosis and intervention. By studying genetically homogeneous groups of individuals with reciprocal deletions and duplications who are willing to participate in long-term neuropsychometric and neuroimaging protocols, researchers will gain invaluable insight into the primary and secondary effects of genes on neurodevelopmental functions.
Author Contributions
W.D. and J.H. contributed to the conceptualization of the review; H.Y. drafted the manuscript; J.S., F.H. and Z.W. reviewed the literature about the theme; G.J., W.D. and J.H. together reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (81673179), the Science and Technology Commission of Shanghai Municipality (21DZ2202000, 19140903100), Shanghai Municipal Health Commission (2020YJZX0213), Pudong Municipal Health Commission (PW2020D-11), Public health disciplines in the second round of medical discipline construction project of the Pudong Health Commission (PWYgts2021-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Blank, R.; Barnett, A.L.; Cairney, J.; Green, D.; Kirby, A.; Polatajko, H.; Rosenblum, S.; Smits-Engelsman, B.; Sugden, D.; Wilson, P.; et al. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder. Dev. Med. Child. Neurol. 2019, 61, 242–285. [Google Scholar] [CrossRef]
- Hua, J.; Du, W.; Dai, X.; Wu, M.; Cai, X.; Shen, M.; Zhu, L. International clinical practice recommendations on the definition, diagnosis, assessment, intervention, and psychosocial aspects of developmental coordination disorder—Chinese (Mandarin) translation. Dev. Med. Child. Neurol. 2020, 61, E1–E35. [Google Scholar] [CrossRef] [PubMed]
- Harrowell, I.; Hollén, L.; Lingam, R.; Emond, A. The impact of developmental coordination disorder on educational achievement in secondary school. Res. Dev. Disabil. 2018, 72, 13–22. [Google Scholar] [CrossRef]
- Cleaton, M.A.M.; Lorgelly, P.K.; Kirby, A. Developmental coordination disorder: The impact on the family. Qual. Life Res. 2019, 28, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Lust, J.M.; Steenbergen, B.; Diepstraten, J.A.E.M.; Wilson, P.H.; Schoemaker, M.M.; Poelma, M.J. The subtypes of developmental coordination disorder. Dev. Med. Child. Neurol. 2022, 64, 1366–1374. [Google Scholar] [CrossRef]
- Kadesjö, B.; Gillberg, C. Attention deficits and clumsiness in Swedish 7-year-old children. Dev. Med. Child. Neurol. 1998, 40, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Lingam, R.; Hunt, L.; Golding, J.; Jongmans, M.; Emond, A. Prevalence of developmental coordination disorder using the DSM-IV at 7 years of age: A UK population-based study. Pediatrics 2009, 123, e693–e700. [Google Scholar] [CrossRef]
- Green, D.; Charman, T.; Pickles, A.; Chandler, S.; Loucas, T.; Simonoff, E.; Baird, G. Impairment in movement skills of children with autistic spectrum disorders. Dev. Med. Child. Neurol. 2009, 51, 311–316. [Google Scholar] [CrossRef]
- Sumner, E.; Leonard, H.C.; Hill, E.L. Overlapping Phenotypes in Autism Spectrum Disorder and Developmental Coordination Disorder: A Cross-Syndrome Comparison of Motor and Social Skills. J. Autism Dev. Disord. 2016, 46, 2609–2620. [Google Scholar] [CrossRef]
- van Hoorn, J.F.; Schoemaker, M.M.; Stuive, I.; Dijkstra, P.U.; Rodrigues Trigo Pereira, F.; van der Sluis, C.K.; Hadders-Algra, M. Risk factors in early life for developmental coordination disorder: A scoping review. Dev. Med. Child. Neurol. 2021, 63, 511–519. [Google Scholar] [CrossRef]
- Pettersson, E.; Anckarsäter, H.; Gillberg, C.; Lichtenstein, P. Different neurodevelopmental symptoms have a common genetic etiology. J. Child. Psychol. Psychiatry 2013, 54, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Carlström, E.; Råstam, M.; Gillberg, C.; Anckarsäter, H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am. J. Psychiatry 2010, 167, 1357–1363. [Google Scholar] [CrossRef]
- Moruzzi, S.; Pesenti-Gritti, P.; Brescianini, S.; Salemi, M.; Battaglia, M.; Ogliari, A. Clumsiness and psychopathology: Causation or shared etiology? A twin study with the CBCL 6–18 questionnaire in a general school-age population sample. Hum. Mov. Sci. 2010, 29, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.C.; Piek, J.P.; Hay, D. DCD and ADHD: A genetic study of their shared aetiology. Hum. Mov. Sci. 2006, 25, 110–124. [Google Scholar] [CrossRef]
- Brown-Lum, M.; Izadi-Najafabadi, S.; Oberlander, T.F.; Rauscher, A.; Zwicker, J.G. Differences in White Matter Microstructure Among Children with Developmental Coordination Disorder. JAMA Netw. Open. 2020, 3, e201184. [Google Scholar] [CrossRef]
- Grohs, M.N.; Lebel, C.; Carlson, H.L.; Craig, B.T.; Dewey, D. Subcortical brain structure in children with developmental coordination disorder: A T1-weighted volumetric study. Brain Imaging Behav. 2021, 15, 2756–2765. [Google Scholar] [CrossRef]
- Van Dyck, D.; Deconinck, N.; Aeby, A.; Baijot, S.; Coquelet, N.; Trotta, N.; Rovai, A.; Goldman, S.; Urbain, C.; Wens, V.; et al. Atypical resting-state functional brain connectivity in children with developmental coordination disorder. Neuroimage Clin. 2022, 33, 102928. [Google Scholar] [CrossRef]
- Reynolds, J.E.; Billington, J.; Kerrigan, S.; Williams, J.; Elliott, C.; Winsor, A.M.; Codd, L.; Bynevelt, M.; Licari, M.K. Mirror neuron system activation in children with developmental coordination disorder: A replication functional MRI study. Res. Dev. Disabil. 2019, 84, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lust, J.M.; van Schie, H.T.; Wilson, P.H.; van der Helden, J.; Pelzer, B.; Steenbergen, B. Activation of Mirror Neuron Regions Is Altered in Developmental Coordination Disorder (DCD)-Neurophysiological Evidence Using an Action Observation Paradigm. Front. Hum. Neurosci. 2019, 13, 232. [Google Scholar] [CrossRef]
- de la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 345–361. [Google Scholar] [CrossRef]
- Fahey, M.C.; Maclennan, A.H.; Kretzschmar, D.; Gecz, J.; Kruer, M.C. The genetic basis of cerebral palsy. Dev. Med. Child. Neurol. 2017, 59, 462–469. [Google Scholar] [CrossRef]
- Kaur, M.; Srinivasan, S.M.; Bhat, A.N. Comparing motor performance, praxis, coordination, and interpersonal synchrony between children with and without Autism Spectrum Disorder (ASD). Res. Dev. Disabil. 2018, 72, 79–95. [Google Scholar] [CrossRef]
- Lim, Y.H.; Licari, M.; Spittle, A.J.; Watkins, R.E.; Zwicker, J.G.; Downs, J.; Finlay-Jones, A. Early Motor Function of Children with Autism Spectrum Disorder: A Systematic Review. Pediatrics 2021, 147, e2020011270. [Google Scholar] [CrossRef] [PubMed]
- Iverson, J.M.; Shic, F.; Wall, C.A.; Chawarska, K.; Curtin, S.; Estes, A.; Gardner, J.M.; Hutman, T.; Landa, R.J.; Levin, A.R.; et al. Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. J. Abnorm. Psychol. 2019, 128, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.L.; Farmer, C.; Bal, V.; Robinson, E.B.; Willsey, A.J.; Werling, D.M.; Havdahl, K.A.; Sanders, S.J.; Thurm, A. Identification of Developmental and Behavioral Markers Associated with Genetic Abnormalities in Autism Spectrum Disorder. Am. J. Psychiatry 2017, 174, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Stessman, H.A.; Bernier, R.; Eichler, E.E. A genotype-first approach to defining the subtypes of a complex disease. Cell 2014, 156, 872–877. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Zoghbi, H.Y. Lessons learned from studying syndromic autism spectrum disorders. Nat. Neurosci. 2016, 19, 1408–1417. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Budarf, M.L.; Emanuel, B.S. A genetic etiology for DiGeorge syndrome: Consistent deletions and microdeletions of 22q11. Am. J. Hum. Genet. 1992, 50, 924–933. [Google Scholar] [PubMed]
- McDonald-McGinn, D.M.; Sullivan, K.E.; Marino, B.; Philip, N.; Swillen, A.; Vorstman, J.A.S.; Zackai, E.H.; Emanuel, B.S.; Vermeesch, J.R.; Morrow, B.E.; et al. 22q11.2 deletion syndrome. Nat. Rev. Dis. Primers 2015, 1, 15071. [Google Scholar] [CrossRef]
- Schneider, M.; Debbané, M.; Bassett, A.S.; Chow, E.W.C.; Fung, W.L.A.; van den Bree, M.; Owen, M.; Murphy, K.C.; Niarchou, M.; Kates, W.R.; et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: Results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am. J. Psychiatry 2014, 171, 627–639. [Google Scholar] [CrossRef]
- Cunningham, A.C.; Delport, S.; Cumines, W.; Busse, M.; Linden, D.E.J.; Hall, J.; Owen, M.J.; van den Bree, M.B.M. Developmental coordination disorder, psychopathology and IQ in 22q11.2 deletion syndrome. Br. J. Psychiatry 2018, 212, 27–33. [Google Scholar] [CrossRef]
- Cunningham, A.C.; Hill, L.; Mon-Williams, M.; Peall, K.J.; Linden, D.E.J.; Hall, J.; Owen, M.J.; van den Bree, M.B.M. Using kinematic analyses to explore sensorimotor control impairments in children with 22q11.2 deletion syndrome. J. Neurodev. Disord. 2019, 11, 8. [Google Scholar] [CrossRef]
- Ross, J.L.; Roeltgen, D.P.; Stefanatos, G.; Benecke, R.; Zeger, M.P.D.; Kushner, H.; Ramos, P.; Elder, F.F.; Zinn, A.R. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am. J. Med. Genet. A 2008, 146A, 708–719. [Google Scholar] [CrossRef]
- Martin, S.; Cordeiro, L.; Richardson, P.; Davis, S.; Tartaglia, N. The Association of Motor Skills and Adaptive Functioning in XXY/Klinefelter and XXYY Syndromes. Phys. Occup. Ther. Pediatr. 2019, 39, 446–459. [Google Scholar] [CrossRef]
- Engwerda, A.; Frentz, B.; den Ouden, A.L.; Flapper, B.C.T.; Swertz, M.A.; Gerkes, E.H.; Plantinga, M.; Dijkhuizen, T.; van Ravenswaaij-Arts, C.M.A. The phenotypic spectrum of proximal 6q deletions based on a large cohort derived from social media and literature reports. Eur. J. Hum. Genet. 2018, 26, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, G.; Bigoni, S.; Buldrini, B.; Garani, G.; Clauser, L.; Galiè, M.; Ferlini, A.; Fini, S. Double Interstitial Deletion of the Long Arm of Chromosome 6 in a Patient with Pierre Robin Sequence, Dysmorphisms, and Severe Developmental Delay. Mol. Syndromol. 2017, 9, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Peter, B.; Lancaster, H.; Vose, C.; Fares, A.; Schrauwen, I.; Huentelman, M. Two unrelated children with overlapping 6q25.3 deletions, motor speech disorders, and language delays. Am. J. Med. Genet. A 2017, 173, 2659–2669. [Google Scholar] [CrossRef]
- Zhong, M.-L.; Song, Y.-M.; Zou, C.-C. 6q25.1-q25.3 Microdeletion in a Chinese Girl. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 109–113. [Google Scholar] [CrossRef]
- Inoue, T.; Sato, K.; Ohashi, H.; Motojima, T.; Takizawa, T. Interstitial 6q25 microdeletion syndrome: 46,XX,del(6)(q25.2q26). Pediatr. Int. 2019, 61, 618–620. [Google Scholar] [CrossRef]
- Bernier, R.; Hudac, C.M.; Chen, Q.; Zeng, C.; Wallace, A.S.; Gerdts, J.; Earl, R.; Peterson, J.; Wolken, A.; Peters, A.; et al. Developmental trajectories for young children with 16p11.2 copy number variation. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, M.J.; Chakrabarti, L.; Eng, J.; Rhodes, C.H.; Lutz, T.; Nieto, A.; Liggitt, H.D.; Warner, S.; Fields, J.; Stöger, R.; et al. Deletion of the Chd6 exon 12 affects motor coordination. Mamm. Genome 2010, 21, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Pochareddy, S.; Choi, J.; An, J.-Y.; Sheppard, B.; Peng, M.; Li, Z.; Dastmalchi, C.; Santpere, G.; Sousa, A.M.M.; et al. Whole-Genome and RNA Sequencing Reveal Variation and Transcriptomic Coordination in the Developing Human Prefrontal Cortex. Cell Rep. 2020, 31, 107489. [Google Scholar] [CrossRef]
- Nielsen, J.; Wohlert, M. Sex chromosome abnormalities found among 34,910 newborn children: Results from a 13-year incidence study in Arhus, Denmark. Birth Defects Orig Artic Ser. 1990, 26, 209–223. [Google Scholar] [PubMed]
- Cordeiro, L.; Tartaglia, N.; Roeltgen, D.; Ross, J. Social deficits in male children and adolescents with sex chromosome aneuploidy: A comparison of XXY, XYY, and XXYY syndromes. Res. Dev. Disabil. 2012, 33, 1254–1263. [Google Scholar] [CrossRef]
- Kolaitis, G.; Bouwkamp, C.G.; Papakonstantinou, A.; Otheiti, I.; Belivanaki, M.; Haritaki, S.; Korpa, T.; Albani, Z.; Terzioglou, E.; Apostola, P.; et al. A boy with conduct disorder (CD), attention deficit hyperactivity disorder (ADHD), borderline intellectual disability, and 47,XXY syndrome in combination with a 7q11.23 duplication, 11p15.5 deletion, and 20q13.33 deletion. Child. Adolesc. Psychiatry Ment. Health 2016, 10, 33. [Google Scholar] [CrossRef]
- Albajara Sáenz, A.; Villemonteix, T.; Van Schuerbeek, P.; Baijot, S.; Septier, M.; Defresne, P.; Delvenne, V.; Passeri, G.; Raeymaekers, H.; Victoor, L.; et al. Motor Abnormalities in Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder Are Associated with Regional Grey Matter Volumes. Front. Neurol. 2021, 12, 666980. [Google Scholar] [CrossRef]
- Fliers, E.A.; Vasquez, A.A.; Poelmans, G.; Rommelse, N.; Altink, M.; Buschgens, C.; Asherson, P.; Banaschewski, T.; Ebstein, R.; Gill, M.; et al. Genome-wide association study of motor coordination problems in ADHD identifies genes for brain and muscle function. World J. Biol. Psychiatry 2012, 13, 211–222. [Google Scholar] [CrossRef]
- Mountford, H.S.; Hill, A.; Barnett, A.L.; Newbury, D.F. Genome-Wide Association Study of Motor Coordination. Front. Hum. Neurosci. 2021, 15, 669902. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.M.; Long, T.M.; Biasini, F.J. The intersection of gross motor abilities and participation in children with autism spectrum disorder. Infants Young Child. 2021, 34, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, A.; Johansson, A.-M.; Rudolfsson, T.; Rönnqvist, L.; von Hofsten, C.; Rosander, K.; Domellöf, E. Motor planning and movement execution during goal-directed sequential manual movements in 6-year-old children with autism spectrum disorder: A kinematic analysis. Res. Dev. Disabil. 2021, 115, 104014. [Google Scholar] [CrossRef]
- Campione, G.C.; Piazza, C.; Villa, L.; Molteni, M. Three-Dimensional Kinematic Analysis of Prehension Movements in Young Children with Autism Spectrum Disorder: New Insights on Motor Impairment. J. Autism Dev. Disord. 2016, 46, 1985–1999. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.K.; Roberts, T.P.; Sherr, E.H.; Snyder, L.G.; Spiro, J.E. 16p11.2 deletion syndrome. Curr. Opin. Genet. Dev. 2021, 68, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bernier, R.; Steinman, K.J.; Reilly, B.; Wallace, A.S.; Sherr, E.H.; Pojman, N.; Mefford, H.C.; Gerdts, J.; Earl, R.; Hanson, E.; et al. Clinical phenotype of the recurrent 1q21.1 copy-number variant. Genet. Med. 2016, 18, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Casto, C.; Dipasquale, V.; Ceravolo, I.; Gambadauro, A.; Aliberto, E.; Galletta, K.; Granata, F.; Ceravolo, G.; Falzia, E.; Riva, A.; et al. Prominent and Regressive Brain Developmental Disorders Associated with Nance-Horan Syndrome. Brain Sci. 2021, 11, 1150. [Google Scholar] [CrossRef]
- Buja, A.; Volfovsky, N.; Krieger, A.M.; Lord, C.; Lash, A.E.; Wigler, M.; Iossifov, I. Damaging de novo mutations diminish motor skills in children on the autism spectrum. Proc. Natl. Acad. Sci. USA 2018, 115, E1859–E1866. [Google Scholar] [CrossRef]
- Kawamura, A.; Katayama, Y.; Kakegawa, W.; Ino, D.; Nishiyama, M.; Yuzaki, M.; Nakayama, K.I. The autism-associated protein CHD8 is required for cerebellar development and motor function. Cell Rep. 2021, 35, 108932. [Google Scholar] [CrossRef]
- Xiao, R.; Zhong, H.; Li, X.; Ma, Y.; Zhang, R.; Wang, L.; Zang, Z.; Fan, X. Abnormal Cerebellar Development Is Involved in Dystonia-Like Behaviors and Motor Dysfunction of Autistic BTBR Mice. Front. Cell Dev. Biol. 2020, 8, 231. [Google Scholar] [CrossRef]
- Armstrong, E.C.; Caruso, A.; Servadio, M.; Andreae, L.C.; Trezza, V.; Scattoni, M.L.; Fernandes, C. Assessing the developmental trajectory of mouse models of neurodevelopmental disorders: Social and communication deficits in mice with Neurexin 1α deletion. Genes. Brain Behav. 2020, 19, e12630. [Google Scholar] [CrossRef]
- Cunningham, A.C.; Hall, J.; Owen, M.J.; van den Bree, M.B.M. Coordination difficulties, IQ and psychopathology in children with high-risk copy number variants. Psychol. Med. 2021, 51, 290–299. [Google Scholar] [CrossRef]
- Martin, N.C.; Piek, J.; Baynam, G.; Levy, F.; Hay, D. An examination of the relationship between movement problems and four common developmental disorders. Hum. Mov. Sci. 2010, 29, 799–808. [Google Scholar] [CrossRef]
- Sadeghi, I.; Gispert, J.D.; Palumbo, E.; Muñoz-Aguirre, M.; Wucher, V.; D’Argenio, V.; Santpere, G.; Navarro, A.; Guigo, R.; Vilor-Tejedor, N. Brain transcriptomic profiling reveals common alterations across neurodegenerative and psychiatric disorders. Comput. Struct. Biotechnol. J. 2022, 20, 4549–4561. [Google Scholar] [CrossRef] [PubMed]
- Papiol, S.; Fatjó-Vilas, M.; Schulze, T.G. Neurological soft signs in patients with schizophrenia: Current knowledge and future perspectives in the post-genomics era. Transl. Dev. Psychiatry 2016, 4, 30071. [Google Scholar] [CrossRef]
- Jones, P.; Rodgers, B.; Murray, R.; Marmot, M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994, 344, 1398–1402. [Google Scholar] [CrossRef]
- Burton, B.K.; Thorup, A.A.E.; Jepsen, J.R.; Poulsen, G.; Ellersgaard, D.; Spang, K.S.; Christiani, C.J.; Hemager, N.; Gantriis, D.; Greve, A.; et al. Impairments of motor function among children with a familial risk of schizophrenia or bipolar disorder at 7 years old in Denmark: An observational cohort study. Lancet Psychiatry 2017, 4, 400–408. [Google Scholar] [CrossRef]
- Filatova, S.; Koivumaa-Honkanen, H.; Hirvonen, N.; Freeman, A.; Ivandic, I.; Hurtig, T.; Khandaker, G.M.; Jones, P.B.; Moilanen, K.; Miettunen, J. Early motor developmental milestones and schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2017, 188, 13–20. [Google Scholar] [CrossRef]
- Cox, D.M.; Butler, M.G. The 15q11.2 BP1-BP2 microdeletion syndrome: A review. Int. J. Mol. Sci. 2015, 16, 4068–4082. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Iturza, N.; Lo, A.C.; Shah, D.; Armendáriz, M.; Vannelli, A.; Mercaldo, V.; Trusel, M.; Li, K.W.; Gastaldo, D.; Santos, A.R.; et al. The autism- and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour. Nat. Commun. 2019, 10, 3454. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.A.; Lewis, A.J. Offspring of Parents with Schizophrenia: A Systematic Review of Developmental Features Across Childhood. Harv. Rev. Psychiatry 2016, 24, 104–117. [Google Scholar] [CrossRef]
- Breuss, M.W.; Hansen, A.H.; Landler, L.; Keays, D.A. Brain-specific knockin of the pathogenic Tubb5 E401K allele causes defects in motor coordination and prepulse inhibition. Behav. Brain Res. 2017, 323, 47–55. [Google Scholar] [CrossRef]
- Neelam, K.; Garg, D.; Marshall, M. A systematic review and meta-analysis of neurological soft signs in relatives of people with schizophrenia. BMC Psychiatry 2011, 11, 139. [Google Scholar] [CrossRef]
- Snitz, B.E.; Macdonald, A.W.; Carter, C.S. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr. Bull. 2006, 32, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Bombin, I.; Arango, C.; Buchanan, R.W. Significance and meaning of neurological signs in schizophrenia: Two decades later. Schizophr. Bull. 2005, 31, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Kushima, I.; Aleksic, B.; Nakatochi, M.; Shimamura, T.; Okada, T.; Uno, Y.; Morikawa, M.; Ishizuka, K.; Shiino, T.; Kimura, H.; et al. Comparative Analyses of Copy-Number Variation in Autism Spectrum Disorder and Schizophrenia Reveal Etiological Overlap and Biological Insights. Cell Rep. 2018, 24, 2838–2856. [Google Scholar] [CrossRef]
- Mosca, S.J.; Langevin, L.M.; Dewey, D.; Innes, A.M.; Lionel, A.C.; Marshall, C.C.; Scherer, S.W.; Parboosingh, J.S.; Bernier, F.P. Copy-number variations are enriched for neurodevelopmental genes in children with developmental coordination disorder. J. Med. Genet. 2016, 53, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Moessner, R.; Marshall, C.R.; Sutcliffe, J.S.; Skaug, J.; Pinto, D.; Vincent, J.; Zwaigenbaum, L.; Fernandez, B.; Roberts, W.; Szatmari, P.; et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Hum. Genet. 2007, 81, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Law, P.-P.; Holland, M.L. DNA methylation at the crossroads of gene and environment interactions. Essays Biochem. 2019, 63, 717–726. [Google Scholar] [CrossRef]
- Christensen, L.H.; Høyer, B.B.; Pedersen, H.S.; Zinchuk, A.; Jönsson, B.A.G.; Lindh, C.; Dürr, D.W.; Bonde, J.P.; Toft, G. Prenatal smoking exposure, measured as maternal serum cotinine, and children’s motor developmental milestones and motor function: A follow-up study. Neurotoxicology 2016, 53, 236–245. [Google Scholar] [CrossRef]
- Edwards, J.; Berube, M.; Erlandson, K.; Haug, S.; Johnstone, H.; Meagher, M.; Sarkodee-Adoo, S.; Zwicker, J.G. Developmental coordination disorder in school-aged children born very preterm and/or at very low birth weight: A systematic review. J. Dev. Behav. Pediatr. 2011, 32, 678–687. [Google Scholar] [CrossRef]
- Dewey, D.; Creighton, D.E.; Heath, J.A.; Wilson, B.N.; Anseeuw-Deeks, D.; Crawford, S.G.; Sauve, R. Assessment of developmental coordination disorder in children born with extremely low birth weights. Dev. Neuropsychol. 2011, 36, 42–56. [Google Scholar] [CrossRef]
- Williams, J.; Lee, K.J.; Anderson, P.J. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2010, 52, 232–237. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Two distinct forms of minor neurological dysfunction: Perspectives emerging from a review of data of the Groningen Perinatal Project. Dev. Med. Child. Neurol. 2002, 44, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Barnett, A.L.; Lin, Y.; Guan, H.; Sun, Y.; Williams, G.J.; Fu, Y.; Zhou, Y.; Du, W. Association of Gestational Age at Birth with Subsequent Neurodevelopment in Early Childhood: A National Retrospective Cohort Study in China. Front. Pediatr. 2022, 10, 860192. [Google Scholar] [CrossRef]
- Uusitalo, K.; Haataja, L.; Nyman, A.; Ripatti, L.; Huhtala, M.; Rautava, P.; Lehtonen, L.; Parkkola, R.; Lahti, K.; Koivisto, M.; et al. Preterm children’s developmental coordination disorder, cognition and quality of life: A prospective cohort study. BMJ Paediatr. Open. 2020, 4, e000633. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-X.; Li, H.-F.; Wu, M.-Q.; Geng, S.-S.; Ke, L.; Lou, B.-W.; Du, W.; Hua, J. Associations of preterm and early-term birth with suspected developmental coordination disorder: A national retrospective cohort study in children aged 3–10 years. World J. Pediatr. WJP 2023, 19, 261–272. [Google Scholar] [CrossRef]
- Zwicker, J.G.; Yoon, S.W.; Mackay, M.; Petrie-Thomas, J.; Rogers, M.; Synnes, A.R. Perinatal and neonatal predictors of developmental coordination disorder in very low birthweight children. Arch. Dis. Child. 2013, 98, 118–122. [Google Scholar] [CrossRef]
- Gima, H.; Nakamura, T. Association between General Movements Assessment and Later Motor Delay (excluding Cerebral Palsy) in Low-Birth-Weight Infants. Brain Sci. 2022, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Barnett, A.L.; Williams, G.J.; Dai, X.; Sun, Y.; Li, H.; Chen, G.; Wang, L.; Feng, J.; Liu, Y.; et al. Association of Gestational Age at Birth with Subsequent Suspected Developmental Coordination Disorder in Early Childhood in China. JAMA Netw. Open. 2021, 4, e2137581. [Google Scholar] [CrossRef]
- Hollund, I.M.H.; Olsen, A.; Skranes, J.; Brubakk, A.-M.; Håberg, A.K.; Eikenes, L.; Evensen, K.A.I. White matter alterations and their associations with motor function in young adults born preterm with very low birth weight. Neuroimage Clin. 2018, 17, 241–250. [Google Scholar] [CrossRef]
- Park, B.; Khanam, R.; Vinayachandran, V.; Baqui, A.H.; London, S.J.; Biswal, S. Epigenetic biomarkers and preterm birth. Environ. Epigenet 2020, 6, dvaa005. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Field, A.E.; Robertson, N.A.; Wang, T.; Havas, A.; Ideker, T.; Adams, P.D. DNA Methylation Clocks in Aging: Categories, Causes, and Consequences. Mol. Cell 2018, 71, 882–895. [Google Scholar] [CrossRef]
- Parets, S.E.; Bedient, C.E.; Menon, R.; Smith, A.K. Preterm birth and its long-term effects: Methylation to mechanisms. Biology 2014, 3, 498–513. [Google Scholar] [CrossRef]
- Brown, C.W.; Olson, H.C.; Croninger, R.G. Maternal Alcohol Consumption during Pregnancy and Infant Social, Mental, and Motor Development. J. Early Interv. 2010, 32, 110–126. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Barr, H.M.; Martin, D.C.; Herman, C.S. Effects of maternal alcohol, nicotine, and caffeine use during pregnancy on infant mental and motor development at 8 months. Alcohol.-Clin. Exp. Res. 1980, 4, 152–164. [Google Scholar] [CrossRef]
- Cai, J.; Shen, Y.; Meng, X.; Zhao, Y.; Niu, Y.; Chen, R.; Du, W.; Quan, G.; Barnett, A.L.; Jones, G.; et al. Association of developmental coordination disorder with early-life exposure to fine particulate matter in Chinese preschoolers. Innovation 2023, 4, 100347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Al-Ahmady, Z.S.; Du, W.; Duan, J.; Liao, Z.; Sun, Q.; Wei, Z.; Hua, J. Maternal exposure to PM2.5 induces cognitive impairment in offspring via cerebellar neuroinflammation and oxidative stress. Ecotoxicol. Environ. Saf. 2023, 249, 114425. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, T.; Zhou, J.; Cao, Z.; Liao, Z.; Zhao, Y.; Su, X.; He, J.; Hua, J. Prenatal exposure to concentrated ambient PM2.5 results in spatial memory defects regulated by DNA methylation in male mice offspring. Environ. Sci. Pollut. Res. Int. 2023, 30, 35142–35152. [Google Scholar] [CrossRef]
- Faebo Larsen, R.; Hvas Mortensen, L.; Martinussen, T.; Nybo Andersen, A.-M. Determinants of developmental coordination disorder in 7-year-old children: A study of children in the Danish National Birth Cohort. Dev. Med. Child. Neurol. 2013, 55, 1016–1022. [Google Scholar] [CrossRef]
- Lingam, R.; Golding, J.; Jongmans, M.J.; Hunt, L.P.; Ellis, M.; Emond, A. The association between developmental coordination disorder and other developmental traits. Pediatrics 2010, 126, e1109–e1118. [Google Scholar] [CrossRef] [PubMed]
- Lingam, R.; Jongmans, M.J.; Ellis, M.; Hunt, L.P.; Golding, J.; Emond, A. Mental health difficulties in children with developmental coordination disorder. Pediatrics 2012, 129, e882–e891. [Google Scholar] [CrossRef]
- Hands, B.; Kendall, G.; Larkin, D.; Parker, H. Perinatal Risk Factors for Mild Motor Disability. Int. J. Disabil. Dev. Educ. 2009, 56, 317–331. [Google Scholar] [CrossRef]
- Foulder-Hughes, L.; Cooke, R. Do mainstream schoolchildren who were born preterm have motor problems? Br. J. Occup. Ther. 2003, 66, 9–16. [Google Scholar] [CrossRef]
- Foulder-Hughes, L.A.; Cooke, R.W.I. Motor, cognitive, and behavioural disorders in children born very preterm. Dev. Med. Child. Neurol. 2003, 45, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; François, O.; Lepeule, J. Epigenetic Alterations of Maternal Tobacco Smoking during Pregnancy: A Narrative Review. Int. J. Environ. Res. Public. Health 2021, 18, 5083. [Google Scholar] [CrossRef] [PubMed]
- Ashrap, P.; Sánchez, B.N.; Téllez-Rojo, M.M.; Basu, N.; Tamayo-Ortiz, M.; Peterson, K.E.; Meeker, J.D.; Watkins, D.J. In utero and peripubertal metals exposure in relation to reproductive hormones and sexual maturation and progression among girls in Mexico City. Environ. Res. 2019, 177, 108630. [Google Scholar] [CrossRef]
- Huang, L.; Huang, S.; Luo, X.; Li, L.; Chen, X.; Zan, G.; Tan, Y.; Liu, C.; Hou, Q.; Ge, X.; et al. Associations of prenatal exposure to multiple metals with testicular volume and anogenital distance in infant boys: A longitudinal cohort study. Environ. Int. 2020, 143, 105900. [Google Scholar] [CrossRef]
- Wang, S.; Meng, H.; Shang, N.; Guo, J.; Zhang, T.; Zhang, S.; Zhao, Y.; Zhang, H.; Zhang, Q.; Niu, Q. The Relationship between Plasma Al Levels and Multi-domain Cognitive Performance among In-service Aluminum-exposed Workers at the SH Aluminum Factory in China: A Cross-sectional Study. Neurotoxicology 2020, 76, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, L.; Huang, S.; Wei, L.; Cao, D.; Zan, G.; Tan, Y.; Wang, S.; Yang, M.; Tian, L.; et al. Association of both prenatal and early childhood multiple metals exposure with neurodevelopment in infant: A prospective cohort study. Environ. Res. 2022, 205, 112450. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef]
- Farías, P.; Hernández-Bonilla, D.; Moreno-Macías, H.; Montes-López, S.; Schnaas, L.; Texcalac-Sangrador, J.L.; Ríos, C.; Riojas-Rodríguez, H. Prenatal Co-Exposure to Manganese, Mercury, and Lead, and Neurodevelopment in Children during the First Year of Life. Int. J. Environ. Res. Public. Health 2022, 19, 13020. [Google Scholar] [CrossRef]
- Boucher, O.; Muckle, G.; Ayotte, P.; Dewailly, E.; Jacobson, S.W.; Jacobson, J.L. Altered fine motor function at school age in Inuit children exposed to PCBs, methylmercury, and lead. Environ. Int. 2016, 95, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.N.d.S.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.P.; Freitas Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; et al. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. 2019, 51, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. Int. 2022, 29, 62067–62092. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).