Clinical, Neuroimaging and Robotic Measures Predict Long-Term Proprioceptive Impairments following Stroke
Abstract
:1. Introduction
1.1. Proprioception and Its Importance after Stroke
1.2. Predictors of Proprioceptive Impairment
1.3. Aims and Hypothesis
2. Materials and Methods
2.1. Participant Recruitment
2.1.1. Study Inclusion Criteria
2.1.2. Study Exclusion Criteria
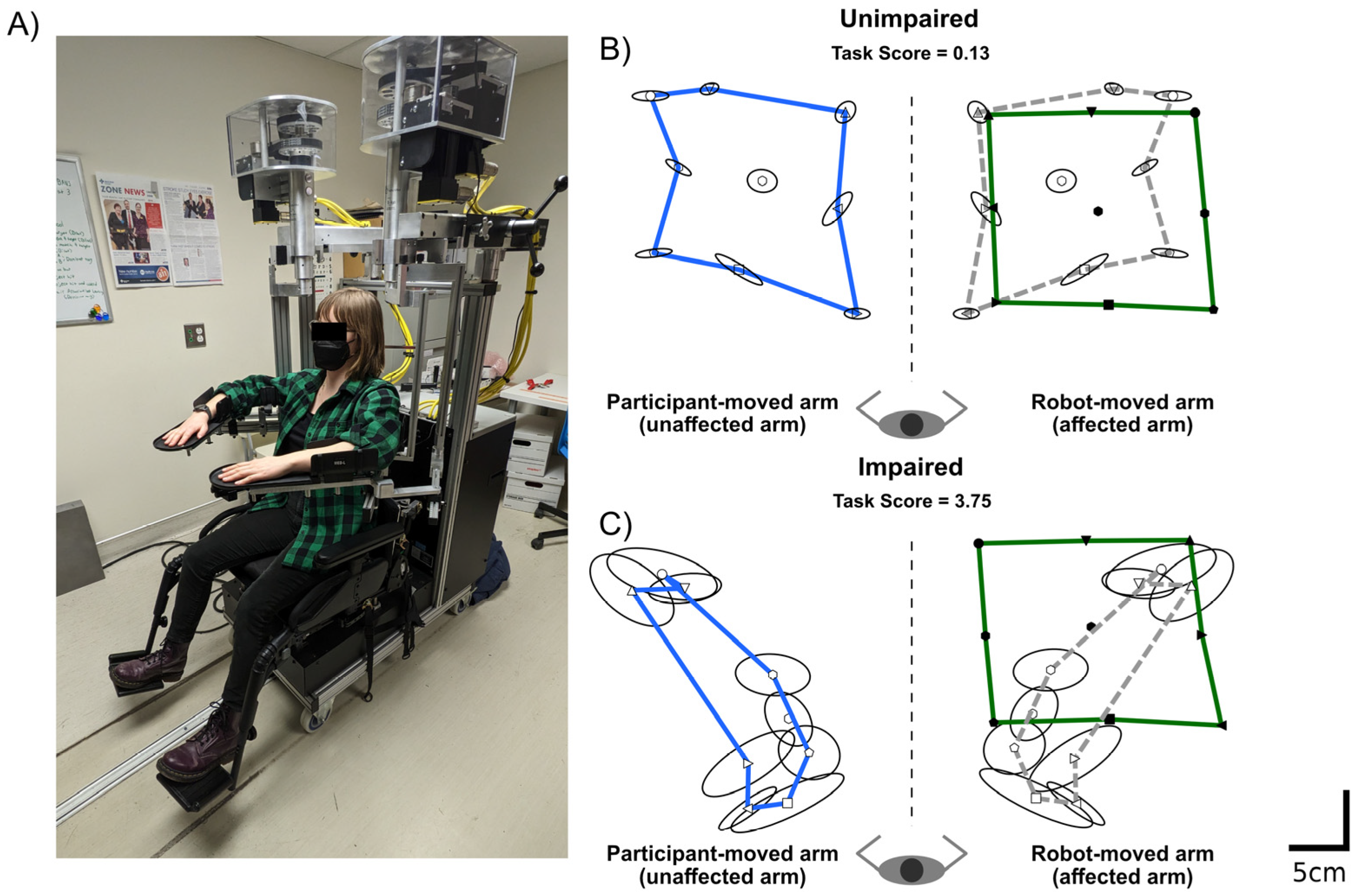
2.2. Robotic Assessment of Proprioception
2.3. Quantifying Proprioceptive Performance and Impairments
2.4. Clinical Assessment
2.5. Neuroimaging
Neuroimaging Measures
2.6. Statistical Analysis
3. Results
3.1. Participant Demographics and Other Predictors
3.2. Examining the Linear Relationships between Clinical, Neuroimaging and Robotic Features and Six-Month APM Task Scores
3.3. Examining Differences in Clinical, Robotic and Neuroimaging Features between Those Impaired and Unimpaired on the APM Task Score
3.4. Classification Models
3.4.1. Single Modality Models
3.4.2. Augmented Model
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherrington, C.S. On the proprioceptive system, especially in its reflex aspect. Brain 1906, 29, 467–482. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Dukelow, S.P.; Herter, T.M.; Moore, K.D.; Demers, M.J.; Glasgow, J.I.; Bagg, S.D.; Norman, K.E.; Scott, S.H. Quantitative assessment of limb position sense following stroke. Neurorehabilit. Neural Repair 2010, 24, 178–187. [Google Scholar] [CrossRef]
- Carey, L.M.; Oke, L.E.; Matyas, T.A. Impaired limb position sense after stroke: A quantitative test for clinical use. Arch. Phys. Med. Rehabil. 1996, 77, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Rand, D. Proprioception deficits in chronic stroke: Upper extremity function and daily living. PLoS ONE 2018, 13, e0195043. [Google Scholar] [CrossRef] [Green Version]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.B.; Tallis, R.C. Sensory loss in hospital-admitted people with stroke: Characteristics, associated factors, and relationship with function. Neurorehabilit. Neural Repair 2018, 22, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Dukelow, S.P.; Herter, T.M.; Bagg, S.D.; Scott, S.H. The independence of deficits in position sense and visually guided reaching following stroke. J. Neuroeng. Rehabil. 2012, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Mostafavi, S.M.; Mousavi, P.; Dukelow, S.P.; Scott, S.H. Robot-based assessment of motor and proprioceptive function identifies biomarkers for prediction of functional independence measures. J. Neuroeng. Rehabil. 2015, 12, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semrau, J.A.; Herter, T.M.; Scott, S.H.; Dukelow, S.P. Examining differences in patterns of sensory and motor recovery after stroke with robotics. Stroke 2015, 46, 3459–3469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findlater, S.E.; Hawe, R.L.; Semrau, J.A.; Kenzie, J.M.; Yu, A.Y.; Scott, S.H.; Dukelow, S.P. Lesion locations associated with persistent proprioceptive impairment in the upper limbs after stroke. Neuroimage Clin. 2018, 20, 955–971. [Google Scholar] [CrossRef]
- Findlater, S.E.; Dukelow, S.P. Upper Extremity Proprioception After Stroke: Bridging the Gap Between Neuroscience and Rehabilitation. J. Mot. Behav. 2017, 49, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.L.; Tommasino, P.; Budhota, A.; Campolo, D. Upper extremity proprioception in healthy aging and stroke populations, and the effects of therapist- and robot-based rehabilitation therapies on proprioceptive function. Front. Hum. Neurosci. 2015, 9, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumpa, L.U.; Cahill, L.S.; Carey, L.M. Somatosensory assessment and treatment after stroke: An evidence-practice gap. Aust. Occup. J. 2015, 62, 93–104. [Google Scholar] [CrossRef]
- Otaka, E.; Otaka, Y.; Kasuga, S.; Nishimoto, A.; Yamazaki, K.; Kawakami, M.; Ushiba, J.; Liu, M. Reliability of the thumb localizing test and its validity against quantitative measures with a robotic device in patients with hemiparetic stroke. PLoS ONE 2020, 15, e0236437. [Google Scholar] [CrossRef]
- Fisher, G.; De Oliveira, C.Q.; Verhagen, A.; Gandevia, S.; Kennedy, D. Proprioceptive impairment in unilateral neglect after stroke: A systematic review. SAGE Open Med. 2020, 8, 205031212095107. [Google Scholar] [CrossRef]
- Chilvers, M.J.; Hawe, R.L.; Scott, S.H.; Dukelow, S.P. Investigating the neuroanatomy underlying proprioception using a stroke model. J. Neurol. Sci. 2021, 430, 120029. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; De Bruyn, N.; Lafosse, C.; Van Dijk, M.; Michielsen, M.; Thijs, L.; Truyens, V.; Oostra, K.; Krumlinde-Sundholm, L.; Peeters, A.; et al. Somatosensory impairments in the upper limb poststroke: Distribution and association with motor function and visuospatial neglect. Neurorehabilit. Neural Repair 2016, 30, 731–742. [Google Scholar] [CrossRef]
- Semrau, J.A.; Wang, J.C.; Herter, T.M.; Scott, S.H.; Dukelow, S.P. Relationship between visuospatial neglect and kinesthetic deficits after stroke. Neurorehabilit. Neural Repair 2015, 29, 318–328. [Google Scholar] [CrossRef]
- Hawe, R.L.; Findlater, S.E.; Kenzie, J.M.; Hill, M.D.; Scott, S.H.; Dukelow, S.P.; Sunderland, A.; Marquine, M.J.; Attix, D.K.; Goldstein, L.B.; et al. Differential impact of acute lesions versus white matter hyperintensities on stroke recovery. J. Am. Heart Assoc. 2018, 7, e009360. [Google Scholar] [CrossRef] [Green Version]
- Kenzie, J.M.; Semrau, J.A.; Findlater, S.E.; Yu, A.Y.; Desai, J.A.; Herter, T.M.; Hill, M.D.; Scott, S.H.; Dukelow, S.P. Localization of impaired kinesthetic processing post-stroke. Front. Hum. Neurosci. 2016, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Findlater, S.E.; Desai, J.A.; Semrau, J.A.; Kenzie, J.M.; Rorden, C.; Herter, T.M.; Scott, S.H.; Dukelow, S.P. Central perception of position sense involves a distributed neural network—Evidence from lesion-behavior analyses. Cortex 2016, 79, 42–56. [Google Scholar] [CrossRef]
- Meyer, S.; Kessner, S.S.; Cheng, B.; Bönstrup, M.; Schulz, R.; Hummel, F.C.; De Bruyn, N.; Peeters, A.; Van Pesch, V.; Duprez, T.; et al. Voxel-based lesion-symptom mapping of stroke lesions underlying somatosensory deficits. Neuroimage Clin. 2016, 10, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Ramsey, L.; Metcalf, N.V.; Rengachary, J.; Shulman, G.L.; Shimony, J.S.; Corbetta, M. Stronger prediction of motor recovery and outcome post-stroke by cortico-spinal tract integrity than functional connectivity. PLoS ONE 2018, 13, e0202504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feys, H.; De Weerdt, W.; Nuyens, G.; Van De Winckel, A.; Selz, B.; Kiekens, C. Predicting motor recovery of the upper limb after stroke rehabilitation: Value of a clinical examination. Physiother. Res. Int. 2000, 5, 1–18. [Google Scholar] [CrossRef]
- Chen, C.-L.; Tang, F.-T.; Chen, H.-C.; Chung, C.-Y.; Wong, M.-K. Brain lesion size and location: Effects on motor recovery and functional outcome in stroke patients. Arch. Phys. Med. Rehabil. 2000, 81, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Kollen, B. Predicting improvement in the upper paretic limb after stroke: A longitudinal prospective study. Restor. Neurol. Neurosci. 2007, 25, 453–460. [Google Scholar] [PubMed]
- DeVetten, G.; Coutts, S.B.; Hill, M.D.; Goyal, M.; Eesa, M.; O’Brien, B.; Demchuk, A.M.; Kirton, A. Acute corticospinal tract wallerian degeneration is associated with stroke outcome. Stroke 2010, 41, 751–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinear, C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010, 9, 1228–1232. [Google Scholar] [CrossRef]
- Boyd, L.A.; Hayward, K.S.; Ward, N.S.; Stinear, C.M.; Rosso, C.; Fisher, R.J.; Carter, A.R.; Leff, A.P.; Copland, D.A.; Carey, L.M.; et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int. J. Stroke 2017, 12, 480–493. [Google Scholar] [CrossRef] [Green Version]
- van Heugten, C.M.; Dekker, J.; Deelman, B.G.; De Stehmann-Saris, A.K. A diagnostic test for apraxia in stroke patients: Internal consistency and diagnostic value. Clin. Neuropsychol. 1999, 13, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.H.; Dukelow, S.P. Potential of robots as next-generation technology for clinical assessment of neurological disorders and upper-limb therapy. J. Rehabil. Res. Dev. 2011, 48, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Kinarm Dexterit-E 3.9; Addendum: Kinarm Standard Tests Summary. BKIN Technologies Ltd.: Kingston, ON, Canada, 2021. Available online: https://kinarm.com/download/kst-summary-analysis-version-3-9/ (accessed on 4 June 2022).
- Scott, S.H.; Lowrey, C.R.; Brown, I.E.; Dukelow, S.P. Assessment of neurological impairment and recovery using statistical models of neurologically healthy behavior. Neurorehabilit. Neural Repair 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simmatis, L.E.R.; Early, S.; Moore, K.D.; Appaqaq, S.; Scott, S.H. Statistical measures of motor, sensory and cognitive performance across repeated robot-based testing. J. Neuroeng. Rehabil. 2020, 17, 86. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K.; Fukutake, T.; Kawamura, M. “Thumb localizing test” for detecting a lesion in the posterior column-medial lemniscal system. J. Neurol. Sci. 1999, 167, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Cockburn, J.; Halligan, P. Development of a behavioral test of visuospatial neglect. Arch. Phys. Med. Rehabil. 1987, 68, 98–102. [Google Scholar]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar]
- Rorden, C.; Bonilha, L.; Fridriksson, J.; Bender, B.; Karnath, H.O. Age-specific CT and MRI templates for spatial normalization. Neuroimage 2012, 61, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Bates, E.; Wilson, S.M.; Saygin, A.P. Voxel-Based Lesion-Symptom Mapping. Nat. Neurosci. 2003, 6, 448–450. [Google Scholar] [CrossRef]
- Geva, S.; Jones, P.S.; Crinion, J.T.; Price, C.J.; Baron, J.-C.; Warburton, E.A. The neural correlates of inner speech defined by voxel-based lesion-symptom mapping. Brain 2011, 134, 3071–3082. [Google Scholar] [CrossRef]
- Kalénine, S.; Buxbaum, L.J.; Coslett, H.B. Critical brain regions for action recognition: Lesion symptom mapping in left hemisphere stroke. Brain 2010, 133, 3269–3280. [Google Scholar] [CrossRef]
- Reid, J.M.; Gubitz, G.J.; Dai, D.; Kydd, D.; Eskes, G.; Reidy, Y.; Christian, C.; Counsell, C.E.; Dennis, M.; Phillips, S.J. Predicting functional outcome after stroke by modelling baseline clinical and CT variables. Age Ageing 2010, 39, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Forkert, N.D.; Verleger, T.; Cheng, B.; Thomalla, G.; Hilgetag, C.C.; Fiehler, J. Multiclass support vector machine-based lesion mapping predicts functional outcome in ischemic stroke patients. PLoS ONE 2015, 10, e0129569. [Google Scholar] [CrossRef]
- Meijer, R.; Ihnenfeldt, D.S.; De Groot, I.J.M.; Van Limbeek, J.; Vermeulen, M.; De Haan, R.J. Prognostic factors for ambulation and activities of daily living in the subacute phase after stroke. A systematic review of the literature. Clin. Rehabil. 2003, 17, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.; Ziegler, A.; König, I.R.; Diener, H.C. Predicting functional outcome and survival after acute ischemic stroke. J. Neurol. 2002, 249, 888–895. [Google Scholar] [CrossRef]
- Thijs, V.N.; Lansberg, M.G.; Beaulieu, C.; Marks, M.P.; Moseley, M.E.; Albers, G.W. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke 2000, 31, 2597–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakkel, G.; Wagenaar, R.C.; Kollen, B.J.; Lankhorst, G.J. Predicting disability in stroke—A critical review of the literature. Age Ageing 1996, 25, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Johnston, K.C.; Barrett, K.M.; Ding, Y.H.; Wagner, D.P. Clinical and imaging data at 5 days as a surrogate for 90-day outcome in ischemic stroke. Stroke 2009, 40, 1332–1333. [Google Scholar] [CrossRef] [Green Version]
- Scrutinio, D.; Lanzillo, B.; Guida, P.; Mastropasqua, F.; Monitillo, V.; Pusineri, M.; Formica, R.; Russo, G.; Guarnaschelli, C.; Ferretti, C.; et al. Development and validation of a predictive model for functional outcome after stroke rehabilitation: The Maugeri model. Stroke 2017, 48, 3308–3315. [Google Scholar] [CrossRef]
- Kent, D.M.; Selker, H.P.; Ruthazer, R.; Bluhmki, E.; Hacke, W. The stroke-thrombolytic predictive instrument: A predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke 2006, 37, 2957–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijland, R.H.M.; Van Wegen, E.E.H.; Harmeling-Van Der Wel, B.C.; Kwakkel, G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: Early prediction of functional outcome after stroke: The EPOS cohort study. Stroke 2010, 41, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Stinear, C.M.; Barber, P.A.; Petoe, M.; Anwar, S.; Byblow, W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012, 135, 2527–2535. [Google Scholar] [CrossRef] [Green Version]
- Stinear, C.M.; Barber, P.A.; Smale, P.R.; Coxon, J.P.; Fleming, M.K.; Byblow, W.D. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007, 130, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Meyer, S.; Karttunen, A.H.; Thijs, V.; Feys, H.; Verheyden, G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Phys. Ther. 2014, 94, 1220–1231. [Google Scholar] [CrossRef] [Green Version]
- Carey, L.M.; Matyas, T.A.; Oke, L.E. Sensory loss in stroke patients: Effective training of tactile and proprioceptive discrimination. Arch. Phys. Med. Rehabil. 1993, 74, 602–611. [Google Scholar] [CrossRef]
- Cuppone, A.V.; Squeri, V.; Semprini, M.; Masia, L.; Konczak, J. Robot-assisted proprioceptive training with added vibro-tactile feedback enhances somatosensory and motor performance. PLoS ONE 2016, 11, e0164511. [Google Scholar] [CrossRef] [Green Version]
- Cordo, P.; Lutsep, H.; Cordo, L.; Wright, W.G.; Cacciatore, T.; Skoss, R. Assisted movement with enhanced sensation (AMES): Coupling motor and sensory to remediate motor deficits in chronic stroke patients. Neurorehabilit. Neural Repair 2009, 23, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadio, M.; Morasso, P.; Sanguineti, V.; Giannoni, P. Minimally assistive robot training for proprioception enhancement. Exp. Brain Res. 2009, 194, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Santis, D.E.; Ezenzeri, J.; Ecasadio, M.; Emasia, L.; Eriva, A.; Emorasso, P.; Esqueri, V. Robot-assisted training of the kinesthetic sense: Enhancing proprioception after stroke. Front. Hum. Neurosci. 2015, 8, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aman, J.E.; Elangovan, N.; Yeh, I.-L.; Konczak, J. The effectiveness of proprioceptive training for improving motor function: A systematic review. Front. Hum. Neurosci. 2015, 8, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schabrun, S.M.; Hillier, S. Evidence for the retraining of sensation after stroke: A systematic review. Clin. Rehabil. 2009, 23, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, N.; Yeh, I.L.; Holst-Wolf, J.; Konczak, J. A Robot-Assisted Sensorimotor Training Program can Improve Proprioception and Motor Function in Stroke Survivors. In Proceedings of the 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019. [Google Scholar]
- Cho, S.; Ku, J.; Cho, Y.K.; Kim, I.Y.; Kang, Y.J.; Jang, D.P.; Kim, S.I. Development of virtual reality proprioceptive rehabilitation system for stroke patients. Comput. Methods Programs Biomed. 2014, 113, 258–265. [Google Scholar] [CrossRef]
- Nam, H.S.; Koh, S.; Beom, J.; Kim, Y.J.; Park, J.W.; Koh, E.-S.; Chung, S.G.; Kim, S. Recovery of proprioception in the upper extremity by robotic mirror therapy: A clinical pilot study for proof of concept. J. Korean Med. Sci. 2017, 32, 1568. [Google Scholar] [CrossRef] [PubMed]
- Dukelow, S.P. The potential power of robotics for upper extremity stroke rehabilitation. Int. J. Stroke 2017, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Sainburg, R.L. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 2002, 142, 241–258. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Kalakanis, D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J. Neurophysiol. 2000, 83, 2661–2675. [Google Scholar] [CrossRef] [Green Version]
- Bagesteiro, L.B.; Sainburg, R.L. Nondominant arm advantages in load compensation during rapid elbow joint movements. J. Neurophysiol. 2003, 90, 1503–1513. [Google Scholar] [CrossRef]
- Bagesteiro, L.B.; Sainburg, R.L. Handedness: Dominant arm advantages in control of limb dynamics. J. Neurophysiol. 2002, 88, 2408–2421. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sainburg, R.L. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp. Brain Res. 2007, 178, 565–570. [Google Scholar] [CrossRef]
- Amemiya, K.; Naito, E. Importance of human right inferior frontoparietal network connected by inferior branch of superior longitudinal fasciculus tract in corporeal awareness of kinesthetic illusory movement. Cortex 2016, 78, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, E.; Nakashima, T.; Kito, T.; Aramaki, Y.; Okada, T.; Sadato, N. Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur. J. Neurosci. 2007, 25, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Morita, T.; Saito, D.N.; Ban, M.; Shimada, K.; Okamoto, Y.; Kosaka, H.; Okazawa, H.; Asada, M. Development of right-hemispheric dominance of inferior parietal lobule in proprioceptive illusion task. Cereb. Cortex 2017, 27, 5385–5397. [Google Scholar] [CrossRef] [Green Version]
- Naito, E.; Roland, P.E.; Grefkes, C.; Choi, H.J.; Eickhoff, S.B.; Geyer, S.; Zilles, K.; Ehrsson, H.H.; Proske, U.; Gandevia, S.C.; et al. Dominance of the right hemisphere and role of area 2 in human kinesthesia. J. Neurophysiol. 2005, 93, 1020–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkhoff, A.K.; Grefkes, C. Precision medicine in stroke: Towards personalized outcome predictions using artificial intelligence. Brain 2022, 145, 457–475. [Google Scholar] [CrossRef]
- Shmueli, G. To explain or to predict? Stat. Sci. 2010, 25, 289–310. [Google Scholar] [CrossRef]
- Bzdok, D.; Engemann, D.; Thirion, B. Inference and Prediction Diverge in Biomedicine. Patterns 2020, 1, 100119. [Google Scholar] [CrossRef] [PubMed]




| Model | Measures Included |
|---|---|
| Basic model | Age, Sex, Affected Arm |
| Clinical model | Age, Sex, Affected Arm, TLT, BIT, FIM |
| Imaging model | Age, Sex, Affected Arm, VLSM mean Z, Lesion Volume |
| Robotic model | Age, Sex, Affected Arm, Absolute Error X, Absolute Error Y, Variability X, Variability Y, Contraction Expansion X, Contraction Expansion Y, Shift X, Shift Y |
| Augmented model | Age, Sex, Affected Arm, TLT, BIT, FIM, VLSM mean Z, Lesion Volume, Absolute Error X, Absolute Error Y, Variability X, Variability Y, Contraction Expansion X, Contraction Expansion Y, Shift X, Shift Y |
| Age | 60.2 ± 13.0 |
| Sex | Males = 91, Females = 42 |
| Affected Arm | Right = 55, Left = 78 |
| TLT | 0 = 50, 1 = 36, 2 = 33, 3 = 14 |
| BIT | 141 (58–146) |
| FIM | 102 (35–126) |
| Lesion Volume (cc) | 35.1 ± 53.5 |
| VLSM Mean Z | 1.652 ± 1.223 |
| Absolute Error X (z-score) | 1.42 ± 1.30 |
| Absolute Error Y (z-score) | 1.49 ± 1.28 |
| Variability X (z-score) | 2.15 ± 1.93 |
| Variability Y (z-score) | 2.67 ± 2.12 |
| Contraction Expansion X | −1.71 ± 2.20 |
| Contraction Expansion Y | −2.27 ± 3.77 |
| Shift X | −0.40 ± 1.78 |
| Shift Y | −0.64 ± 1.98 |
| APM Task Score (six months) | 1.90 ± 1.55 |
| Accuracy | F1-Score | AUC | Sensitivity | Specificity | AIC | |
|---|---|---|---|---|---|---|
| Basic Model | 63.16 | 0.49 | 0.45 | 0.00 | 98.82 * | 176.94 |
| Clinical model | 78.95 * | 0.78 * | 0.79 | 62.50 | 88.24 | 146.96 |
| Imaging model | 72.18 | 0.71 | 0.74 | 47.92 | 85.88 | 148.43 |
| Robotic model | 77.44 | 0.77 | 0.84 | 68.75* | 82.35 | 120.07 * |
| Augmented model | 76.69 | 0.77 | 0.86* | 64.58 | 83.53 | 126.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilvers, M.J.; Rajashekar, D.; Low, T.A.; Scott, S.H.; Dukelow, S.P. Clinical, Neuroimaging and Robotic Measures Predict Long-Term Proprioceptive Impairments following Stroke. Brain Sci. 2023, 13, 953. https://doi.org/10.3390/brainsci13060953
Chilvers MJ, Rajashekar D, Low TA, Scott SH, Dukelow SP. Clinical, Neuroimaging and Robotic Measures Predict Long-Term Proprioceptive Impairments following Stroke. Brain Sciences. 2023; 13(6):953. https://doi.org/10.3390/brainsci13060953
Chicago/Turabian StyleChilvers, Matthew J., Deepthi Rajashekar, Trevor A. Low, Stephen H. Scott, and Sean P. Dukelow. 2023. "Clinical, Neuroimaging and Robotic Measures Predict Long-Term Proprioceptive Impairments following Stroke" Brain Sciences 13, no. 6: 953. https://doi.org/10.3390/brainsci13060953
APA StyleChilvers, M. J., Rajashekar, D., Low, T. A., Scott, S. H., & Dukelow, S. P. (2023). Clinical, Neuroimaging and Robotic Measures Predict Long-Term Proprioceptive Impairments following Stroke. Brain Sciences, 13(6), 953. https://doi.org/10.3390/brainsci13060953








