Altered Spontaneous Brain Activity in Cirrhotic Patients with Minimal Hepatic Encephalopathy: A Meta-Analysis of Resting-State Functional Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
2.4.1. Voxel-Based Meta-Analysis
2.4.2. Conjunction Analysis between Meta-Analysis Groups
2.4.3. Subgroup Meta-Analysis and Meta-Regression Analysis
3. Results
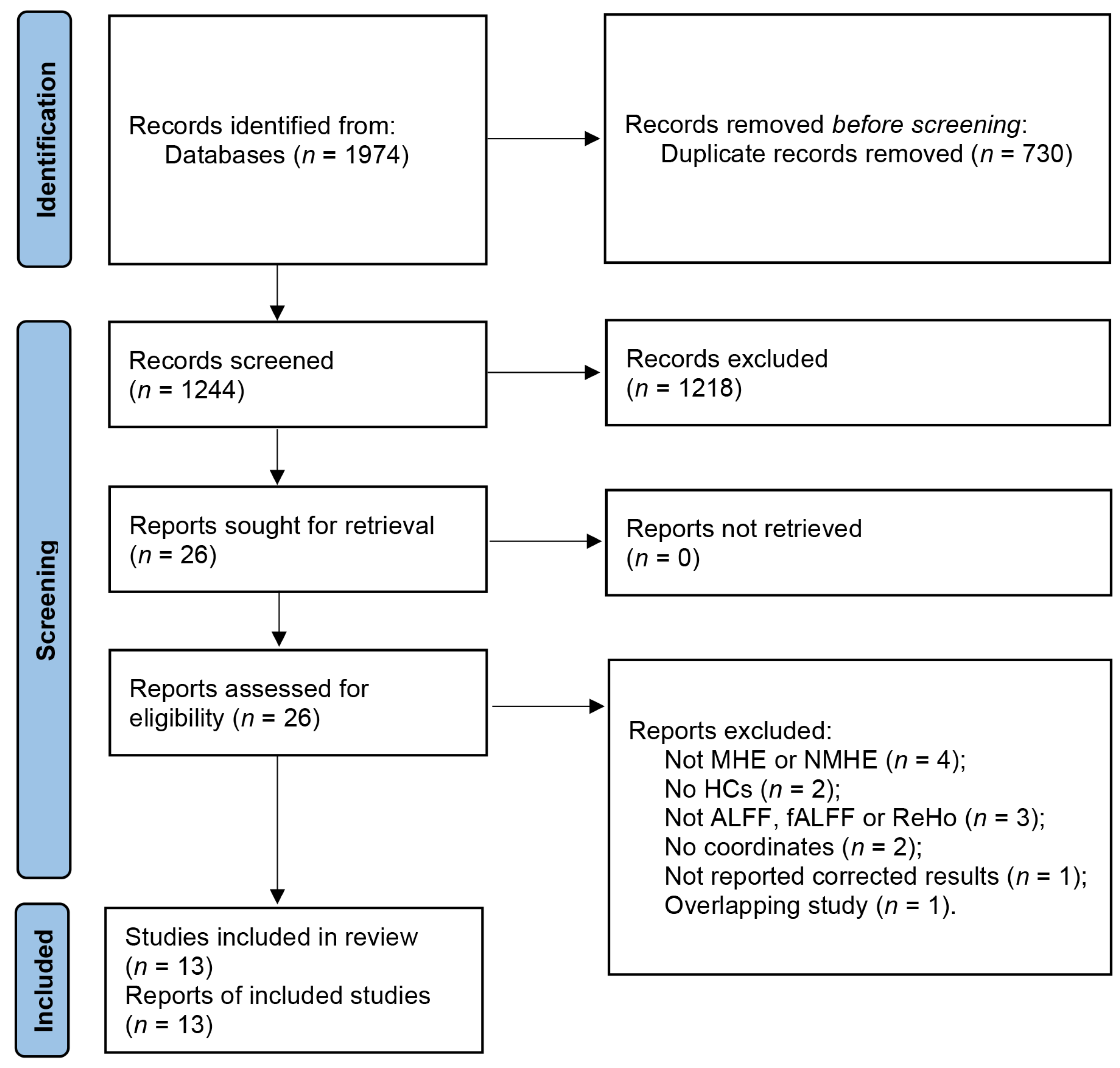
3.1. Included Studies and Sample Characteristics
3.2. Results of the Main Meta-Analysis
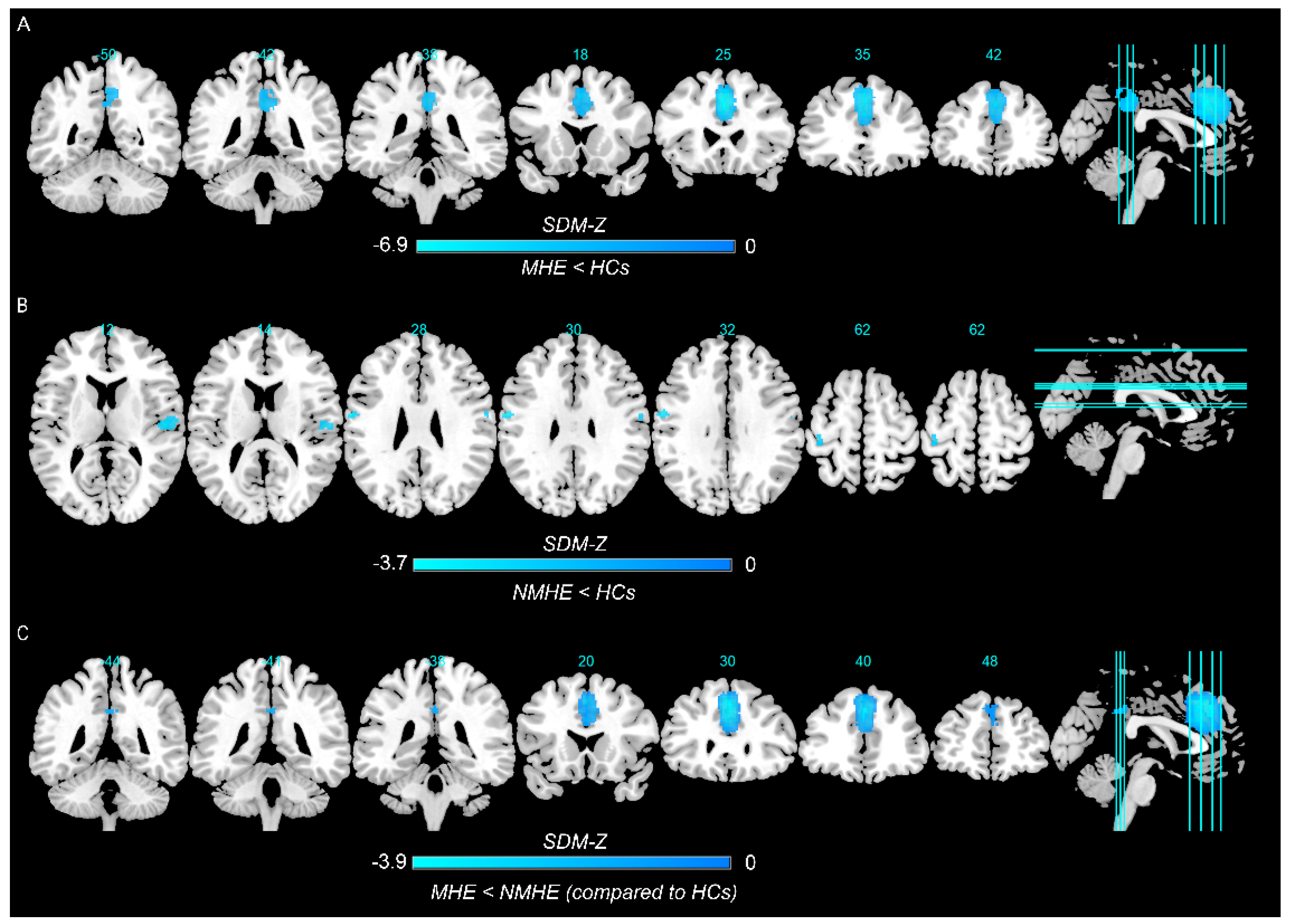
3.2.1. MHE Patients versus HCs
3.2.2. NMHE Patients versus HCs
3.2.3. Conjunction Analysis between MHE and NMHE Patients as Compared to HCs
3.3. Subgroup Meta-Analysis and Meta-Regression Analysis
3.4. Analyses of Heterogeneity and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nardelli, S.; Gioia, S.; Faccioli, J.; Riggio, O.; Ridola, L. Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy. World J. Gastroenterol. 2019, 25, 5257–5265. [Google Scholar] [CrossRef]
- Guo, J.R.; Shi, J.Y.; Dong, Q.Y.; Cao, Y.B.; Li, D.; Chen, H.J. Altered dynamic spontaneous neural activity in minimal hepatic encephalopathy. Front. Neurol. 2022, 13, 963551. [Google Scholar] [CrossRef]
- Karanfilian, B.V.; Park, T.; Senatore, F.; Rustgi, V.K. Minimal Hepatic Encephalopathy. Clin. Liver Dis. 2020, 24, 209–218. [Google Scholar] [CrossRef]
- Rudler, M.; Weiss, N.; Bouzbib, C.; Thabut, D. Diagnosis and Management of Hepatic Encephalopathy. Clin. Liver Dis 2021, 25, 393–417. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D.; Waljee, A.K.; Volk, M.; Carlozzi, N.E.; Lok, A.S. Diagnosis of Minimal Hepatic Encephalopathy: A Systematic Review of Point-of-Care Diagnostic Tests. Am. J. Gastroenterol. 2018, 113, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Krishnarao, A.; Gordon, F.D. Prognosis of Hepatic Encephalopathy. Clin. Liver Dis. 2020, 24, 219–229. [Google Scholar] [CrossRef]
- Ridola, L.; Cardinale, V.; Riggio, O. The burden of minimal hepatic encephalopathy: From diagnosis to therapeutic strategies. Ann. Gastroenterol. 2018, 31, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Scheres, A.; Margulies, D.S.; Kelly, A.M.; Uddin, L.Q.; Shehzad, Z.; Biswal, B.; Walters, J.R.; Castellanos, F.X.; Milham, M.P. Functional connectivity of human striatum: A resting state FMRI study. Cereb. Cortex 2008, 18, 2735–2747. [Google Scholar] [CrossRef]
- Xu, Y.; Zhuo, C.; Qin, W.; Zhu, J.; Yu, C. Altered Spontaneous Brain Activity in Schizophrenia: A Meta-Analysis and a Large-Sample Study. Biomed Res. Int. 2015, 2015, 204628. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Zhu, X.Q.; Jiao, Y.; Li, P.C.; Wang, Y.; Teng, G.J. Abnormal baseline brain activity in low-grade hepatic encephalopathy: A resting-state fMRI study. J. Neurol. Sci. 2012, 318, 140–145. [Google Scholar] [CrossRef]
- Ni, L.; Qi, R.; Zhang, L.J.; Zhong, J.; Zheng, G.; Zhang, Z.; Zhong, Y.; Xu, Q.; Liao, W.; Jiao, Q.; et al. Altered regional homogeneity in the development of minimal hepatic encephalopathy: A resting-state functional MRI study. PLoS ONE 2012, 7, e42016. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, L.; Wu, S.; Zhong, J.; Zhang, Z.; Zhong, Y.; Ni, L.; Zhang, Z.; Li, K.; Jiao, Q.; et al. Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology 2012, 264, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fan, W.; Ye, J.; Han, P. Abnormal Regional Homogeneity and Functional Connectivity of Baseline Brain Activity in Hepatitis B Virus-Related Cirrhosis with and Without Minimal Hepatic Encephalopathy. Front. Hum. Neurosci. 2018, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.J.; Zhou, Z.M.; Zhao, J.N.; Wu, W.; Guo, D.J. Abnormal spontaneous brain activity in minimal hepatic encephalopathy: Resting-state fMRI study. Diagn. Interv. Radiol. 2016, 22, 196–200. [Google Scholar] [CrossRef]
- Albajes-Eizagirre, A.; Solanes, A.; Vieta, E.; Radua, J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage 2019, 186, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cai, M.; Liu, J.; Wang, X.; Ma, J.; Ma, L.; Liu, M.; Zhao, Y.; Wang, H.; Fu, D.; Wang, W.; et al. Spontaneous brain activity abnormalities in migraine: A meta-analysis of functional neuroimaging. Hum. Brain Mapp. 2022, 44, 571–584. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Gong, J.; Qi, Z.; Fu, S.; Tang, G.; Chen, P.; Huang, L.; Wang, Y. Functional and structural brain differences in bipolar disorder: A multimodal meta-analysis of neuroimaging studies. Psychol. Med. 2022, 52, 2861–2873. [Google Scholar] [CrossRef]
- Albajes-Eizagirre, A.; Solanes, A.; Fullana, M.A.; Ioannidis, J.P.A.; Fusar-Poli, P.; Torrent, C.; Sole, B.; Bonnin, C.M.; Vieta, E.; Mataix-Cols, D.; et al. Meta-analysis of Voxel-Based Neuroimaging Studies using Seed-based d Mapping with Permutation of Subject Images (SDM-PSI). J. Vis. Exp. 2019, 27, e59841. [Google Scholar] [CrossRef] [Green Version]
- Radua, J.; Mataix-Cols, D.; Phillips, M.L.; El-Hage, W.; Kronhaus, D.M.; Cardoner, N.; Surguladze, S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 2012, 27, 605–611. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Radua, J.; Grau, M.; van den Heuvel, O.A.; Thiebaut de Schotten, M.; Stein, D.J.; Canales-Rodriguez, E.J.; Catani, M.; Mataix-Cols, D. Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology 2014, 39, 1547–1557. [Google Scholar] [CrossRef] [Green Version]
- Radua, J.; Mataix-Cols, D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry 2009, 195, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Zhu, X.Q.; Yang, M.; Liu, B.; Zhang, Y.; Wang, Y.; Teng, G.J. Changes in the regional homogeneity of resting-state brain activity in minimal hepatic encephalopathy. Neurosci. Lett. 2012, 507, 5–9. [Google Scholar] [CrossRef]
- Ji, J.; Zhao, C.Y.; Liu, Y.Y.; Zhang, D.Q.; Wang, Y.; Ding, X.C.; Wang, X.D. Correlation between changes of amplitude of low-frequency fluctuation and cognitive impairment in patients with mild hepatic encephalopathy. Chin. J. Neuromed. 2020, 19, 1109–1115. [Google Scholar] [CrossRef]
- Jiang, X.P.; Qian, W.W.; Zhang, L.; Zhang, J.Q. Brain resting state function and brain metabolism in patients with hepatic encephalopathy at different stages of development. Radiol. Pract. 2017, 32, 696–700. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zhao, J.N.; Zhou, Z.M.; Guo, D.J. fMRI evaluation of alterations of baseline brain activity in patients with hepatic encephalopathy. Chin. J. Med. Imaging Technol. 2015, 31, 701–705. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Tao, R.; You, Z.L.; Zhang, J.Q.; Wang, J.; Cui, J.G. MRI characteristics of structural and functional brain abnormalities in minimal hepatic encephalopathy. Chin. J. Med. Imaging Technol. 2015, 31, 340–346. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, D.J.; Zhao, J.N.; Zhong, W.J.; Zhang, W.; Wu, W.; Zhang, B.L.; Zhou, Z.M.; Wang, W.Q.; Zhou, H.L. Regional homogeneity of resting-state brain activity in patients with minimal hepatic encephalopathy. J. Chongqing Med. Univ. 2014, 39, 466–469. [Google Scholar] [CrossRef]
- Yang, X.H.; Chen, H.J.; Huang, X.Y.; Liu, W.X.; Wang, M.L.; Ge, X.; Zhang, W.; Dang, P.; Ding, X.C.; Wang, X.D. Homogeneity of cognitive function altered in patients with minimal hepatic encephalopathy: A fMRI based study. Chin. J. Magn. Reson. Imaging 2022, 13, 77–81 + 88. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Zhao, J.N.; Guo, D.J.; Zhang, W.; Wu, W. Functional MRI observation on abnormal brain activity in patients with minimal hepatic encephalopathy based on non-alcoholic cirrhosis. Chin. J. Med. Imaging Technol. 2014, 30, 24–28. [Google Scholar] [CrossRef]
- Briggs, R.G.; Khan, A.B.; Chakraborty, A.R.; Abraham, C.J.; Anderson, C.D.; Karas, P.J.; Bonney, P.A.; Palejwala, A.H.; Conner, A.K.; O’Donoghue, D.L.; et al. Anatomy and White Matter Connections of the Superior Frontal Gyrus. Clin. Anat. 2020, 33, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qin, W.; Liu, H.; Fan, L.; Wang, J.; Jiang, T.; Yu, C. Subregions of the human superior frontal gyrus and their connections. Neuroimage 2013, 78, 46–58. [Google Scholar] [CrossRef]
- du Boisgueheneuc, F.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the left superior frontal gyrus in humans: A lesion study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef] [Green Version]
- Alagapan, S.; Lustenberger, C.; Hadar, E.; Shin, H.W.; Fröhlich, F. Low-frequency direct cortical stimulation of left superior frontal gyrus enhances working memory performance. Neuroimage 2019, 184, 697–706. [Google Scholar] [CrossRef]
- Weissenborn, K.; Giewekemeyer, K.; Heidenreich, S.; Bokemeyer, M.; Berding, G.; Ahl, B. Attention, memory, and cognitive function in hepatic encephalopathy. Metab. Brain Dis. 2005, 20, 359–367. [Google Scholar] [CrossRef]
- Liao, L.M.; Zhou, L.X.; Le, H.B.; Yin, J.J.; Ma, S.H. Spatial working memory dysfunction in minimal hepatic encephalopathy: An ethology and BOLD-fMRI study. Brain Res. 2012, 1445, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, B.; Chen, T.; Diao, W.; Jia, Z. Altered intrinsic brain activity in patients with hepatic encephalopathy. J. Neurosci. Res. 2021, 99, 1337–1353. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhang, W.; Chen, L.; Ren, Y.; Cao, Y.; Sun, T.; Sun, B.; Liu, J.; Wang, J.; Zheng, C. Brain Gray Matter Alterations in Hepatic Encephalopathy: A Voxel-Based Meta-Analysis of Whole-Brain Studies. Front. Hum. Neurosci. 2022, 16, 838666. [Google Scholar] [CrossRef]
- Jiang, B.; He, D.; Guo, Z.; Gao, Z. Effect-size seed-based d mapping of resting-state fMRI for persistent insomnia disorder. Sleep Breath 2020, 24, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Dagher, A.; Owen, A.M.; Boecker, H.; Brooks, D.J. Mapping the network for planning: A correlational PET activation study with the Tower of London task. Brain 1999, 122 Pt 10, 1973–1987. [Google Scholar] [CrossRef] [Green Version]
- Duncan, J.; Owen, A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000, 23, 475–483. [Google Scholar] [CrossRef]
- Fornito, A.; Yucel, M.; Wood, S.; Stuart, G.W.; Buchanan, J.A.; Proffitt, T.; Anderson, V.; Velakoulis, D.; Pantelis, C. Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb. Cortex 2004, 14, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Ridola, L.; Nardelli, S.; Gioia, S.; Riggio, O. Quality of life in patients with minimal hepatic encephalopathy. World J. Gastroenterol. 2018, 24, 5446–5453. [Google Scholar] [CrossRef]
- Stewart, C.A.; Smith, G.E. Minimal hepatic encephalopathy. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 677–685. [Google Scholar] [CrossRef]
- Buganza-Torio, E.; Mitchell, N.; Abraldes, J.G.; Thomas, L.; Ma, M.; Bailey, R.J.; Tandon, P. Depression in cirrhosis—A prospective evaluation of the prevalence, predictors and development of a screening nomogram. Aliment. Pharmacol. Ther. 2019, 49, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Xu, Q.; Zhang, L.J.; Zhong, J.; Zheng, G.; Wu, S.; Zhang, Z.; Liao, W.; Zhong, Y.; Ni, L.; et al. Structural and functional abnormalities of default mode network in minimal hepatic encephalopathy: A study combining DTI and fMRI. PLoS ONE 2012, 7, e41376. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utevsky, A.V.; Smith, D.V.; Huettel, S.A. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014, 34, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoliu, C.; Gonzalez-Escamilla, G.; Atienza, M.; Urios, A.; Gonzalez, O.; Wassel, A.; Aliaga, R.; Giner-Duran, R.; Serra, M.A.; Rodrigo, J.M.; et al. Focal cortical damage parallels cognitive impairment in minimal hepatic encephalopathy. Neuroimage 2012, 61, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, C.; Urios, A.; Forn, C.; Garcia-Panach, J.; Avila, C.; Gimenez-Garzo, C.; Wassel, A.; Serra, M.A.; Giner-Duran, R.; Gonzalez, O.; et al. Reduced white matter microstructural integrity correlates with cognitive deficits in minimal hepatic encephalopathy. Gut 2014, 63, 1028–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supekar, K.; de Los Angeles, C.; Ryali, S.; Cao, K.; Ma, T.; Menon, V. Deep learning identifies robust gender differences in functional brain organization and their dissociable links to clinical symptoms in autism. Br. J. Psychiatry 2022, 13, 202–209. [Google Scholar] [CrossRef]
| Patient Information | Healthy Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Types | No | Female (%) | Mean Age (Year) | NCT-A (s) | DST (Score) | Ammonia (μmol/L) | Methods | No | Female (%) | Mean Age (Year) | NCT-A (s) | DST (Score) |
| Chen et al., 2012 [10] | MHE | 22 | 9.09 | 53.10 ± 7.70 | NA | 22.80 ± 6.10 | NA | ALFF | 19 | 15.79 | 51.30 ± 7.80 | NA | 43.60 ± 9.30 |
| NMHE | 18 | 11.11 | 50.60 ± 8.80 | NA | 40.40 ± 11.10 | NA | ALFF | 19 | 15.79 | 51.30 ± 7.80 | NA | 43.60 ± 9.30 | |
| Chen et al., 2012 [24] | MHE | 18 | 11.11 | 54.80 ± 6.10 | NA | 23.10 ± 4.40 | NA | ReHo | 18 | 11.11 | 51.60 ± 7.90 | NA | 43.70 ± 9.50 |
| Ji et al., 2020 [25] | MHE | 31 | 38.71 | 43.51 ± 7.24 | 70.60 ± 34.9 | 21.10 ± 4.10 | 71.72 ± 8.50 | ALFF | 33 | 48.48 | 46.87 ± 7.24 | 20.65 ± 8.43 | 44.05 ± 11.00 |
| NMHE | 28 | 46.43 | 45.32 ± 8.17 | 34.24 ± 5.15 | 44.86 ± 9.05 | 55.20 ± 6.53 | ALFF | 33 | 48.48 | 46.87 ± 7.24 | 20.65 ± 8.43 | 44.05 ± 11.00 | |
| Jiang et al., 2017 [26] | MHE | 22 | 31.82 | 53.60 ± 1.50 | NA | NA | NA | fALFF | 13 | 38.46 | 53.80 ± 1.70 | NA | NA |
| Ni et al., 2012 [11] | MHE | 20 | 35.00 | 55.00 ± 7.00 | 72.80 ± 16.71 | 23.15 ± 8.17 | 69.06 ± 26.13 | ReHo | 25 | 48.00 | 55.00 ± 8.00 | 46.32 ± 9.09 | 44.68 ± 8.28 |
| NMHE | 27 | 25.93 | 51.00 ± 6.00 | 45.78 ± 8.53 | 40.11 ± 8.80 | 51.10 ± 33.54 | ReHo | 25 | 48.00 | 55.00 ± 8.00 | 46.32 ± 9.09 | 44.68 ± 8.28 | |
| Qi et al., 2012 [12] | MHE | 14 | 21.43 | 56.57 ± 9.19 | NA | NA | 38.58 ± 25.55 | ALFF | 17 | 29.41 | 54.35 ± 9.10 | NA | NA |
| Shi et al., 2015 [27] | MHE | 12 | 33.33 | 53.60 ± 9.40 | 86.92 ± 32.04 | 23.67 ± 7.08 | 20.83 ± 8.02 | ALFF | 12 | 41.67 | 53.60 ± 8.40 | 42.92 ± 11.38 | 41.33 ± 10.25 |
| Shi et al., 2015 [28] | MHE | 32 | 28.13 | 45.31 ± 8.96 | 117 ± 29.50 | 27.19 ± 5.13 | NA | ReHo | 34 | 29.41 | 46.62 ± 8.78 | 77.00 ± 17.00 | 44.26 ± 5.58 |
| NMHE | 30 | 30.00 | 43.57 ± 10.24 | 85.00 ± 13.00 | 41.70 ± 4.85 | NA | ReHo | 34 | 29.41 | 46.62 ± 8.78 | 77.00 ± 17.00 | 44.26 ± 5.58 | |
| Sun et al., 2018 [13] | MHE | 30 | 20.00 | 48.80 ± 12.20 | NA | NA | NA | ReHo | 64 | 28.13 | 46.80 ± 9.70 | NA | NA |
| NMHE | 32 | 12.50 | 46.30 ± 9.20 | NA | NA | NA | ReHo | 64 | 28.13 | 46.80 ± 9.70 | NA | NA | |
| Wu et al., 2014 [29] | MHE | 17 | 47.06 | 55.58 ± 10.41 | NA | NA | NA | ReHo | 17 | 47.06 | 55.11 ± 10.19 | NA | NA |
| Yang et al., 2022 [30] | MHE | 25 | 36.00 | 47.80 ± 9.60 | 70.30 ± 14.40 | 20.80 ± 5.00 | NA | ReHo | 30 | 37.00 | 44.90 ± 7.20 | 36.40 ± 8.80 | 46.00 ± 9.90 |
| NMHE | 27 | 33.00 | 47.30 ± 9.50 | 38.10 ± 12.90 | 43.60 ± 4.80 | NA | ReHo | 30 | 37.00 | 44.90 ± 7.20 | 36.40 ± 8.80 | 46.00 ± 9.90 | |
| Zhong et al., 2016 [14] | MHE | 14 | 50.00 | 54.57 ± 10.57 | 57.63 ± 30.96 | 23.29 ± 10.99 | NA | ALFF/fALFF | 14 | 50.00 | 50.86 ± 9.38 | 21.33 ± 3.80 | 48.86 ± 10.29 |
| Zhou et al., 2014 [31] | NMHE | 17 | 47.06 | 48.00 (37–70) | 30.48 ± 5.44 | 42.59 ± 6.36 | 30.24 ± 8.94 | ALFF | 14 | 50.00 | 49.50 (36–68) | 21.33 ± 3.80 | 48.86 ± 10.29 |
| Maximum | Cluster | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Regions | MNI Coordinates, x, y, z | SDM Value | p-Value | No. of Voxels | Breakdown (no. of Voxels) | Q (p-Value) | I2 (%) | Egger’s Test p Value |
| MHE vs. HCs | ||||||||
| MHE > HCs | ||||||||
| None | ||||||||
| MHE < HCs | ||||||||
| Left superior frontal gyrus | −2, 28, 40 | −6.884 | ~0 | 1736 | Left superior frontal gyrus, medial, BA 8, BA 9, BA 24, BA 32 (596); Left median cingulate/paracingulate gyri, BA 24, BA 32 (310) | 4.400495 (0.728953) | 4.494176 | 0.446 |
| Left median cingulate/paracingulate gyri | 0, −42, 36 | −4.656 | 0.003000021 | 467 | Left median cingulate/paracingulate gyri, BA 23 (119);Right precuneus (135) | 7.236337 (0.523467) | 7.436439 | 0.142 |
| NMHE vs. HCs | ||||||||
| NMHE > HCs | ||||||||
| None | ||||||||
| NMHE < HCs | ||||||||
| Right rolandic operculum | 52, −12, 10 | −3.215 | 0.0006513 | 125 | Right rolandic operculum, BA 48 (99); Right superior temporal gyrus, BA 48 (7) | 1.675306 (0.41893) | 0.653348 | 0.895 |
| Left precentral gyrus | −58, −6, 30 | −3.679 | 0.000117183 | 123 | Left precentral gyrus, BA 3, BA 4 (47); Left postcentral gyrus, BA 3, BA 4, BA 43, BA 48 (76) | 0 (0.524043) | 6.151155 | 0.943 |
| Right postcentral gyrus | 58, −6, 30 | −2.842 | 0.002242744 | 12 | Right postcentral gyrus, BA 3, BA 4, BA 43 (12) | 1.104866 (0.399276) | 3.466042 | 0.958 |
| MHE vs. NMHE as compared to HCs | ||||||||
| MHE > NMHE | ||||||||
| None | ||||||||
| MHE < NMHE | ||||||||
| Left superior frontal gyrus | 0, 30, 42 | −3.821 | 0.000999987 | 1935 | Left superior frontal gyrus, medial, BA 8, BA 9, BA 24, BA 32 (657); Left median cingulate/paracingulate gyri, BA 24, BA 32 (88) | 8.954032 (0.667304) | 2.479716 | 0.623 |
| Left median cingulate/paracingulate gyri | 0, −42, 36 | −2.084 | 0.04400003 | 63 | Left median cingulate/paracingulate gyri, BA 23 (40); Right median cingulate/paracingulate gyri, BA 23 (20) | 16.213957 (0.429249) | 23.814262 | 0.804 |
| Right precuneus | 4, −54, 44 | −2.134 | 0.046999991 | 24 | Right precuneus (24) | 15.727232 (0.418632) | 13.443153 | 0.786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, B.; Liang, S.; Tang, S.; Liang, H.; Zhang, Y.; Liang, Z. Altered Spontaneous Brain Activity in Cirrhotic Patients with Minimal Hepatic Encephalopathy: A Meta-Analysis of Resting-State Functional Imaging. Brain Sci. 2023, 13, 960. https://doi.org/10.3390/brainsci13060960
Qin B, Liang S, Tang S, Liang H, Zhang Y, Liang Z. Altered Spontaneous Brain Activity in Cirrhotic Patients with Minimal Hepatic Encephalopathy: A Meta-Analysis of Resting-State Functional Imaging. Brain Sciences. 2023; 13(6):960. https://doi.org/10.3390/brainsci13060960
Chicago/Turabian StyleQin, Bin, Shuolin Liang, Shiting Tang, Huo Liang, Yunli Zhang, and Zhijian Liang. 2023. "Altered Spontaneous Brain Activity in Cirrhotic Patients with Minimal Hepatic Encephalopathy: A Meta-Analysis of Resting-State Functional Imaging" Brain Sciences 13, no. 6: 960. https://doi.org/10.3390/brainsci13060960






