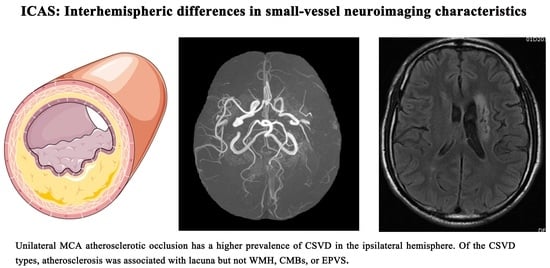
Asymmetry of Lacunae between Brain Hemispheres Is Associated with Atherosclerotic Occlusions of Middle Cerebral Artery
Abstract
1. Introduction
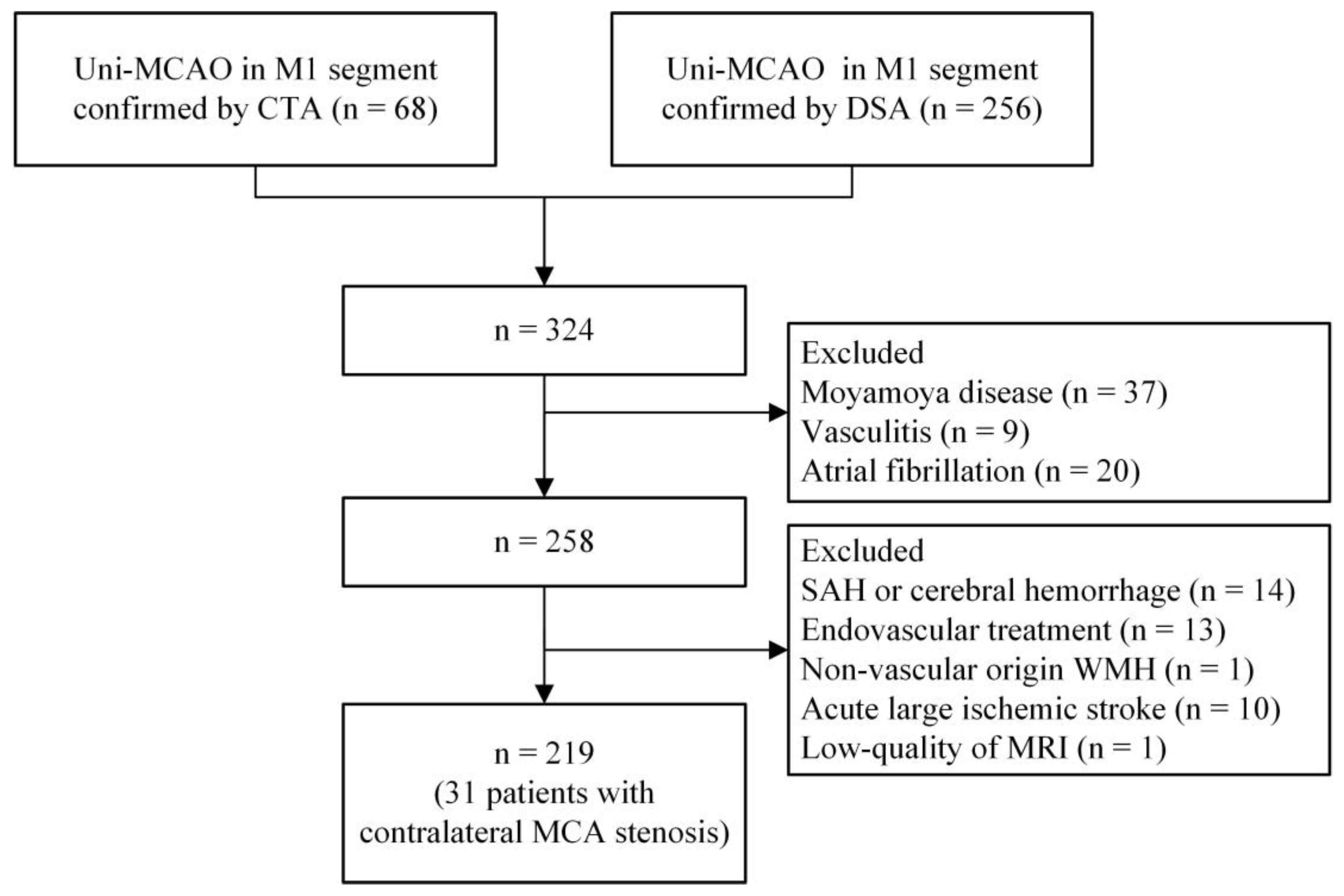
2. Materials and Methods
2.1. Subjects
2.2. Clinical Data
2.3. CSVD Assessment
2.4. Collateral Grading
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Interhemispheric Differences in Small-Vessel Neuroimaging Characteristics
3.3. Association of Demographics with the Heterogeneity of CSVD between Hemispheres
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutierrez, J.; Turan, T.N.; Hoh, B.L.; Chimowitz, M.I. Intracranial atherosclerotic stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2022, 21, 355–368. [Google Scholar] [CrossRef]
- Derdeyn, C.P. Hemodynamics and oxygen extraction in chronic large artery steno-occlusive disease: Clinical applications for predicting stroke risk. J. Cereb. Blood Flow Metab. 2018, 38, 1584–1597. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, M.P.; Lau, K.K.; Mordasini, P.; Tsang, A.C.O.; Heldner, M.R. Intracranial Atherosclerotic Stenoses: Pathophysiology, Epidemiology, Risk Factors and Current Therapy Options. Adv. Ther. 2020, 37, 1829–1865. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, H.; Pan, M.; Dai, F. Potential risk factors of persistent postural-perceptual dizziness: A pilot study. J. Neurol. 2022, 269, 3075–3085. [Google Scholar] [CrossRef]
- Du, H.; Chen, C.; Ye, C.; Lin, F.; Wei, J.; Xia, P.; Chen, R.; Wu, S.; Yuan, Q.; Chen, H.; et al. Association between steno-occlusive middle cerebral artery and basal ganglia perivascular spaces. Front. Neurol. 2020, 11, 293. [Google Scholar] [CrossRef]
- Park, J.H.; Kwon, H.M.; Lee, J.; Kim, D.S.; Ovbiagele, B. Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur. J. Neurol. 2015, 22, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.F.; Yan, S.; Li, M.L.; Han, F.; Wang, Q.; Zhou, L.X.; Ni, J.; Yao, M.; Zhang, S.-Y.; Cui, L.-Y.; et al. Intracranial arterial dolichoectasia and stenosis: Risk factors and relation to cerebral small vessel disease. Stroke 2018, 49, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Wahlund, L.O.; Barkhof, F.; Fazekas, F.; Bronge, L.; Augustin, M.; Sjögren, M.; Wallin, A.; Ader, H.; Leys, D.; Pantoni, L.; et al. A New Rating Scale for Age-Related White Matter Changes Applicable to MRI and CT. Stroke 2001, 32, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- van Swieten, J.C.; Hijdra, A.; Koudstaal, P.J.; van Gijn, J. Grading white matter lesions on CT and MRI: A simple scale. J. Neurol. Neurosurg. Psychiatry 1990, 53, 1080–1083. [Google Scholar] [CrossRef] [PubMed]
- Doubal, F.N.; MacLullich, A.M.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef]
- Arba, F.; Leigh, R.; Inzitari, D.; Warach, S.J.; Luby, M.; Lees, K.R. Blood-brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurology 2017, 89, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Singer, O.C.; Berkefeld, J.; Nolte, C.H.; Bohner, G.; Reich, A.; Wiesmann, M.; Groeschel, K.; Boor, S.; Neumann-Haefelin, T.; Hofmann, E.; et al. Collateral Vessels in Proximal Middle Cerebral Artery Occlusion: The endostroke Study. Radiology 2015, 274, 851–858. [Google Scholar] [CrossRef]
- Tan, I.; Demchuk, A.; Hopyan, J.; Zhang, L.; Gladstone, D.; Wong, K.; Martin, M.; Symons, S.; Fox, A.; Aviv, R. CT Angiography Clot Burden Score and Collateral Score: Correlation with Clinical and Radiologic Outcomes in Acute Middle Cerebral Artery Infarct. Am. J. Neuroradiol. 2009, 30, 525–531. [Google Scholar] [CrossRef]
- Sallustio, F.; Motta, C.; Pizzuto, S.; Diomedi, M.; Giordano, A.; D’Agostino, V.C.; Samà, D.; Mangiafico, S.; Saia, V.; Legramante, J.M.; et al. CT angiography-based collateral flow and time to reperfusion are strong predictors of outcome in endovascular treatment of patients with stroke. J. NeuroInterventional Surg. 2017, 9, 940–943. [Google Scholar] [CrossRef]
- Gupta, A.; Giambrone, A.; Gialdini, G.; Finn, C.B.; Delgado, D.; Gutierrez, J.; Wright, C.; Beiser, A.B.; Seshadri, S.; Pandya, A.; et al. Abstract WP165: Silent Brain Infarction and Risk of Future Stroke: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 719–725. [Google Scholar] [CrossRef]
- Fu, J.H.; Lu, C.Z.; Hong, Z.; Dong, Q.; Luo, Y.; Wong, K.S. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 793–796. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Heijer, T.D.; Koudstaal, P.J.; Oudkerk, M.; Hofman, A.; Breteler, M.M. Incidence and Risk Factors of Silent Brain Infarcts in the Population-Based Rotterdam Scan Study. Stroke 2003, 34, 392–396. [Google Scholar] [CrossRef]
- Baradaran, H.; Gialdini, G.; Mtui, E.; Askin, G.; Kamel, H.; Gupta, A. Silent Brain Infarction in Patients with Asymptomatic Carotid Artery Atherosclerotic Disease. Stroke 2016, 47, 1368–1370. [Google Scholar] [CrossRef]
- Das, A.S.; Regenhardt, R.W.; Vernooij, M.W.; Blacker, D.; Charidimou, A.; Viswanathan, A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J. Stroke 2019, 21, 121–138. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Magnoni, M.; D’Ascenzo, F.; Anselmino, M.; Anzalone, N.; Rocca, M.A.; Falini, A.; Filippi, M.; Camici, P.G. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 223, 681–687. [Google Scholar] [CrossRef]
- Shi, Y.; Thrippleton, M.J.; Makin, S.D.; Marshall, I.; Geerlings, M.I.; de Craen, A.J.; van Buchem, M.A.; Wardlaw, J.M. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2016, 36, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Liebeskind, D.S.; Cotsonis, G.; Nizam, A.; Feldmann, E.; Sangha, R.S.; Campo-Bustillo, I.; Romano, J.G.; on behalf of the MyRIAD Investigators. Predictors of Early Infarct Recurrence in Patients with Symptomatic Intracranial Atherosclerotic Disease. Stroke 2021, 52, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, H.; Mtui, E.; Richardson, J.E.; Delgado, D.; Gupta, A. Hemispheric Differences in Leukoaraiosis in Patients with Carotid Artery Stenosis: A Systematic Review. Clin. Neuroradiol. 2017, 27, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Moroni, F.; Magnoni, M.; Rocca, M.A.; Messina, R.; Anzalone, N.; De Filippis, C.; Scotti, I.; Besana, F.; Spagnolo, P.; et al. Relation between characteristics of carotid atherosclerotic plaques and brain white matter hyperintensities in asymptomatic patients. Sci. Rep. 2017, 7, 10559. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, M.E.; Perrotta, P.; Augimeri, A.; Rocca, F.; Quattrone, A.; Cherubini, A. Automatic Detection of White Matter Hyperintensities in Healthy Aging and Pathology Using Magnetic Resonance Imaging: A Review. Neuroinformatics 2015, 13, 261–276. [Google Scholar] [CrossRef]
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473. [Google Scholar] [CrossRef]
- Gouveia-Freitas, K.; Bastos-Leite, A.J. Perivascular spaces and brain waste clearance systems: Relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology 2021, 63, 1581–1597. [Google Scholar] [CrossRef]
- Troili, F.; Cipollini, V.; Moci, M.; Morena, E.; Palotai, M.; Rinaldi, V.; Romano, C.; Ristori, G.; Giubilei, F.; Salvetti, M.; et al. Perivascular unit: This must be the place. The anatomical crossroad between the immune, vascular and nervous system. Front. Neuroanat. 2020, 14, 17. [Google Scholar] [CrossRef]
- Shulyatnikova, T.; Hayden, M.R. Why are perivascular spaces important? Medicina 2023, 59, 917. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Wei, G.; Cheng, M.; Jiang, H. Association between enlarged perivascular spaces and internal carotid artery stenosis: A study in patients diagnosed by digital subtraction angiography. J. Stroke Cerebrovasc. Dis. 2020, 29, 104635. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vernooij, M.W.; Cordonnier, C.; Viswanathan, A.; Salman, R.A.-S.; Warach, S.; Launer, L.J.; Van Buchem, M.A.; Breteler, M.M. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 2009, 8, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; French, S.; Ji, P.; Kim, R.C. Cerebral microbleeds in the elderly: A pathological analysis. Stroke 2010, 41, 2782–2785. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Kwok, C.; Benavente, O. Cerebral Microbleeds: Histopathological Correlation of Neuroimaging. Cerebrovasc. Dis. 2011, 32, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, C.L.; Qiu, Y.M.; Chen, A.Q.; Li, Y.N.; Hu, B. Dysfunction of the blood-brain barrier in cerebral microbleeds: From bedside to bench. Aging Dis. 2021, 12, 1898–1919. [Google Scholar] [CrossRef]
- Shu, M.-J.; Zhai, F.-F.; Zhang, D.-D.; Han, F.; Zhou, L.; Ni, J.; Yao, M.; Zhang, S.-Y.; Cui, L.-Y.; Jin, Z.-Y.; et al. Metabolic syndrome, intracranial arterial stenosis and cerebral small vessel disease in community-dwelling populations. Stroke Vasc. Neurol. 2021, 6, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rutten-Jacobs, L.; Liu, M.; Markus, H.S.; Traylor, M. Causal impact of type 2 diabetes mellitus on cerebral small vessel disease: A mendelian randomization analysis. Stroke 2018, 49, 1325–1331. [Google Scholar] [CrossRef]
- Evans, L.E.; Taylor, J.L.; Smith, C.J.; Pritchard, H.A.T.; Greenstein, A.S.; Allan, S.M. Cardiovascular comorbidities, inflammation, and cerebral small vessel disease. Cardiovasc. Res. 2021, 117, 2575–2588. [Google Scholar] [CrossRef]
- van Harten, B.; de Leeuw, F.E.; Weinstein, H.C.; Scheltens, P. Brain imaging in patients with diabetes—A systematic review. Diabetes Care 2006, 29, 2539–2548. [Google Scholar] [CrossRef]
- Coll, E.; Botey, A.; Alvarez, L.; Poch, E.; Quintó, L.; Saurina, A.; Vera, M.; Piera, C.; Darnell, A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am. J. Kidney Dis. 2000, 36, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, S.; Jing, J.; Cai, X.; Jin, A.; Yang, Y.; Wang, S.; Meng, X.; Lin, J.; Mei, L.; et al. Association of serum cystatin c with cerebral small vessel disease in community-based population. Stroke 2022, 53, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ye, X.; Cai, J.; Gao, T.; Zhao, G.; Zhang, W.; Tong, L.; Gao, F. Association of renal dysfunction with remote diffusion-weighted imaging lesions and total burden of cerebral small vessel disease in patients with primary intracerebral hemorrhage. Front. Aging Neurosci. 2018, 10, 171. [Google Scholar] [CrossRef]
- Yang, S.; Cai, J.; Lu, R.; Wu, J.; Zhang, M.; Zhou, X. Association between serum cystatin c level and total magnetic resonance imaging burden of cerebral small vessel disease in patients with acute lacunar stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 186–191. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Safar, M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 2005, 46, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Leung, T.W. Collateral flow in intracranial atherosclerotic disease. Transl. Stroke Res. 2023, 14, 38–52. [Google Scholar] [CrossRef]
- Eker, O.F.; Rascle, L.; Cho, T.-H.; Mechtouff, L.; Derex, L.; Ong, E.; Berthezene, Y.; Nighoghossian, N. Does small vessel disease burden impact collateral circulation in ischemic stroke treated by mechanical thrombectomy? Stroke 2019, 50, 1582–1585. [Google Scholar] [CrossRef]


| Demographic Characteristics | Patients (n = 219) |
|---|---|
| Age, years | 57 (49~63) |
| Male, n (%) | 156 (71.2%) |
| Symptomatic, n (%) | 162 (74.0%) |
| Vascular risk factors | |
| Hypertension, n (%) | 152 (69.4%) |
| Diabetes, n (%) | 59 (26.9%) |
| Hyperlipidemia, n (%) | 41 (18.7%) |
| Prior stroke, n (%) | 41 (18.7%) |
| CHD, n (%) | 11 (5.0%) |
| Current smoker, n (%) | 103 (47.0%) |
| Current drinker, n (%) | 75 (34.2%) |
| Laboratory parameters | |
| TC, mg/dL | 3.54 (2.99~4.25) |
| LDL, mg/dL | 2.12 (1.66~2.80) |
| HDL, mg/dL | 0.96 (0.83~1.08) |
| Triglycerides, mg/dL | 1.22 (0.96~1.68) |
| Urea, mmol/L | 4.8 (4~5.72) |
| Creatinine, µmol/L | 73.83 ± 16.67 |
| Cystatin C, mg/L | 0.98 (0.88~1.14) |
| eGFR, mL/min/1.73 m2 | 93.88 ± 15.53 |
| Collateral status, n (%) | |
| Poor collateral, n (%) | 37 (16.9%) |
| CSVD Features | Contralateral Hemisphere | Ipsilateral Hemisphere | p Value |
|---|---|---|---|
| WMH | |||
| ARWMC score, median (IQR) | 1 (0~3) | 1 (0~3) | 0.508 |
| Lacunae | |||
| Presence of lacunae, n (%) | 55 (25.1%) | 95 (43.4%) | <0.001 |
| Two or more lacunae, n (%) | 21 (9.6%) | 43 (29.7%) | <0.001 |
| No. of lacunae, median (IQR) | 0 (0~1) | 0 (0~2) | <0.001 |
| EPVS | |||
| EPVS grade, median (IQR) | 1 (0~1) | 1 (0~1) | 0.872 |
| CSVD scores | |||
| CSVD score, median (IQR) | 0 (0~1) | 0 (0~1) | 0.004 |
| CSVD Features | Contralateral Hemisphere | Ipsilateral Hemisphere | p Value |
|---|---|---|---|
| WMH | |||
| ARWMC score, median (IQR) | 0 (0~3) | 1 (0~2) | 0.75 |
| Lacunae | |||
| Prescence of lacunae, n (%) | 11 (50%) | 11 (50%) | 1 |
| Two or more lacunae, n (%) | 4 (12.9%) | 9 (29%) | 0.119 |
| No. of lacunae, median (IQR) | 0 (0~1) | 0 (0~2) | 0.698 |
| EPVS | |||
| EPVS grade, median (IQR) | 0 (0~0) | 0 (0~0) | 0.471 |
| CSVD | |||
| Total CSVD score, median (IQR) | 0 (0~1) | 0 (0~1) | 0.429 |
| CSVD Features | Contralateral Hemisphere | Ipsilateral Hemisphere | p Value |
|---|---|---|---|
| No. of CMB | 0 (0~0) | 0 (0~0) | 0.246 |
| Variables | No. of Lacunae | CSVD Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Age | 1.025 (0.996~1.055) | 0.086 | 1.022 (0.989, 1.056) | 0.188 | ||||
| Male sex | 0.658 (0.348~1.241) | 0.196 | 0.613 (0.292, 1.29) | 0.197 | ||||
| Symptomatic | 0.906 (0.483~1.697) | 0.757 | 0.909 (0.449, 1.842) | 0.791 | ||||
| Poor collateral | 1.15 (0.553~2.389) | 0.708 | 0.892 (0.38, 2.095) | 0.793 | ||||
| Hypertension | 1.16 (0.632~2.13) | 0.633 | 1.218 (0.607, 2.442) | 0.579 | ||||
| Diabetes | 2.046 (1.11~3.772) | 0.022 | 2.029 (1.039~3.961) | 0.038 | 1.9 (0.973,3.711) | 0.060 | 2.219 (1.067, 4.615) | 0.033 |
| Hyperlipidemia | 0.719 (0.344~1.506) | 0.382 | 0.627 (0.26, 1.515) | 0.300 | ||||
| Prior stroke | 1.393 (0.695~2.79) | 0.350 | 1.475 (0.689, 3.156) | 0.317 | ||||
| CHD | 0.174 (0.022~1.383) | 0.198 | 0.316 (0.039, 2.53) | 0.278 | ||||
| Current smoker | 1.876 (1.07~3.288) | 0.028 | 2.301 (1.207, 4.385) | 0.011 | ||||
| Current drinker | 1.786 (1.002~3.182) | 0.049 | 2.269 (1.195, 4.308) | 0.012 | ||||
| TC | 0.945 (0.712~1.255) | 0.695 | 0.885 (0.638, 1.226) | 0.461 | ||||
| LDL | 0.937 (0.676~1.299) | 0.696 | 0.878 (0.604, 1.277) | 0.496 | ||||
| HDL | 1.101 (0.302~4.009) | 0.884 | 1.209 (0.284, 5.149) | 0.798 | ||||
| Triglycerides | 0.848 (0.59~1.221) | 0.376 | 0.642 (0.374, 1.103) | 0.109 | ||||
| Urea | 0.99 (0.95~1.032) | 0.627 | 0.993 (0.957, 1.03) | 0.692 | ||||
| Creatinine | 1.012 (0.995~1.029) | 0.176 | 1.017 (0.998, 1.036) | 0.079 | ||||
| Cystatin C | 4.94 (1.109~21.996) | 0.036 | 5.725 (1.252~26.173) | 0.024 | 8.042 (1.554, 41.613) | 0.013 | 9.674 (1.801, 51.969) | 0.008 |
| eGFR | 0.985 (0.967~1.003) | 0.107 | 0.982 (0.962, 1.003) | 0.087 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Huang, H.; Yu, Z.; Luo, X.; Xu, S. Asymmetry of Lacunae between Brain Hemispheres Is Associated with Atherosclerotic Occlusions of Middle Cerebral Artery. Brain Sci. 2023, 13, 1016. https://doi.org/10.3390/brainsci13071016
Wu L, Huang H, Yu Z, Luo X, Xu S. Asymmetry of Lacunae between Brain Hemispheres Is Associated with Atherosclerotic Occlusions of Middle Cerebral Artery. Brain Sciences. 2023; 13(7):1016. https://doi.org/10.3390/brainsci13071016
Chicago/Turabian StyleWu, Lingshan, Hao Huang, Zhiyuan Yu, Xiang Luo, and Shabei Xu. 2023. "Asymmetry of Lacunae between Brain Hemispheres Is Associated with Atherosclerotic Occlusions of Middle Cerebral Artery" Brain Sciences 13, no. 7: 1016. https://doi.org/10.3390/brainsci13071016
APA StyleWu, L., Huang, H., Yu, Z., Luo, X., & Xu, S. (2023). Asymmetry of Lacunae between Brain Hemispheres Is Associated with Atherosclerotic Occlusions of Middle Cerebral Artery. Brain Sciences, 13(7), 1016. https://doi.org/10.3390/brainsci13071016