Functional Overlay Model of Persistent Post-Concussion Syndrome
Abstract
:1. Introduction
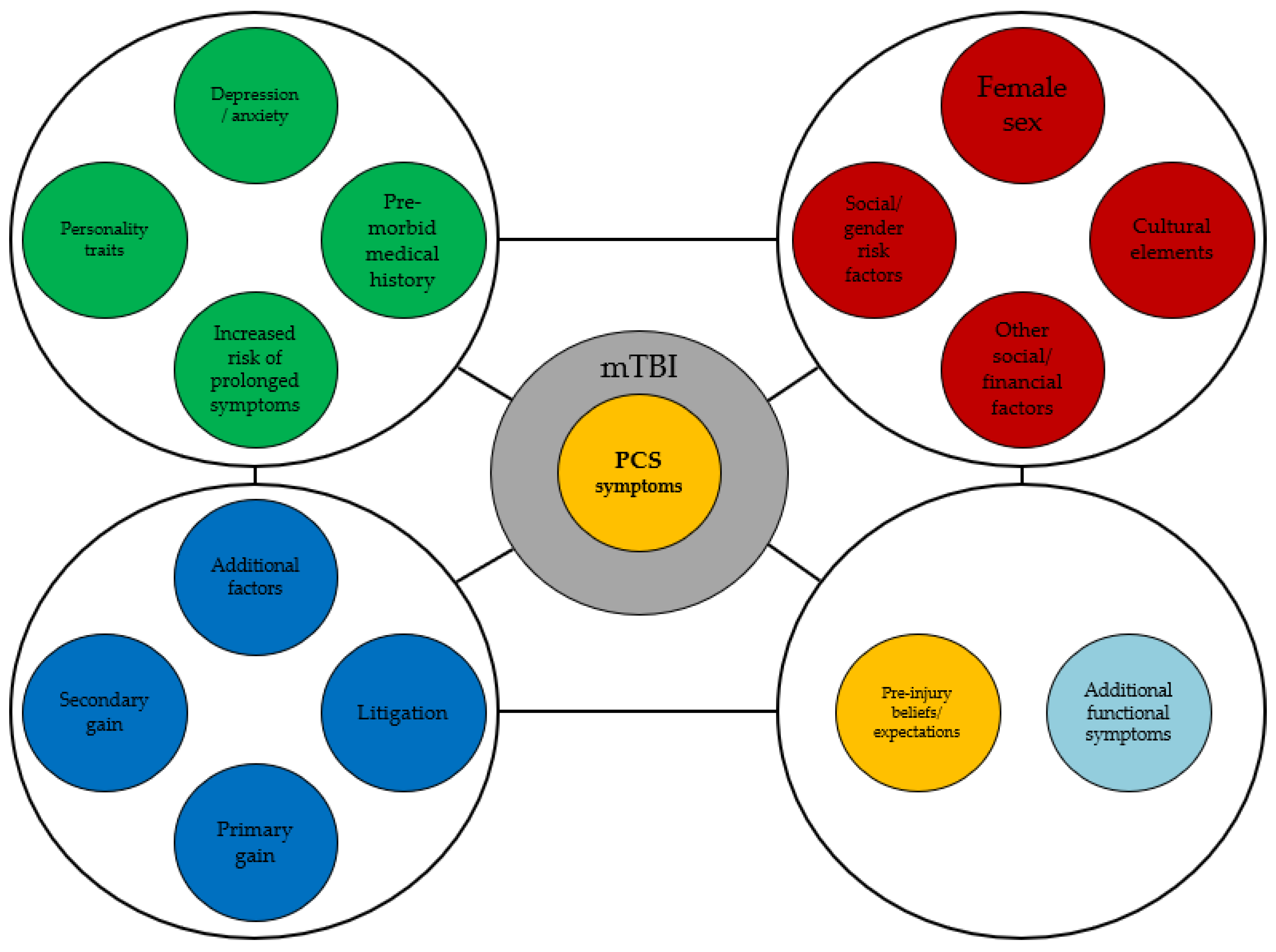
2. Mechanisms and Predisposing Factors for Persistent Post-Concussion Syndrome
3. Mechanisms and Predisposing Factors for Functional Neurological Disorder
4. Beliefs and Expectations in Post-Concussion Syndrome
5. Illness Beliefs in Functional Neurological Disorders
6. Similarities in Symptoms and Underlying Mechanisms
7. FND Overlay Model of PCS
8. Limitations of FND Diagnostic Criteria
9. Critiques and Limitations of the FND Model for PPCS
10. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bazarian, J.J.; Wong, T.; Harris, M.; Leahey, N.; Mookerjee, S.; Dombovy, M. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj. 1999, 13, 173. [Google Scholar] [CrossRef]
- Strauss, I.; Savitsky, N. Head injury, Neurologic and psychiatric aspects. Arch. Neurol. Psychiatry 1934, 31, 893. [Google Scholar] [CrossRef]
- Evans, R.W. Persistent post-traumatic headache, postconcussion syndrome, and whiplash injuries, the evidence for a non-traumatic basis with an historical review. Headache 2010, 50, 716. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Karr, J.E.; Gardner, A.J.; Silverberg, N.D.; Terry, D.P. Results of scoping review do not support mild traumatic brain injury being associated with a high incidence of chronic cognitive impairment: Commentary on McInnes et al. 2017. PLoS ONE 2019, 14, e0218997. [Google Scholar] [CrossRef] [Green Version]
- Fesharaki-Zadeh, A. Chronic Traumatic Encephalopathy: A Brief Overview. Front. Neurol. 2019, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- VanItallie, T.B. Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE). Metabolism 2019, 100S, 153943. [Google Scholar] [CrossRef]
- Dwyer, B.; Katz, D.I. Postconcussion syndrome. Handb. Clin. Neurol. 2018, 158, 163. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.C. Chronic post-traumatic headaches classified and compared with natural headaches. Cephalalgia 1996, 16, 486. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Pedroza, C.; Brown, S.A.; Levin, H.S.; Goodman, H.S.; Merritt, S.G. Postconcussional disorder, Are the DSM-IV criteria an improvement over the ICD-10? J. Nerv. Ment. Dis. 2005, 193, 540. [Google Scholar] [CrossRef] [PubMed]
- de Kruijk, J.R.; Leffers, P.; Meerhoff, S.; Rutten, J.; Twijnstra, A. Effectiveness of bed rest after mild traumatic brain injury, a randomised trial of no versus six days of bed rest. J. Neurol. Neurosurg. Psychiatry 2002, 73, 167. [Google Scholar] [CrossRef]
- Hughes, D.G.; Jackson, A.; Mason, D.L.; Berry, E.; Hollis, S.; Yates, D.W. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury, correlation with neuropsychological tests and delayed recovery. Neuroradiology 2004, 46, 550. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Levin, H.S.; Contant, C.F.; Song, J.X. Postconcussional disorder following mild to moderate traumatic brain injury, anxiety, depression, and social support as risk factors and comorbidities. J. Clin. Exp. Neuropsychol. 2001, 23, 792–808. [Google Scholar] [CrossRef]
- Eisenberg, M.A.; Andrea, J.; Meehan, W.; Mannix, R. Time interval between concussions and symptom duration. Pediatrics 2013, 132, 8–17. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Association: London, UK; Washington, DC, USA, 2013. [Google Scholar]
- Stone, J.; Carson, A.; Duncan, R.; Roberts, R.; Warlow, C.; Hibberd, C.; Coleman, R.; Cull, R.; Murray, G.; Pelosi, A.; et al. Who is referred to neurology clinics?--the diagnoses made in 3781 new patients. Clin. Neurol. Neurosurg. 2010, 112, 747–751. [Google Scholar] [CrossRef]
- Carson, A.; Stone, J.; Hibberd, C.; Murray, G.; Duncan, R.; Coleman, R.; Warlow, C.; Roberts, R.; Pelosi, A.; Cavanagh, J.; et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J. Neurol. Neurosurg. Psychiatry 2011, 82, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Gelauff, J.; Stone, J.; Edwards, M.; Carson, A. The prognosis of functional (psychogenic) motor symptoms, a systematic review. J. Neurol. Neurosurg. Psychiatry 2014, 85, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.; Rickards, H.; Cavanna, A.E. Prognosis and outcome predictors in psychogenic nonepileptic seizures. Epilepsy Res. Treat. 2011, 2011, 274736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.; Carson, A.; Sharpe, M. Functional symptoms in neurology, management. J. Neurol. Neurosurg. Psychiatry. 2005, 76, i13. [Google Scholar] [CrossRef] [Green Version]
- Stone, J. The bare essentials, Functional symptoms in neurology. Pract. Neurol. 2009, 9, 179. [Google Scholar] [CrossRef]
- Rosebush, P.I.; Mazurek, M.F. Treatment of conversion disorder in the 21st century, have we moved beyond the couch? Curr. Treat. Options Neurol. 2011, 13, 255. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Atabaki, S. Predicting postconcussion syndrome after minor traumatic brain injury. Acad. Emerg. Med. 2001, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Fenton, G.; McClelland, R.; Montgomery, A.; MacFlynn, G.; Rutherford, W. The postconcussional syndrome, social antecedents and psychological sequelae. Br. J. Psychiatry 1993, 162, 493. [Google Scholar] [CrossRef]
- Iverson, G.L. Outcome from mild traumatic brain injury. Curr. Opin. Psychiatry 2005, 18, 301. [Google Scholar] [CrossRef]
- Streifer, M.; Brown, A.M.; Porfido, T.; Anderson, E.Z.; Buckman, J.F.; Esopenko, C. The Potential Role of the Cervical Spine in Sports-Related Concussion: Clinical Perspectives and Considerations for Risk Reduction. J. Orthopaedic. Sports Physical. Ther. 2019, 49, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Eckner, J.T.; Oh, Y.K.; Joshi, M.S.; Richardson, J.K.; Ashton-Miller, J.A. Effect of neck muscle strength and anticipatory cervical muscle activation on the kinematic response of the head to impulsive loads. Am. J. Sports Med. 2014, 42, 566–576. [Google Scholar] [CrossRef] [Green Version]
- Porfido, T.; de Souza, N.L.; Brown, A.M.; Buckman, J.F.; Fanning, B.D.; Parrott, J.S.; Esopenko, C. The relation between neck strength and psychological distress: Preliminary evidence from collegiate soccer athletes. Concussion 2021, 14, 6. [Google Scholar] [CrossRef]
- Bretzin, A.C.; Mansell, J.L.; Tierney, R.T.; McDevitt, J.K. Sex Differences in Anthropometrics and Heading Kinematics among Division I Soccer Athletes. Sports Health 2017, 9, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Lishman, W.A. Physiogenesis and psychogenesis in the ‘post-concussional syndrome’. Br. J. Psychiatry 1988, 153, 460. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.; Greco, T.; Prins, M.L. Concussion, pathophysiology and clinical translation. Handb. Clin. Neurol. 2018, 158, 51. [Google Scholar] [CrossRef]
- Ashina, H.; Porreca, F.; Anderson, T.; Amin, F.D.; Ashina, M.; Schytz, H.W.; Dodick, D.W. Post-traumatic headache, epidemiology and pathophysiological insights. Nat. Rev. Neurol. 2019, 15, 607. [Google Scholar] [CrossRef]
- Zhou, Y.; Kierans, A.; Kenul, D.; Ge, Y.; Rath, J.; Reaume, J.; Grossman, R.I.; Lui, Y.W. Mild traumatic brain injury, longitudinal regional brain volume changes. Radiology 2013, 267, 880. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Smith-Seemiller, L.; Isaac, G.; Duffy, J. Tc-HMPAO SPECT in persistent post-concussion syndrome after mild head injury, comparison with MRI/CT. Brain Inj. 1997, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Korn, A.; Golan, H.; Melamed, I.; Pascual-Marqui, R.; Friedman, A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J. Clin. Neurophysiol. 2005, 22, 1. [Google Scholar] [CrossRef]
- Umile, E.M.; Sandel, M.E.; Alavi, A.; Terry, C.M.; Plotkin, R.C. Dynamic imaging in mild traumatic brain injury, support for the theory of medial temporal vulnerability. Arch. Phys. Med. Rehabil. 2002, 83, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.H.; Kareken, D.A.; Fastenau, P.S.; Trexler, L.E.; Hutchins, G.D. A study of persistent post- concussion symptoms in mild head trauma using positron emission tomography. J. Neurol. Neurosurg. Psychiatry 2003, 74, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogduk, N. The neck and headaches. Neurol. Clin. 2004, 22, 151. [Google Scholar] [CrossRef]
- Chen, J.K.; Johnston, K.M.; Collie, A.; McCrory, P.; Ptito, A. A validation of the postconcussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.K.; Johnston, K.M.; Petrides, M.; Ptito, A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch. Gen. Psychiatry 2008, 65, 81. [Google Scholar] [CrossRef] [Green Version]
- Niogi, S.N.; Mukherjee, P.; Ghajar, J.; Johnson, C.E.; Kolster, R.; Lee, H.; Suh, M.; Zimmerman, R.D.; Manley, G.T.; McCandliss, B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 2008, 131, 3209. [Google Scholar] [CrossRef] [Green Version]
- Shumskaya, E.; Andriessen, T.M.; Norris, D.G.; Vos, P.E. Abnormal whole-brain functional networks in homogeneous acute mild traumatic brain injury. Neurology 2012, 79, 175. [Google Scholar] [CrossRef]
- Levin, H.S.; Williams, D.H.; Eisenberg, H.M.; High, W.M., Jr.; Guinto, F.C., Jr. Serial MRI and neurobehavioural findings after mild to moderate closed head injury. J. Neurol. Neurosurg. Psychiatry 1992, 55, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilde, E.A.; McCauley, S.R.; Hunter, J.V.; Bigler, E.D.; Chu, Z.; Wang, Z.J.; Hanten, G.R.; Troyanskaya, M.; Yallampalli, R.; Li, X.; et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008, 70, 948. [Google Scholar] [CrossRef] [PubMed]
- Metting, Z.; Rödiger, L.A.; Stewart, R.E.; Oudkerk, M.; De Keyser, J.; van der Naalt, J. Perfusion computed tomography in the acute phase of mild head injury, regional dysfunction and prognostic value. Ann. Neurol. 2009, 66, 809. [Google Scholar] [CrossRef]
- Brand, N.; Jolles, J. Information processing in depression and anxiety. Psychol. Med. 1987, 17, 145. [Google Scholar] [CrossRef]
- Nicholson, K.; Martelli, M.F.; Zasler, N.D. Does pain confound interpretation of neuropsychological test results? NeuroRehabilitation 2001, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Uomoto, J.M.; Katon, W.J. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics 2001, 42, 48. [Google Scholar] [CrossRef]
- Hou, R.; Moss-Morris, R.; Peveler, R.; Mogg, K.; Bradley, B.P.; Belli, A. When a minor head injury results in enduring symptoms, a prospective investigation of risk factors for postconcussional syndrome after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2012, 83, 217. [Google Scholar] [CrossRef]
- Tatrow, K.; Blanchard, E.B.; Hickling, E.J.; Silverman, D.J. Posttraumatic headache, biopsychosocial comparisons with multiple control groups. Headache 2003, 43, 755. [Google Scholar] [CrossRef]
- Deb, S.; Lyons, I.; Koutzoukis, C.; Ali, I.; McCarthy, G. Rate of psychiatric illness 1 year after traumatic brain injury. Am. J. Psychiatry 1999, 156, 374. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-H.; Chen, P.C.; Wang, T.C.; Kuo, T.Y.; Cheng, C.Y.; Yang, Y.H. Endocrine dysfunction following traumatic brain injury: A 5-year follow-up nationwide-based study. Sci. Rep. 2016, 6, 32987. [Google Scholar] [CrossRef]
- Krishna, G.; Bromberg, C.; Connell, E.C.; Mian, E.; Hu, C.; Lifshitz, J.; Adelson, P.D.; Thomas, T.C. Traumatic Brain Injury-Induced Sex-Dependent Changes in Late-Onset Sensory Hypersensitivity and Glutamate Neurotransmission. Front. Neurol. 2020, 11, 749. [Google Scholar] [CrossRef]
- Blaya, M.O.; Raval, A.P.; Bramlett, H.M. Traumatic brain injury in women across lifespan. Neurobiol. Dis. 2022, 164, 105613. [Google Scholar] [CrossRef]
- Mahajan, C.; Prabhakar, H.; Bilotta, F. Endocrine Dysfunction after Traumatic Brain Injury: An Ignored Clinical Syndrome? Neurocrit. Care 2023. [Google Scholar] [CrossRef]
- Prencipe, N.; Marinelli, L.; Varaldo, E.; Cuboni, D.; Berton, A.M.; Bioletto, F.; Bona, C.; Gasco, V.; Grottoli, S. Isolated anterior pituitary dysfunction in adulthood. Front. Endocrinol. 2023, 14, 1100007. [Google Scholar] [CrossRef]
- Gilis-Januszewska, A.; Kluczyński, Ł.; Hubalewska-Dydejczyk, A. Traumatic brain injuries induced pituitary dysfunction: A call for algorithms. Endocr. Connect. 2020, 9, R112–R123. [Google Scholar] [CrossRef] [Green Version]
- Borgaro, S.R.; Prigatano, G.P.; Kwasnica, C.; Rexer, J.L. Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Inj. 2003, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Reuber, M. Towards an integrative theory of psychogenic non- epileptic seizures (PNES). Clin. Psychol. Rev. 2016, 47, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Reuber, M.; Brown, R.J. Understanding psychogenic nonepileptic seizures—Phenomenology, semiology and the Integrative Cognitive Model. Seizure 2017, 44, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raynor, G.; Baslet, G. A historical review of functional neurological disorder and comparison to contemporary models. Epilepsy Behav. Rep. 2021, 16, 100489. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, C.; Hoeritzauer, I.; Cabreira, V.; Aybek, S.; Adams, C.; Alty, J.; Ball, H.A.; Baker, J.; Bullock, K.; Burness, C.; et al. Functional neurological disorder is a feminist issue. J. Neurol. Neurosurg. Psychiatry 2023, jnnp-2022-330192, Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Mark, V.W. Functional neurological disorder: Extending the diagnosis to other disorders, and proposing an alternate disease term-Attentionally-modifiable disorder. NeuroRehabilitation 2022, 50, 179–207. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.J.; Adams, R.A.; Brown, H.; Pareés, I.; Friston, K.J. A Bayesian account of “hysteria”. Brain 2012, 135, 3495–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voon, V.; Brezing, C.; Gallea, C.; Hallett, M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov. Disord. 2011, 26, 2396–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowska, K.; Chudleigh, C.; Cruz, C.; Lim, M.; McClure, G.; Savage, B.; Shah, U.; Cook, A.; Scher, S.; Carrive, P.; et al. Psychogenic non-epileptic seizures in children and adolescents, part I—Diagnostic formulations. Clin. Child Psychol. Psychiatry 2018, 23, 140–159. [Google Scholar] [CrossRef] [Green Version]
- Reuber, M.; Howlett, S.; Khan, A.; Grünewald, R.A. Non-epileptic seizures and other functional neurological symptoms, predisposing, precipitating, and perpetuating factors. Psychosomatics 2007, 48, 230–238. [Google Scholar] [CrossRef]
- Sharpe, D.; Faye, C. Non-epileptic seizures and child sexual abuse, a critical review of the literature. Clin. Psychol. Rev. 2006, 26, 1020–1040. [Google Scholar] [CrossRef]
- Roelofs, K.; Keijsers, G.P.J.; Hoogduin, K.A.L.; Näring, G.W.; Moene, F.C. Childhood abuse in patients with conversion disorder. Am. J. Psychiatry 2002, 159, 1908–1913. [Google Scholar] [CrossRef] [Green Version]
- Selkirk, M.; Duncan, R.; Oto, M.; Pelosi, A. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia 2008, 49, 1446–1450. [Google Scholar] [CrossRef]
- Perez, D.L.; Matin, N.; Barsky, A.; Costumero-Ramos, V.; Makaretz, S.J.; Young, S.S.; Sepulcre, J.; LaFrance, W.C., Jr.; Keshavan, M.S.; Dickerson, B.C. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J. Neurol. Neurosurg. Psychiatry 2017, 88, 491–497. [Google Scholar] [CrossRef]
- van der Hoeven, R.M.; Broersma, M.; Pijnenborg, G.H.M.; Koops, E.A.; van Laar, T.; Stone, J.; van Beilen, M. Functional (psychogenic) movement disorders associated with normal scores in psychological questionnaires, a case control study. J. Psychosom. Res. 2015, 79, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Dimaro, L.V.; Dawson, D.L.; Roberts, N.A.; Brown, I.; Moghaddam, N.G.; Reuber, M. Anxiety and avoidance in psychogenic nonepileptic seizures, the role of implicit and explicit anxiety. Epilepsy Behav. 2014, 33, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Demartini, B.; Goeta, D.; Barbieri, V.; Ricciardi, L.; Canevini, M.P.; Turner, K.; D’Agostino, A.; Romito, L.; Gambini, O. Psychogenic non-epileptic seizures and functional motor symptoms, a common phenomenology? J. Neurol. Sci. 2016, 368, 49–54. [Google Scholar] [CrossRef]
- Graham, J.R. MMPI-2, Assessing Personality and Psychopathology, 4th ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Sar, V.; Akyüz, G.; Kundakçı, T.; Kiziltan, E.; Dogan, O. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am. J. Psychiatry 2004, 161, 2271–2276. [Google Scholar] [CrossRef]
- Dixit, R.; Popescu, A.; Bagic, A.; Ghearing, G.; Hendrickson, R. Medical comorbidities in patients with psychogenic nonepileptic spells (PNES) referred for video-EEG monitoring. Epilepsy Behav. 2013, 28, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Marzooqi, S.M.; Baker, G.A.; Reilly, J.; Salmon, P. The perceived health status of people with psychologically derived non-epileptic attack disorder and epilepsy, a comparative study. Seizure 2004, 13, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Gunstaf, J.; Suhr, J. Perception of illness, Nonspecificity of Postconcussion Syndrome symptom expectation. J. Int. Neuropsychol. Soc. 2002, 8, 37–47. [Google Scholar] [CrossRef]
- Kirkwood, M.W.; Peterson, R.L.; Connery, A.K.; Baker, D.A.; Grubenhoff, J.A. Postconcussive symptom exaggeration after pediatric mild traumatic brain injury. Pediatrics 2014, 133, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.T.; Brickell, T.A.; French, L.M. Examination of the Mild Brain Injury Atypical Symptom Scale and the Validity-10 Scale to detect symptom exaggeration in US military service members. J. Clin. Exp. Neuropsychol. 2015, 37, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, R.J.; Seyranian, G.D.; Ruff, R.M. The Use of Significant Others to Enhance the Detection of Malingerers From Traumatically Brain-Injured Patients. Arch. Clin. Neuropsychol. 2000, 15, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Neal, J.; Strothkamp, S.; Bedingar, E.; Cordero, P.; Wagner, B.; Vagnini, V.; Jiang, Y. Discriminating Fake From True Brain Injury Using Latency of Left Frontal Neural Responses During Old/New Memory Recognition. Front. Neurosci. 2019, 13, 988. [Google Scholar] [CrossRef]
- Lippa, S.M.; Axelrod, B.N.; Lange, R.T. The Mild Brain Injury Atypical Symptoms (mBIAS) scale in a mixed clinical sample. J. Clin. Exp. Neuropsychol. 2016, 38, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.B.; Nelson, L.; Armistead-Jehle, P.; Bowles, A.O. Utility of the Mild Brain Injury Atypical Symptoms Scale as a Screening Measure for Symptom Over-Reporting in Operation Enduring Freedom/Operation Iraqi Freedom Service Members with Post-Concussive Complaints. Arch. Clin. Neuropsychol. 2011, 26, 718–727. [Google Scholar] [CrossRef] [Green Version]
- Fobian, A.D.; Elliott, L. A review of functional neurological symptom disorder etiology and the integrated etiological summary model. J. Psychiatry Neurosci. 2019, 44, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, M.J.; Fotopoulou, A.; Pareés, I. Neurobiology of functional (psychogenic) movement disorders. Curr. Opin. Neurol. 2013, 26, 442–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.J. Psychological mechanisms of medically unexplained symptoms, an integrative conceptual model. Psychol. Bull. 2004, 130, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Picon, E.L.; Perez, D.L.; Burke, M.J.; Debert, C.T.; Iverson, G.L.; Panenka, W.J.; Silverberg, N.D. Unexpected symptoms after concussion, Potential links to functional neurological and somatic symptom disorders. J. Psychosom. Res. 2021, 151, 110661. [Google Scholar] [CrossRef]
- Davidson, B.S.; Noteboom, L.; Pierro, H.; Kantor, C.; Stoot, D.; Stoot, F.; Linseman, D.; Hale, T.; Gorgens, K. Post-Concussion Assessment as a diagnostic and mechanistic framework for treating patients with Long COVID. medRxiv 2022. [Google Scholar] [CrossRef]
- Teodoro, T.; Chen, J.; Gelauff, J.; Edwards, M.J. Functional neurological disorder in people with long COVID: A systematic review. Eur. J. Neurol. 2023, 30, 1505–1514. [Google Scholar] [CrossRef]
- Rytter, H.M.; Graff, H.J.; Henriksen, H.K.; Aaen, N.; Hartvigsen, J.; Hoegh, M.; Nisted, I.; Næss-Schmidt, E.T.; Pedersen, L.L.; Schytz, H.; et al. Nonpharmacological Treatment of Persistent Postconcussion Symptoms in Adults: A Systematic Review and Meta-analysis and Guideline Recommendation. JAMA Netw. Open 2021, 4, e2132221. [Google Scholar] [CrossRef] [PubMed]
- Renga, V. Clinical Evaluation and Treatment of Patients with Postconcussion Syndrome. Neurol. Res. Int. 2021, 2021, 5567695. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, G.S.; Nielsen, G.; Teodoro, T.; Yogarajah, M.; Coebergh, J.A.; Dilley, M.D.; Martino, D.; Edwards, M.J. Management of functional neurological disorder. J. Neurol. 2020, 267, 2164–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, M.A.; Dworetzky, B.A.; Baslet, G. Functional neurological disorder: Engaging patients in treatment. Epilepsy Behav. Rep. 2021, 16, 100499. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, S.; Chowdhury, I.; Sinanaj, A.; Ewang, I.; Blain, C.; Teodoro, T.; Edwards, M.; Yogarajah, M. A Service Evaluation of the Experiences of Patients with Functional Neurological Disorders within the NHS. Front. Neurol. 2021, 12, 656466. [Google Scholar] [CrossRef] [PubMed]
- Coxon, A. Exploring Mechanisms of Change in a Pilot Randomised Trial of a Distant Delivery Mindfulness Intervention for People with Parkinson’s Disease. Unpublished. Ph.D. Thesis, University of London, London, UK, 2018. Available online: https://openaccess.city.ac.uk/ (accessed on 28 June 2023).
- Espay, A.J.; Aybek, S.; Carson, A.; Edwards, M.J.; Goldstein, L.H.; Hallett, M.; LaFaver, K.; LaFrance, W.C., Jr.; Lang, A.E.; Nicholson, T.; et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol. 2018, 75, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Sandri, A.; Geroin, C.; Bombieri, F.; Riello, M.; Menaspà, Z.; Bonetto, C.; Smania, N.; Tinazzi, M. Improvement in motor symptoms, physical fatigue, and self-rated change perception in functional motor disorders: A prospective cohort study of a 12-week telemedicine program. J. Neurol. 2022, 269, 5940–5953. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudis, I.; Chatzikonstantinou, S.; Petridis, F.; Palade, O.D.; Ciobica, A.; Balmus, I.-M. Functional Overlay Model of Persistent Post-Concussion Syndrome. Brain Sci. 2023, 13, 1028. https://doi.org/10.3390/brainsci13071028
Mavroudis I, Chatzikonstantinou S, Petridis F, Palade OD, Ciobica A, Balmus I-M. Functional Overlay Model of Persistent Post-Concussion Syndrome. Brain Sciences. 2023; 13(7):1028. https://doi.org/10.3390/brainsci13071028
Chicago/Turabian StyleMavroudis, Ioannis, Simela Chatzikonstantinou, Foivos Petridis, Octavian Dragos Palade, Alin Ciobica, and Ioana-Miruna Balmus. 2023. "Functional Overlay Model of Persistent Post-Concussion Syndrome" Brain Sciences 13, no. 7: 1028. https://doi.org/10.3390/brainsci13071028




