Individual Differences in Emotion Attenuation Brought by Indirect Replies Is Related to Resting-State Brain Activity
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Participants
2.3. Materials
Pretests of Materials
2.4. Procedure
2.4.1. Behavioral Test
2.4.2. Image Acquisition and Data Preprocessing
2.5. Data Analysis
2.5.1. Behavioral Data Analysis
2.5.2. ALFF/fALFF Calculation and ALFF/fALFF-Behavior Analysis Procedures
2.5.3. Functional Connectivity (FC) Analysis and FC-Behavior Analysis Procedures
3. Results
3.1. Behavioral Results
3.2. Regional ALFF/fALFF Analysis and ALFF/fALFF-Behavior Analysis
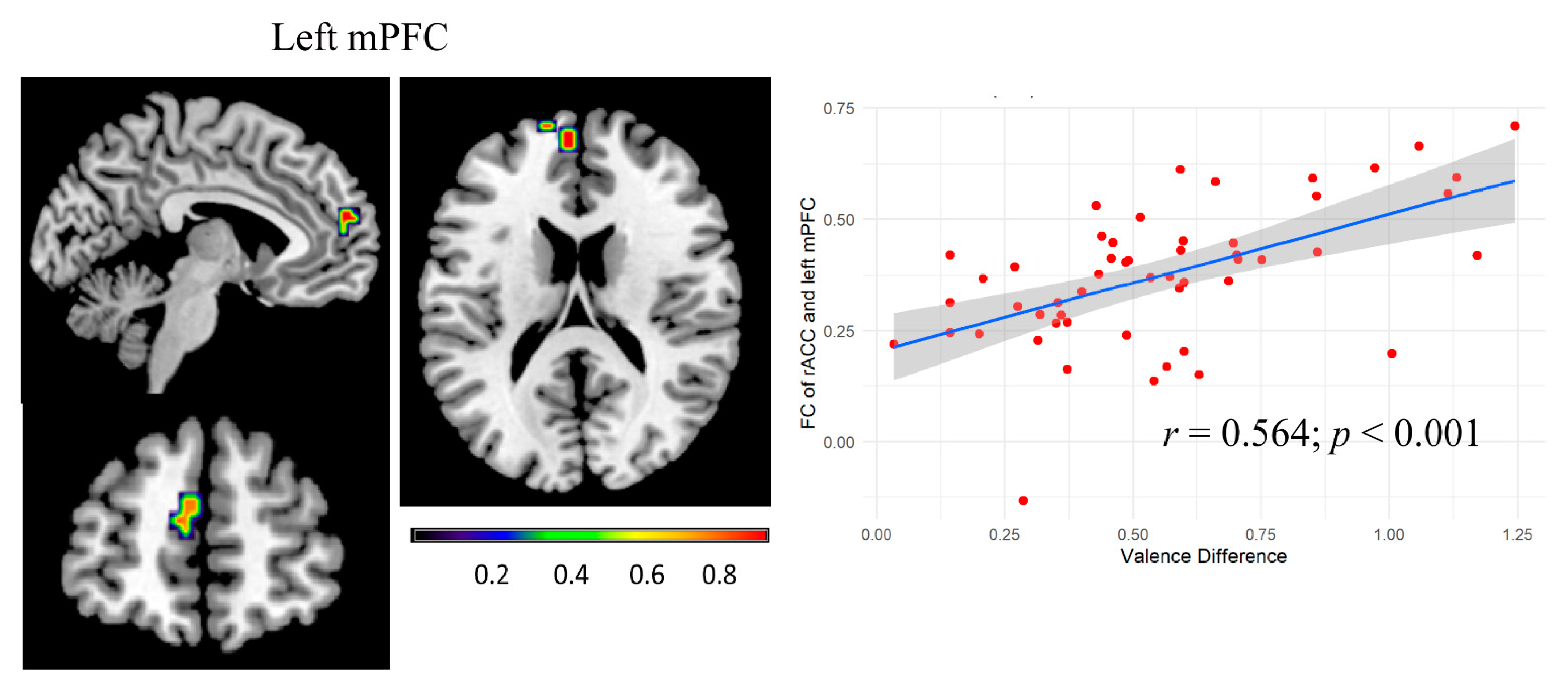
3.3. Functional Connectivity Analysis and Connectivity–Behavior Analysis
4. Discussion
4.1. Face-Saving Indirect Replies Attenuate Recipients’ Negative Emotion Experience
4.2. The Intrinsic Brain Activity Underlying Individual Differences in the Attenuation of Negative Emotions Brought by Indirect Replies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtgraves, T. Interpreting Indirect Replies. Cogn. Psychol. 1998, 37, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Wood, J.S.; Le-Luan, E.; Yao, B.; Haigh, M. ‘It’s hard to write a good article’: The online comprehension of excuses as indirect replies. Q. J. Exp. Psychol. 2018, 71, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, X.; Yang, X.; Yang, Y. Conventionality determines the time course of indirect replies comprehension: An ERP study. Brain Lang. 2023, 239, 105253. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Levinson, S.C. Politeness: Some Universals in Language Usage; Cambridge University Press: New York, NY, USA, 1987. [Google Scholar]
- Brown, R.; Gilman, A. Politeness theory and Shakespeare’s four major tragedies. Lang. Soc. 1989, 18, 159–212. [Google Scholar] [CrossRef]
- Hauptman, M.; Blank, I.; Fedorenko, E. Non-literal language processing is jointly supported by the language and Theory of Mind networks: Evidence from a novel meta-analytic fMRI approach. Cortex 2023, 162, 96–114. [Google Scholar] [CrossRef]
- Reyes-Aguilar, A.; Valles-Capetillo, E.; Giordano, M. A quantitative meta-analysis of neuroimaging studies of pragmatic language comprehension: In search of a universal neural substrate. Neuroscience 2018, 395, 60–88. [Google Scholar] [CrossRef]
- Bašnáková, J.; Weber, K.; Petersson, K.M.; van Berkum, J.; Hagoort, P. Beyond the Language Given: The Neural Correlates of Inferring Speaker Meaning. Cereb. Cortex 2014, 24, 2572–2578. [Google Scholar] [CrossRef]
- Feng, W.; Wu, Y.; Jan, C.; Yu, H.; Jiang, X.; Zhou, X. Effects of contextual relevance on pragmatic inference during conversation: An fMRI study. Brain Lang. 2017, 171, 52–61. [Google Scholar] [CrossRef]
- Feng, W.; Yu, H.; Zhou, X. Understanding particularized and generalized conversational implicatures: Is theory-of-mind necessary? Brain Lang. 2021, 212, 104878. [Google Scholar] [CrossRef]
- Jang, G.; Yoon, S.A.; Lee, S.E.; Park, H.; Kim, J.; Ko, J.H.; Park, H.J. Everyday conversation requires cognitive inference: Neural bases of comprehending implicated meanings in conversations. NeuroImage 2013, 81, 61–72. [Google Scholar] [CrossRef]
- Shibata, M.; Abe, J.I.; Itoh, H.; Shimada, K.; Umeda, S. Neural processing associated with comprehension of an indirect reply during a scenario reading task. Neuropsychologia 2011, 49, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- van Ackeren, M.J.; Smaragdi, A.; Rueschemeyer, S.-A. Neuronal interactions between mentalizing and action systems during indirect request processing. Soc. Cogn. Affect. Neurosci. 2016, 11, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F.; Satpute, A.B. Large-scale brain networks in affective and social neuroscience: Towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 2013, 23, 361–372. [Google Scholar] [CrossRef]
- Bašnáková, J.; van Berkum, J.; Weber, K.; Hagoort, P. A job interview in the MRI scanner: How does indirectness affect addressees and overhearers? Neuropsychologia 2015, 76, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Becker, B.; Camilleri, J.; Wang, L.; Yu, S.Q.; Eickhoff, S.B.; Feng, C.L. A domain-general brain network underlying emotional and cognitive interference processing: Evidence from coordinate-based and functional connectivity meta-analyses. Brain Struct. Funct. 2018, 223, 3813–3840. [Google Scholar] [CrossRef] [PubMed]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. NeuroImage 2014, 87, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Herwig, U.; Opialla, S.; Hittmeyer, A.; Jäncke, L.; Rufer, M.; Holtforth, M.G.; Brühl, A.B. Mindfulness and emotion regulation—An fMRI study. Soc. Cogn. Affect. Neurosci. 2014, 9, 776–785. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef]
- Webb, T.L.; Miles, E.; Sheeran, P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol. Bull. 2012, 138, 775. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, Z.; Yang, X.; Yang, Y. How working memory capacity modulates the time course of indirect replies comprehension: An event-related potential study. Lang. Cogn. Neurosci. 2021, 36, 1246–1257. [Google Scholar] [CrossRef]
- Holtgraves, T. Styles of language use: Individual and cultural variability in conversational indirectness. J. Personal. Soc. Psychol. 1997, 73, 624–637. [Google Scholar] [CrossRef]
- Marti, L. Indirectness and politeness in Turkish–German bilingual and Turkish monolingual requests. J. Pragmat. 2006, 38, 1836–1869. [Google Scholar] [CrossRef]
- Tannen, D. Indirectness in discourse: Ethnicity as conversational style. Discourse Process. 1981, 4, 221–238. [Google Scholar] [CrossRef]
- Lewis, C.M.; Baldassarre, A.; Committeri, G.; Romani, G.L.; Corbetta, M. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. USA 2009, 106, 17558–17563. [Google Scholar] [CrossRef]
- Branco, P.; Seixas, D.; Deprez, S.; Kovacs, S.; Peeters, R.; Castro, S.L.; Sunaert, S. Resting-state functional magnetic resonance imaging for language preoperative planning. Front. Hum. Neurosci. 2016, 10, 11. [Google Scholar] [CrossRef]
- Mennes, M.; Zuo, X.-N.; Kelly, C.; Di Martino, A.; Zang, Y.-F.; Biswal, B.; Castellanos, F.X.; Milham, M.P. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. NeuroImage 2011, 54, 2950–2959. [Google Scholar] [CrossRef]
- Wei, T.; Liang, X.; He, Y.; Zang, Y.; Han, Z.; Caramazza, A.; Bi, Y. Predicting conceptual processing capacity from spontaneous neuronal activity of the left middle temporal gyrus. J. Neurosci. 2012, 32, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; De Beuckelaer, A.; Wang, X.; Liu, L.; Song, Y.; Liu, J. Regional amplitude of the low-frequency fluctuations at rest predicts word-reading skill. Neuroscience 2015, 298, 318–328. [Google Scholar] [CrossRef]
- Zang, Y.F.; He, Y.; Zhu, C.Z.; Cao, Q.J.; Sui, M.Q.; Liang, M.; Tian, L.X.; Jiang, T.Z.; Wang, Y.F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007, 29, 83–91. [Google Scholar]
- Zou, Q.H.; Wu, C.W.; Stein, E.A.; Zang, Y.F.; Yang, Y.H. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage 2009, 48, 515–524. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Hampson, M.; Tokoglu, F.; Sun, Z.; Schafer, R.J.; Skudlarski, P.; Gore, J.C.; Constable, R.T. Connectivity–behavior analysis reveals that functional connectivity between left BA39 and Broca’s area varies with reading ability. NeuroImage 2006, 31, 513–519. [Google Scholar] [CrossRef]
- Yu, M.; Xu, M.; Li, X.; Chen, Z.; Song, Y.; Liu, J. The shared neural basis of music and language. Neuroscience 2017, 357, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zang, Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of restingstate fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef]
- Yan, C.G.; Wang, X.-D.; Zuo, X.-N.; Zang, Y.-F. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.R.A.; Sabuncu, M.R.; Buckner, R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 2012, 59, 431–438. [Google Scholar] [CrossRef]
- Zuo, X.N.; Di Martino, A.; Kelly, C.; Shehzad, Z.E.; Gee, D.G.; Klein, D.F.; Castellanos, F.X.; Biswal, B.B.; Milham, M.P. The oscillating brain: Complex and reliable. NeuroImage 2010, 49, 1432–1445. [Google Scholar] [CrossRef]
- Jiang, H.; White, M.P.; Greicius, M.D.; Waelde, L.C.; Spiegel, D. Brain Activity and Functional Connectivity Associated with Hypnosis. Cereb. Cortex 2017, 27, 4083–4093. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.G.; Cheung, B.; Kelly, C.; Colcombe, S.; Craddock, R.C.; Di Martino, A.; Li, Q.; Zuo, X.-N.; Castellanos, F.X.; Milham, M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 2013, 76, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-M.; Xu, S.-X.; Sun, Y.; Wang, K.-Y.; Wang, C.; Zhang, J.; Xia, J.-X.; Zhang, L.; Tan, B.-J.; Xie, X.-H. Electroconvulsive therapy changes the regional resting state function measured by regional homogeneity (ReHo) and amplitude of low frequency fluctuations (ALFF) in elderly major depressive disorder patients: An exploratory study. Psychiatry Res. Neuroimaging 2017, 264, 13–21. [Google Scholar] [CrossRef]
- Hamann, S.; Mao, H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport 2002, 13, 15–19. [Google Scholar] [CrossRef]
- Heller, A.S.; Johnstone, T.; Shackman, A.J.; Light, S.N.; Peterson, M.J.; Kolden, G.G.; Kalin, N.H.; Davidson, R.J. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci. USA 2009, 106, 22445–22450. [Google Scholar] [CrossRef]
- Chiu, P.H.; Holmes, A.J.; Pizzagalli, D.A. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. NeuroImage 2008, 42, 988–997. [Google Scholar] [CrossRef]
- Dalgleish, T. The emotional brain. Nat. Rev. Neurosci. 2004, 5, 583–589. [Google Scholar] [CrossRef]
- Luan Phan, K.; Liberzon, I.; Welsh, R.C.; Britton, J.C.; Taylor, S.F. Habituation of Rostral Anterior Cingulate Cortex to Repeated Emotionally Salient Pictures. Neuropsychopharmacology 2003, 28, 1344–1350. [Google Scholar] [CrossRef]
- Mohanty, A.; Engels, A.S.; Herrington, J.D.; Heller, W.; Ringo Ho, M.-H.; Banich, M.T.; Webb, A.G.; Warren, S.L.; Miller, G.A. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 2007, 44, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Sripada, R.K.; Marx, C.E.; King, A.P.; Rajaram, N.; Garfinkel, S.N.; Abelson, J.L.; Liberzon, I. DHEA Enhances Emotion Regulation Neurocircuits and Modulates Memory for Emotional Stimuli. Neuropsychopharmacology 2013, 38, 1798–1807. [Google Scholar] [CrossRef]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hoptman, M.J.; Zuo, X.-N.; Butler, P.D.; Javitt, D.C.; D’Angelo, D.; Mauro, C.J.; Milham, M.P. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr. Res. 2010, 117, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zhang, J.; Wen, H.; Zhang, Y.; Kang, H.; Wang, X.; Li, W.; He, H.; Peng, Y. Altered Spontaneous Brain Activity in Children with Early Tourette Syndrome: A Resting-state fMRI Study. Sci. Rep. 2017, 7, 4808. [Google Scholar] [CrossRef] [PubMed]
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.J.; Bailey, A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 2009, 30, 2313–2335. [Google Scholar] [CrossRef]
- Frith, C.D.; Frith, U. The Neural Basis of Mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef]
- Koster-Hale, J.; Saxe, R. Theory of Mind: A Neural Prediction Problem. Neuron 2013, 79, 836–848. [Google Scholar] [CrossRef]
- Leopold, A.; Krueger, F.; dal Monte, O.; Pardini, M.; Pulaski, S.J.; Solomon, J.; Grafman, J. Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Soc. Cogn. Affect. Neurosci. 2012, 7, 871–880. [Google Scholar] [CrossRef]
- Mar, R.A. The Neural Bases of Social Cognition and Story Comprehension. Annu. Rev. Psychol. 2011, 62, 103–134. [Google Scholar] [CrossRef]
- Schnell, K.; Bluschke, S.; Konradt, B.; Walter, H. Functional relations of empathy and mentalizing: An fMRI study on the neural basis of cognitive empathy. NeuroImage 2011, 54, 1743–1754. [Google Scholar] [CrossRef]
- Van Overwalle, F. Social cognition and the brain: A meta-analysis. Hum. Brain Mapp. 2009, 30, 829–858. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Baetens, K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. NeuroImage 2009, 48, 564–584. [Google Scholar] [CrossRef]
- Tettamanti, M.; Vaghi, M.M.; Bara, B.G.; Cappa, S.F.; Enrici, I.; Adenzato, M. Effective connectivity gateways to the Theory of Mind network in processing communicative intention. NeuroImage 2017, 155, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.W. Interpreting What Speakers Say and Implicate. Brain Lang. 1999, 68, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Spotorno, N.; Koun, E.; Prado, J.; Van Der Henst, J.B.; Noveck, I.A. Neural evidence that utterance-processing entails mentalizing: The case of irony. NeuroImage 2012, 63, 25–39. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, P.; Wen, J.; Wang, J.; Li, H.; Biswal, B.B. Hippocampus-based static functional connectivity mapping within white matter in mild cognitive impairment. Brain Struct. Funct. 2022, 227, 2285–2297. [Google Scholar] [CrossRef]
- Sun, N.; Li, Y.; Zhang, A.; Yang, C.; Liu, P.; Liu, Z.; Wang, Y.; Jin, R.; Zhang, K. Fractional amplitude of low-frequency fluctuations and gray matter volume alterations in patients with bipolar depression. Neurosci. Lett. 2020, 730, 135030. [Google Scholar] [CrossRef]



| Condition | Cover Story | Dialogue | |
|---|---|---|---|
| You are talking with Zhang Zheng, who is an acquaintance of yours. You hosted a party last week and Zhang Zheng attended the party. You decide to ask Zhang Zheng how he felt about the party. | Question | Reply | |
| Indirect Reply | You: Did you enjoy yourself at my party? | Zhang Zheng: I prefer to be quiet. | |
| Direct Reply | Zhang Zheng: I didn’t have a good time. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, M.; Yang, X.; Yang, Y. Individual Differences in Emotion Attenuation Brought by Indirect Replies Is Related to Resting-State Brain Activity. Brain Sci. 2023, 13, 1053. https://doi.org/10.3390/brainsci13071053
Zhang X, Xu M, Yang X, Yang Y. Individual Differences in Emotion Attenuation Brought by Indirect Replies Is Related to Resting-State Brain Activity. Brain Sciences. 2023; 13(7):1053. https://doi.org/10.3390/brainsci13071053
Chicago/Turabian StyleZhang, Xiuping, Maoyao Xu, Xiaohong Yang, and Yufang Yang. 2023. "Individual Differences in Emotion Attenuation Brought by Indirect Replies Is Related to Resting-State Brain Activity" Brain Sciences 13, no. 7: 1053. https://doi.org/10.3390/brainsci13071053
APA StyleZhang, X., Xu, M., Yang, X., & Yang, Y. (2023). Individual Differences in Emotion Attenuation Brought by Indirect Replies Is Related to Resting-State Brain Activity. Brain Sciences, 13(7), 1053. https://doi.org/10.3390/brainsci13071053





