Factors Affecting the Quality of Sleep and Social Participation of Stroke Patients
Abstract
:1. Introduction
2. Method
2.1. Research Subjects and Period
2.2. Research Tools
2.2.1. Pittsburgh Sleep Quality Index
2.2.2. Beck Depression Inventory
2.2.3. Beck Anxiety Inventory
2.2.4. Stress Scale
2.2.5. Reintegration to Normal Living Index
2.3. Analysis Methods
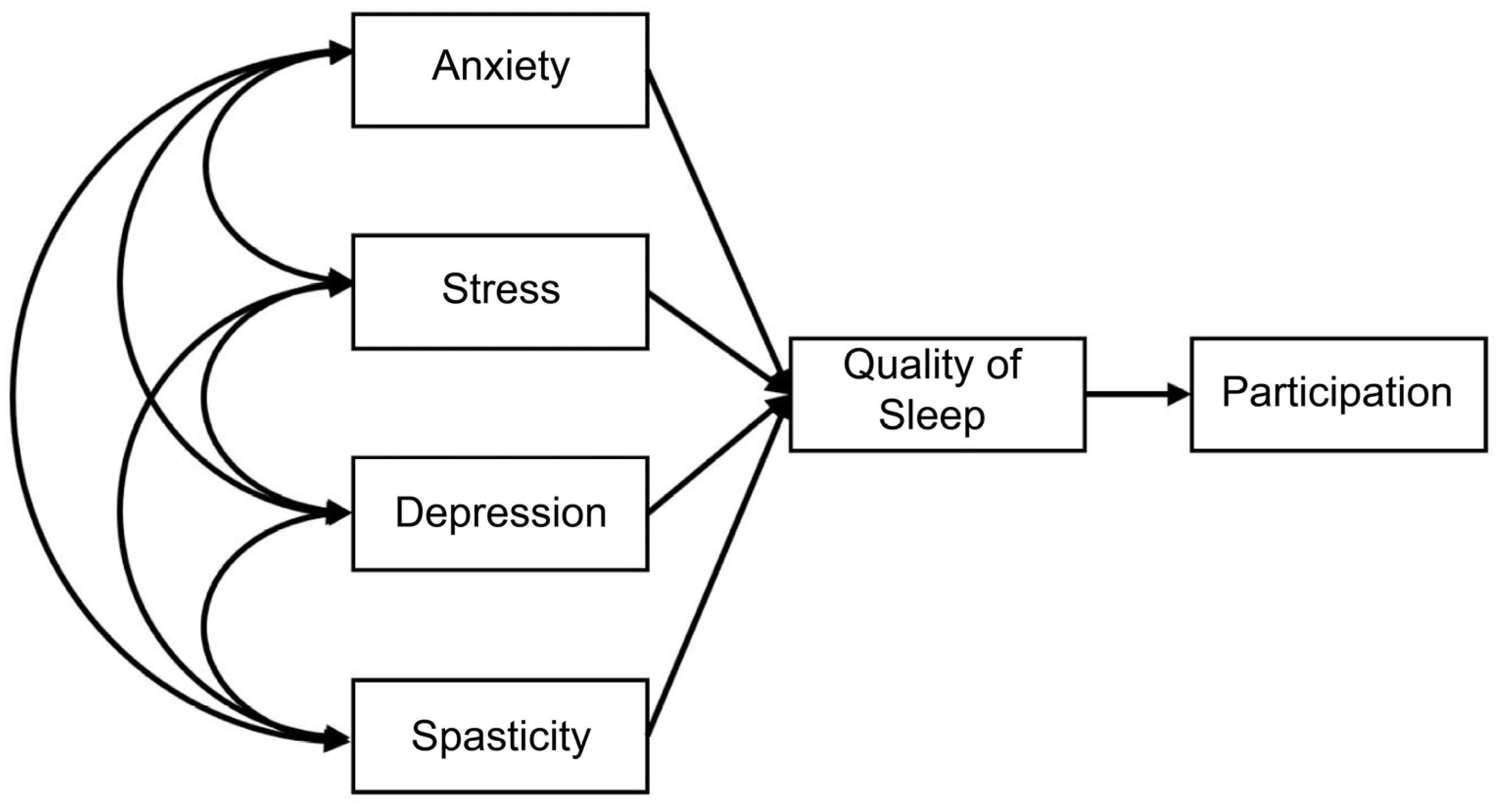
2.3.1. Path Analysis
2.3.2. Prediction Model
2.3.3. Analysis Methods
3. Results
3.1. Participants’ General Characteristics
3.2. Correlations between Sleep Quality, Social Participation, Depression, Anxiety, Stress, and Spasticity
3.3. Measurement Model and Model Fit
3.4. Direct, Indirect, and Total Effects of Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 383, 245–255, Corrected in Lancet 2014, 383, 218. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and Epidemiologic Drivers of Global Cardiovascular Mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y. Sleep Disorders after Stroke. Brain Neurorehabil. 2015, 8, 73–80. [Google Scholar] [CrossRef]
- Pasic, Z.; Smajlovic, D.; Dostovic, Z.; Kojic, B.; Selmanovic, S. Incidence and Types of Sleep Disorders in patients with Stroke. Med. Arch. 2011, 65, 225–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djonlagic, I.; Saboisky, J.; Carusona, A.; Stickgold, R.; Malhotra, A. Increased Sleep Fragmentation Leads to Impaired Off-Line Consolidation of Motor Memories in Humans. PLoS ONE 2012, 7, e34106. [Google Scholar] [CrossRef] [Green Version]
- Khot, S.P.; Morgenstern, L.B. Sleep and Stroke. Stroke 2019, 50, 1612–1617. [Google Scholar] [CrossRef]
- Ho, E.C.M.; Siu, A.M.H. Occupational Therapy Practice in Sleep Management: A Review of Conceptual Models and Research Evidence. Occup. Ther. Int. 2018, 2018, 8637498. [Google Scholar] [CrossRef] [Green Version]
- O’Donoghue, N.; McKay, E.A. Exploring the impact of sleep apnoea on daily life and occupational engagement. Br. J. Occup. Ther. 2012, 75, 509–516. [Google Scholar] [CrossRef]
- Scott, C.L.; Phillips, L.H.; Johnston, M.; Whyte, M.M.; MacLeod, M.J. Emotion processing and social participation following stroke: Study protocol. BMC Neurol. 2012, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Youngstrom, M.j.; Jane Brayman, S.J.; Anthony, P.; Brinson, M.; Brownrigg, S.; Clark, G.F.; Roley, S.S.; Sellers, J.; Van Slyke, N.L.; Desmarais, S.M.; et al. Occupational therapy practice framework: Domain and process. Am. J. Occup. Ther. 2002, 56, 609–639, Corrected in Am. J. Occup. Ther. 2003, 57, 115. [Google Scholar]
- Kielhofner, G. A Model of Human Occupation: Theory and Application; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Üstün, T.; Chatterji, S.; Bickenbach, J.; Kostanjsek, N.; Schneider, M. The International Classification of Functioning, Disability and Health: A new tool for understanding disability and health. Disabil. Rehabil. 2003, 25, 565–571. [Google Scholar] [CrossRef]
- Sagen, U.; Vik, T.G.; Moum, T.; Mørland, T.; Finset, A.; Dammen, T. Screening for anxiety and depression after stroke: Comparison of the Hospital Anxiety and Depression Scale and the Montgomery and Åsberg Depression Rating Scale. J. Psychosom. Res. 2009, 67, 325–332. [Google Scholar] [CrossRef] [PubMed]
- De Wit, L.; Putman, K.; Baert, I.; Lincoln, N.B.; Angst, F.; Beyens, H.; Bogaerts, K.; Brinkmann, N.; Connell, L.; Dejaeger, E.; et al. Anxiety and depression in the first six months after stroke. A longitudinal multicentre study. Disabil. Rehabil. 2008, 30, 1858–1866. [Google Scholar] [CrossRef]
- Salah, S.; Rekik, M.; Frih, Z. Association of quality of life and sleep quality in patients with ischemic stroke. J. Diabetes Treat. 2018, 10, 2574–7568. [Google Scholar] [CrossRef]
- Garton, A.L.; Sisti, J.A.; Gupta, V.; Christophe, B.R.; Connolly, E.S. Poststroke Post-Traumatic Stress Disorder. Stroke 2017, 48, 507–512, Corrected in Stroke 2017, 48, e96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuller, K.A.; Jarrett, B.; DeVries, A.C. Stress and social isolation increase vulnerability to stroke. Exp. Neurol. 2012, 233, 33–39. [Google Scholar] [CrossRef]
- Kang, M.-S.; Hong, K.-H.; Jung, H.-R. The Relationship of Quality of Sleep with Stress and Rehabilitation Motivation in Stroke. J. Korean Soc. Occup. Ther. 2017, 25, 59–70. [Google Scholar] [CrossRef]
- Karaca, B. Factors Affecting Poststroke Sleep Disorders. J. Stroke Cerebrovasc. Dis. 2016, 25, 727–732. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, B.; Liu, Q.; Wang, Y.; Zhao, Y.; Zhu, X.; Zhao, B.Y.; Hu, B.; Zhu, M.X. Social support and sleep quality in patients with stroke: The mediating roles of depression and anxiety symptoms. Int. J. Nurs. Pract. 2021, 28, e12939. [Google Scholar] [CrossRef]
- Huzmeli, E.D.; Sarac, E.T. Examination of Sleep Quality, Anxiety and Depression in Stroke Patients. Turk Beyin Damar Hastalik. Derg. 2017, 23, 51–55. [Google Scholar] [CrossRef]
- Davis, J.C.; Falck, R.S.; Best, J.R.; Chan, P.; Doherty, S.; Liu-Ambrose, T. Examining the Inter-relations of Depression, Physical Function, and Cognition with Subjective Sleep Parameters among Stroke Survivors: A Cross-sectional Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, J.Y. A study of the reliability and the validity of the BDI, SDS, and MMPI-D scales. Korean J. Clin. Psychol. 1991, 10, 98–113. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.-M.; Lee, H.-K.; Kim, J.W.; Lee, K. Reliability and Validity of the Korean Version of Beck Depression Inventory-II via the Internet: Results from a University Student Sample. J. Korean Neuropsychiatr. Assoc. 2012, 51, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Yook, S.P.; Kim, Z.S. A clinical study on the Korean version of Beck Anxiety Inventory: Comparative study of patient and non-patient. Kor. J. Clin. Psychol. 1997, 16, 185–197. [Google Scholar]
- Neuman, B. The Neuman System Model: Application to Nursing Education and Practice; Appleton Century Crofts: Norwalk, CT, USA, 1982. [Google Scholar]
- Moon, J.Y.; Cho, B.H. Relationships among rehabilitation motivation, perceived stress and social support in stroke survivors. Korean J. Rehabil. Nurs. 2011, 14, 24–31. [Google Scholar]
- Wood-Dauphinee, S.L.; Opzoomer, M.A.; Williams, J.I.; Marchand, B.; Spitzer, W.O. Assessment of global function: The Reintegration to Normal Living Index. Arch. Phys. Med. Rehabil. 1988, 69, 583–590. [Google Scholar]
- Salter, K.; Foley, N.; Jutai, J.; Bayley, M.; Teasell, R. Assessment of community integration following traumatic brain injury. Brain Inj. 2008, 22, 820–835. [Google Scholar] [CrossRef]
- Cardol, M.; Brandsma, J.W.; De Groot, I.J.M.; van den Bosoe, G.A.M.; de Haan, R.J.; de Jong, B.A. Handicap questionnaires: What do they assess? Disability Rehabil. 1999, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wood-Dauphinee, S.; Williams, J. Reintegration to normal living as a proxy to quality of life. J. Chronic Dis. 1987, 40, 491–499. [Google Scholar] [CrossRef]
- Hitzig, S.L.; Escobar, E.M.R.; Noreau, L.; Craven, B.C. Validation of the Reintegration to Normal Living Index for Community-Dwelling Persons With Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2012, 93, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.B. Principles and Practice of Structural Equation Modeling; The Guilford Press: New York, NY, USA, 2015. [Google Scholar]
- Moon, S.B. Basic Concepts and Applications of Structural Equation Modeling with AMOS 17.0; Hakjisa: Seoul, Republic of Korea, 2009; pp. 383–458. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1998. [Google Scholar]
- Oliveira, G.D.P.; Vago, E.R.L.; Prado, G.F.D.; Coelho, F.M.S. The critical influence of nocturnal breathing complaints on the quality of sleep after stroke: The Pittsburgh Sleep Quality Index and STOP-BANG. Arq. Neuro-Psiquiatr. 2017, 75, 785–788. [Google Scholar] [CrossRef] [Green Version]
- Almhdawi, K.A.; Alazrai, A.; Kanaan, S.; Shyyab, A.A.; Oteir, A.; Mansour, Z.M.; Jaber, H. Post-stroke depression, anxiety, and stress symptoms and their associated factors: A cross-sectional study. Neuropsychol. Rehabil. 2020, 31, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Brown, D.L.; Chervin, R.D.; Case, E.; Morgenstern, L.B.; Lisabeth, L.D. Pre-stroke sleep duration and post-stroke depression. Sleep Med. 2021, 77, 325–329. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Xu, P.; Fan, Q.; Gong, P.; Ding, C.; Sheng, L.; Zhang, X. Association between obstructive sleep apnea and risk of post-stroke depression: A hospital-based study in ischemic stroke patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104876. [Google Scholar] [CrossRef]
- Oh, M.K.; Lee, C.S.; Park, C.S.; Kim, B.J.; Cha, B.S.; Kim, S.M.; Lee, S.J.; Shin, H.S.; Kim, A.R. Clinical characteristics associated with quality of sleep in old stroke patients: Preliminary study. J. Korean Geriatr. Psychiatry 2011, 1, 3–6. [Google Scholar]
- Leppävuori, A.; Pohjasvaara, T.; Vataja, R.; Kaste, M.; Erkinjuntti, T. Insomnia in Ischemic Stroke Patients. Cerebrovasc. Dis. 2002, 14, 90–97. [Google Scholar] [CrossRef]
- Kim, W.O.; Kang, H.S.; Wang, M.J.; Kim, J.H.; Choi, J.Y. Relationships among activity of daily living, depression, and quality of life (QOL) in patients with stroke. J. East-West Nurs. Res. 2007, 13, 138–146. [Google Scholar]
- Bak, H.K. The effects of the health promotion program on functional status of the in-house stroke patients. Korean J. Rehabil. Nurs. 2003, 6, 213–225. [Google Scholar]
- Choi-Kwon, S.; Han, S.W.; Kwon, S.U.; Kim, J.S. Poststroke Fatigue: Characteristics and Related Factors. Cerebrovasc. Dis. 2005, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.V.; Brininger, T.L. Occupational Therapy for Servicemember and Veteran Recovery, Resilience, and Reintegration: Opportunities for Societal Contribution and Professional Transformation. Am. J. Occup. Ther. 2014, 68, 379–380. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.F.; Hahn, M.; Baum, C.; Dromerick, A.W. The Impact of Mild Stroke on Meaningful Activity and Life Satisfaction. J. Stroke Cerebrovasc. Dis. 2006, 15, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol. Neurochir. Polska 2019, 53, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.-M.; Marosszeky, J.E.; Renton, R.; Sandanam, J.; Gandevia, S.C.; Burke, D. Short-term effects of dynamic Lycra splints on upper limb in hemiplegic patients. Arch. Phys. Med. Rehabil. 2000, 81, 1547–1555. [Google Scholar] [CrossRef]
- Park, S.A.; Kim, H.Y. Effects of virtual reality program on recovery of functional in individuals stroke: A systematic review and meta analysis. J. Digit. Converg. 2019, 17, 235–247. [Google Scholar]
- Cho, S.H.; Choi, K.B. The effect of virtual reality programs on upper extremity function in stroke patients: A meta-analysis. J. Korean Acad.-Ind. Coop. Soc. 2020, 21, 429–439. [Google Scholar]
- Yao, K.-W.; Yu, S.; Cheng, S.-P.; Chen, I.-J. Relationships Between Personal, Depression and Social Network Factors and Sleep Quality in Community-Dwelling Older Adults. J. Nurs. Res. 2008, 16, 131–139. [Google Scholar] [CrossRef]
- Cheng, W.; Rolls, E.T.; Ruan, H.; Feng, J. Functional Connectivities in the Brain That Mediate the Association Between Depressive Problems and Sleep Quality. JAMA Psychiatry 2018, 75, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 negative(−) effect.
negative(−) effect.
 negative(−) effect.
negative(−) effect.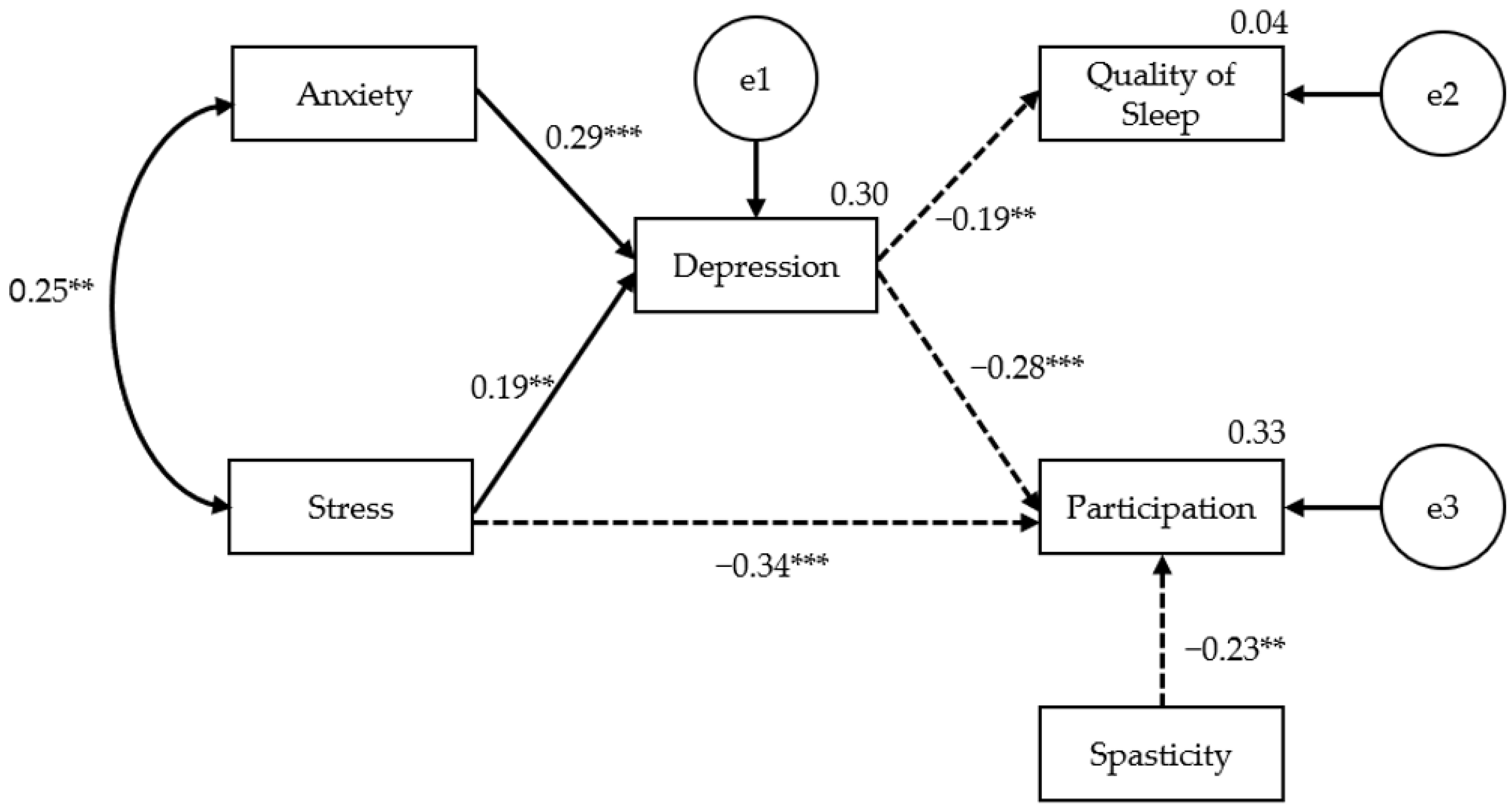
| Characteristics | N (%) | Male (n = 84) | Female (n = 45) | t | |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Social participation rate | 90–100% | 16 (12.4) | 8 (6.2) | 8 (6.2) | 0.375 |
| 80–89% | 8 (6.2) | 8 (6.2) | 0 (0.0) | ||
| 70–79% | 9 (7.0) | 7 (5.4) | 2 (1.6) | ||
| 60–69% | 18 (14.0) | 11 (8.6) | 7 (5.4) | ||
| 50–59% | 14 (10.8) | 10 (7.7) | 4 (3.1) | ||
| 40–49% | 12 (9.3) | 8 (6.2) | 4 (3.1) | ||
| 30–39% | 12 (9.3) | 5 (3.9) | 7 (5.4) | ||
| 20–29% | 13 (10.1) | 11 (8.6) | 2 (1.5) | ||
| 10–19% | 14 (10.8) | 9 (6.9) | 5 (3.9) | ||
| 0–9% | 13 (10.1) | 7 (5.4) | 6 (4.7) | ||
| Sleep quality | Poor | 70 (54.3) | 44 (34.1) | 26 (20.2) | 0.583 |
| High | 59 (45.7) | 40 (31.0) | 19 (14.7) | ||
| Paralyzed side of the body | Rt. | 60 (46.5) | 48 (37.2) | 12 (9.3) | 2.644 ** |
| Lt. | 55 (42.7) | 29 (22.5) | 26 (20.2) | ||
| Both | 3 (2.3) | 1 (0.8) | 2 (1.5) | ||
| Normal | 11 (8.5) | 6 (4.6) | 5 (3.9) | ||
| Diagnosis | Infarction | 70 (54.3) | 43 (33.4) | 27 (20.9) | 0.953 |
| Hemorrhage | 59 (45.7) | 41 (31.7) | 18 (14.0) | ||
| Education | ≥University | 42 (32.5) | 27 (20.9) | 15 (11.6) | 2.144 * |
| High school | 46 (35.7) | 36 (27.9) | 10 (7.8) | ||
| Middle school | 15 (11.6) | 10 (7.7) | 5 (3.9) | ||
| Elementary School | 21 (16.3) | 10 (7.7) | 11 (8.5) | ||
| <Elementary School | 5 (3.9) | 1 (0.8) | 4 (3.1) | ||
| Current Job | Employee | 16 (12.4) | 12 (9.3) | 4 (3.1) | 0.182 |
| Self-employed | 28 (21.7) | 20 (15.5) | 8 (6.2) | ||
| Student | 2 (1.6) | 0 (0.0) | 2 (1.6) | ||
| Housewife | 14 (10.9) | 0 (0.0) | 14 (10.9) | ||
| Retirement | 7 (5.4) | 7 (5.4) | 0 (0.0) | ||
| Retirement (health problem) | 57 (44.1) | 41 (31.7) | 16 (12.4) | ||
| Retirement (other reason) | 4 (3.9) | 4 (3.1) | 1 (0.8) | ||
| Marital status | Married | 79 (61.2) | 56 (43.4) | 23 (17.8) | 2.695 ** |
| Single | 21 (16.3) | 15 (11.6) | 6 (4.7) | ||
| Divorce | 9 (7.0) | 5 (3.9) | 4 (3.1) | ||
| Bereaved | 16 (12.4) | 7 (5.4) | 9 (7.0) | ||
| Separation | 4 (3.1) | 1 (0.8) | 3 (2.3) | ||
| Monthly income (KRW) | >3,000,000 (>USD 2295) | 23 (17.8) | 15 (11.6) | 8 (6.2) | 0.056 |
| 2,000,000–3,000,000 (USD 1530–USD 2295) | 30 (23.3) | 21 (16.3) | 9 (7.0) | ||
| 1,500,000–2,000,000 (USD 1147–USD 1530) | 26 (20.2) | 14 (10.9) | 12 (9.3) | ||
| 1,000,000–1,500,000 (USD 765–USD 1147) | 17 (13.2) | 12 (9.3) | 5 (3.9) | ||
| <1,000,000 (<USD 765) | 33 (25.6) | 22 (17.1) | 11 (8.5) | ||
| Residential area | Metropolis | 81 (62.8) | 55 (42.6) | 26 (20.2) | 0.439 |
| Small-medium cities | 24 (18.6) | 13 (10.1) | 11 (8.5) | ||
| Small town | 24 (18.6) | 16 (12.4) | 8 (6.2) | ||
| Assistance device | None | 54 (41.8) | 34 (26.4) | 20 (15.4) | 0.629 |
| Manual wheelchair | 44 (34.1) | 30 (23.2) | 14 (10.9) | ||
| Cane | 21 (16.3) | 13 (10.1) | 8 (6.2) | ||
| Electric wheelchair | 5 (3.9) | 4 (3.1) | 1 (0.8) | ||
| Walker | 5 (3.9) | 3 (2.3) | 2 (1.6) | ||
| Spasticity | MAS 0 | 43 (33.3) | 27 (20.9) | 16 (12.4) | 1.340 |
| MAS 1 | 31 (24.0) | 16 (12.4) | 15 (11.6) | ||
| MAS 1+ | 24 (18.6) | 18 (14.0) | 6 (4.7) | ||
| MAS 2 | 25 (19.4) | 19 (14.7) | 6 (4.7) | ||
| MAS 3 | 6 (4.7) | 4 (3.1) | 2 (1.6) | ||
| Age (year. M ± SD) | 58.29 ± 15.46 | 0.252 | |||
| Duration from onset (months, M ± SD) | 39.73 ± 51.49 | 0.690 | |||
| Quality of Sleep | Spasticity | Depression | Anxiety | Stress | Participation | |
|---|---|---|---|---|---|---|
| Quality of sleep | 1 | |||||
| Spasticity | 0.094 ** | 1 | ||||
| Depression | −0.195 ** | 0.051 ** | 1 | |||
| Anxiety | −0.145 ** | 0.121 ** | 0.393 ** | 1 | ||
| Stress | −0.118 ** | −0.114 ** | 0.473 ** | 0.252 ** | 1 | |
| Participation | 0.178 * | −0.203 ** | −0.456 ** | −0.314 ** | −0.446 ** | 1 |
| χ2 (p) | df | TLI | NFI | CFI | GFI | RMSEA |
|---|---|---|---|---|---|---|
| 11.712 (0.165) | 8 | 0.935 | 0.904 | 0.965 | 0.971 | 0.060 |
| β | S.E. | C.R. | |||
|---|---|---|---|---|---|
| Depression | ← | Stress | 0.399 | 0.005 | 5.239 *** |
| Depression | ← | Anxiety | 0.292 | 0.112 | 3.837 *** |
| Sleep | ← | Depression | −0.195 | 0.036 | −2.246 * |
| Participation | ← | Depression | −0.283 | 2.018 | −3.456 *** |
| Participation | ← | Spasticity | −0.225 | 1.696 | −3.122 ** |
| Participation | ← | Stress | −0.336 | 0.135 | −4.099 *** |
| Variable | Spasticity | Stress | Anxiety | Depression | |
|---|---|---|---|---|---|
| Depression | Indirect Effects | ||||
| Direct Effects | 0.399 ** | 0.292 ** | |||
| Total Effects | 0.399 ** | 0.292 ** | |||
| Participation | Indirect Effects | −0.113 ** | −0.083 ** | ||
| Direct Effects | −0.225 ** | −0.336 ** | −0.283 ** | ||
| Total Effects | −0.225 ** | −0.449 ** | −0.083 ** | −0.283 ** | |
| Quality of Sleep | Indirect Effects | −0.078 * | −0.057 * | ||
| Direct Effects | −0.195 * | ||||
| Total Effects | −0.078 * | −0.057 * | −0.195 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-S.; Kim, H. Factors Affecting the Quality of Sleep and Social Participation of Stroke Patients. Brain Sci. 2023, 13, 1068. https://doi.org/10.3390/brainsci13071068
Hwang H-S, Kim H. Factors Affecting the Quality of Sleep and Social Participation of Stroke Patients. Brain Sciences. 2023; 13(7):1068. https://doi.org/10.3390/brainsci13071068
Chicago/Turabian StyleHwang, Ho-Sung, and Hee Kim. 2023. "Factors Affecting the Quality of Sleep and Social Participation of Stroke Patients" Brain Sciences 13, no. 7: 1068. https://doi.org/10.3390/brainsci13071068





