Mood Symptoms and Chronic Fatigue Syndrome Due to Relapsing-Remitting Multiple Sclerosis Are Associated with Immune Activation and Aberrations in the Erythron
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Biochemical Assays
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic, Clinical, and Blood Parameters of the Study Groups
3.1.1. Assessment of Immune Profiles
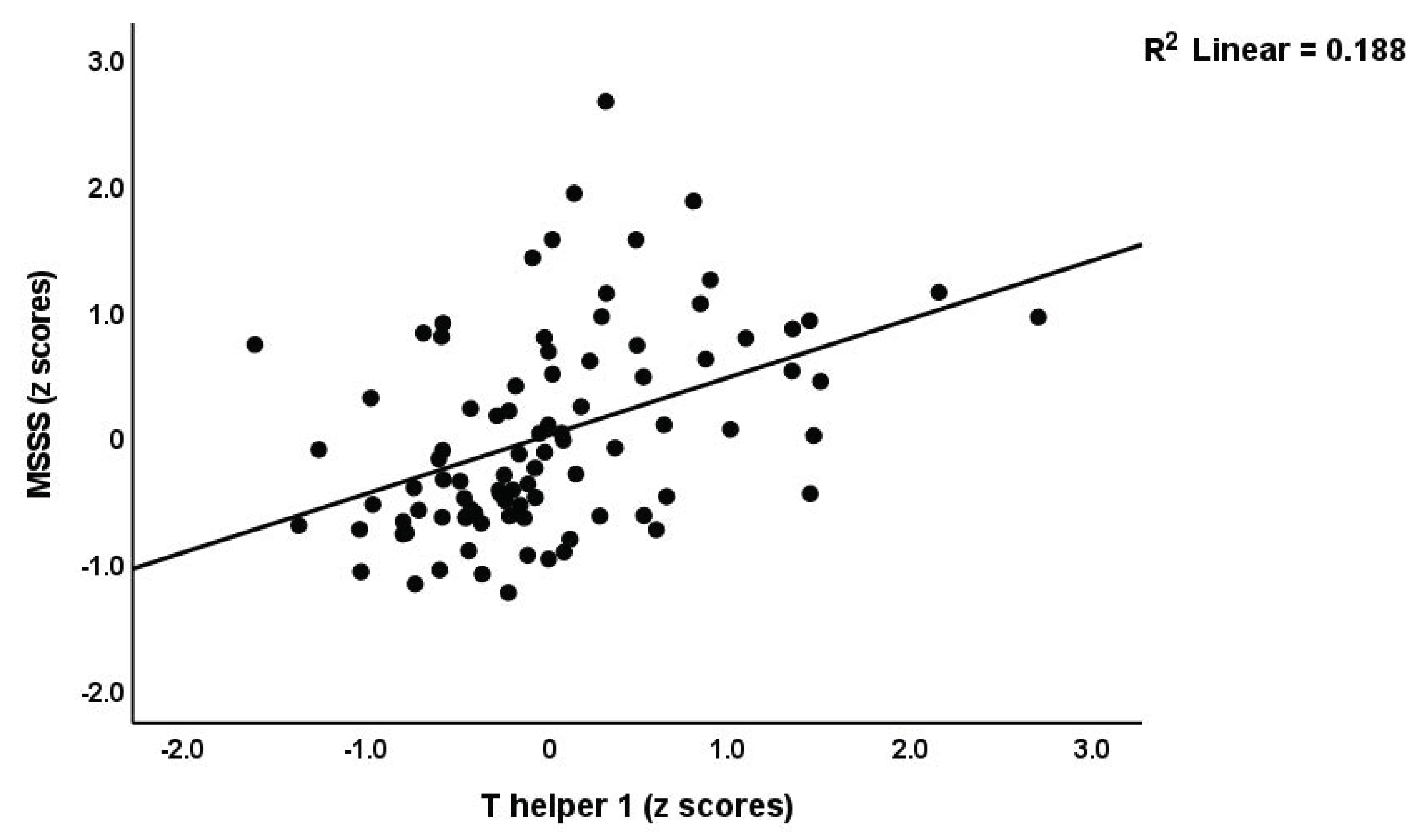
3.1.2. Prediction of Disabilities and Severity of MS by Immune Biomarkers
3.1.3. Prediction of Neuropsychiatric (NP) Symptoms by Erythron Variables and Immune Indices
3.2. Results of Automatic Linear Modeling Analyses with Overfit Prevention
4. Discussion
4.1. Immune Profiles in the Remitted Phase
4.2. Immune Profiles and Depression, Anxiety, and Physiosomatic Symptoms due to MS
4.3. Fatigue and PP due to MS and the Erythron
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havrdová, E.; Preiningerova, J.L. Symptoms and diagnosis of multiple sclerosis. In Personalized Management of Multiple Sclerosis; Future Medicine Ltd.: London, UK, 2013; pp. 36–48. [Google Scholar]
- Kallaur, A.P.; Lopes, J.; Oliveira, S.R.; Simão, A.N.; Reiche, E.M.; de Almeida, E.R.; Morimoto, H.K.; de Pereira, W.L.; Alfieri, D.F.; Borelli, S.D.; et al. Immune-Inflammatory and Oxidative and Nitrosative Stress Biomarkers of Depression Symptoms in Subjects with Multiple Sclerosis: Increased Peripheral Inflammation but Less Acute Neuroinflammation. Mol. Neurobiol. 2016, 53, 5191–5202. [Google Scholar] [CrossRef]
- Jongen, P.J.; Ter Horst, A.T.; Brands, A.M. Cognitive impairment in multiple sclerosis. Minerva Med. 2012, 103, 73–96. [Google Scholar] [PubMed]
- Leray, E.; Moreau, T.; Fromont, A.; Edan, G. Epidemiology of multiple sclerosis. Rev. Neurol. 2016, 172, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, H.K.; Al-Mahadawi, A.; Sheaheed, N.M.; Sami, S.M.; Jamal, A.; Allebban, Z. Epidemiology of multiple sclerosis in Iraq: Retrospective review of 4355 cases and literature review. Neurol. Res. 2022, 44, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Yiangou, Y.; Facer, P.; Durrenberger, P.; Chessell, I.P.; Naylor, A.; Bountra, C.; Banati, R.R.; Anand, P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Roxburgh, R.H.S.R.; Seaman, S.R.; Masterman, T.; Hensiek, A.E.; Sawcer, S.J.; Vukusic, S.; Achiti, I.; Confavreux, C.; Coustans, M.; le Page, E.; et al. Multiple Sclerosis Severity Score. Neurology 2005, 64, 1144. [Google Scholar] [CrossRef]
- Lublin, F.D.; Häring, D.A.; Ganjgahi, H.; Ocampo, A.; Hatami, F.; Čuklina, J.; Aarden, P.; Dahlke, F.; Arnold, D.L.; Wiendl, H.; et al. How patients with multiple sclerosis acquire disability. Brain 2022, 145, 3147–3161. [Google Scholar] [CrossRef]
- Siotto, M.; Filippi, M.M.; Simonelli, I.; Landi, D.; Ghazaryan, A.; Vollaro, S.; Ventriglia, M.; Pasqualetti, P.; Rongioletti, M.C.A.; Squitti, R.; et al. Oxidative Stress Related to Iron Metabolism in Relapsing Remitting Multiple Sclerosis Patients With Low Disability. Front. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef]
- De Carvalho Jennings Pereira, W.L.; Flauzino, T.; Alfieri, D.F.; Oliveira, S.R.; Kallaur, A.P.; Simão, A.N.C.; Lozovoy, M.A.B.; Kaimen-Maciel, D.R.; Maes, M.; Reiche, E.M.V. Prolactin is Not Associated with Disability and Clinical Forms in Patients with Multiple Sclerosis. NeuroMolecular Med. 2020, 22, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Nabavi, S.M.; Fereshtehnejad, S.M.; Ansari, I.; Zerafatjou, N.; Shayegannejad, V.; Mohammadianinejad, S.E.; Farhoudi, M.; Noorian, A.; Razazian, N.; et al. Risk factors of Multiple sclerosis and their Relation with Disease Severity: A Cross-sectional Study from Iran. Arch. Iran Med. 2016, 19, 852–860. [Google Scholar]
- Debouverie, M. Gender as a prognostic factor and its impact on the incidence of multiple sclerosis in Lorraine, France. J. Neurol. Sci. 2009, 286, 14–17. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Semin. Neurol. 2016, 36, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Maes, M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013, 11, 205. [Google Scholar] [CrossRef]
- Bai, Z.; Chen, D.; Wang, L.; Zhao, Y.; Liu, T.; Yu, Y.; Yan, T.; Cheng, Y. Cerebrospinal Fluid and Blood Cytokines as Biomarkers for Multiple Sclerosis: A Systematic Review and Meta-Analysis of 226 Studies with 13,526 Multiple Sclerosis Patients. Front. Neurosci. 2019, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple Immune-Inflammatory and Oxidative and Nitrosative Stress Pathways Explain the Frequent Presence of Depression in Multiple Sclerosis. Mol. Neurobiol. 2018, 55, 6282–6306. [Google Scholar] [CrossRef]
- Ormstad, H.; Simonsen, C.S.; Broch, L.; Maes, D.M.; Anderson, G.; Celius, E.G. Chronic fatigue and depression due to multiple sclerosis: Immune-inflammatory pathways, tryptophan catabolites and the gut-brain axis as possible shared pathways. Mult. Scler. Relat. Disord. 2020, 46, 102533. [Google Scholar] [CrossRef]
- Nagaraj, K.; Taly, A.B.; Gupta, A.; Prasad, C.; Christopher, R. Prevalence of fatigue in patients with multiple sclerosis and its effect on the quality of life. J. Neurosci. Rural Pr. 2013, 4, 278–282. [Google Scholar] [CrossRef]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M.J. Prevalence of depression and anxiety in Multiple Sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Ayache, S.S.; Serratrice, N.; Abi Lahoud, G.N.; Chalah, M.A. Fatigue in Multiple Sclerosis: A Review of the Exploratory and Therapeutic Potential of Non-Invasive Brain Stimulation. Front. Neurol. 2022, 13, 813965. [Google Scholar] [CrossRef]
- Peres, D.S.; Rodrigues, P.; Viero, F.T.; Frare, J.M.; Kudsi, S.Q.; Meira, G.M.; Trevisan, G. Prevalence of depression and anxiety in the different clinical forms of multiple sclerosis and associations with disability: A systematic review and meta-analysis. Brain Behav. Immun. Health 2022, 24, 100484. [Google Scholar] [CrossRef]
- Herring, T.E.; Alschuler, K.N.; Knowles, L.M.; Phillips, K.M.; Morean, W.M.; Turner, A.P.; Ehde, D.M. Differences in correlates of fatigue between relapsing and progressive forms of multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 54, 103109. [Google Scholar] [CrossRef] [PubMed]
- Kahl, K.G.; Kruse, N.; Faller, H.; Weiss, H.; Rieckmann, P. Expression of tumor necrosis factor-alpha and interferon-gamma mRNA in blood cells correlates with depression scores during an acute attack in patients with multiple sclerosis. Psychoneuroendocrinology 2002, 27, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Pokryszko-Dragan, A.; Frydecka, I.; Kosmaczewska, A.; Ciszak, L.; Bilińska, M.; Gruszka, E.; Podemski, R.; Frydecka, D. Stimulated peripheral production of interferon-gamma is related to fatigue and depression in multiple sclerosis. Clin. Neurol. Neurosurg. 2012, 114, 1153–1158. [Google Scholar] [CrossRef]
- Maes, M.; Carvalho, A.F. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol. Neurobiol. 2018, 55, 8885–8903. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Vasupanrajit, A.; Jirakran, K.; Tunvirachaisakul, C.; Solmi, M.; Maes, M. Inflammation and nitro-oxidative stress in current suicidal attempts and current suicidal ideation: A systematic review and meta-analysis. Mol. Psychiatry 2022, 27, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Song, C.; Lin, A.; De Jongh, R.; Van Gastel, A.; Kenis, G.; Bosmans, E.; De Meester, I.; Benoy, I.; Neels, H.; et al. The Effects of Psychological Stress on Humans: Increased Production of Pro-Inflammatory Cytokines and Th1-Like Response in Stress-Induced Anxiety. Cytokine 1998, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kunkl, M.; Frascolla, S.; Amormino, C.; Volpe, E.; Tuosto, L. T Helper Cells: The Modulators of Inflammation in Multiple Sclerosis. Cells 2020, 9, 482. [Google Scholar] [CrossRef]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef]
- Melnikov, M.; Lopatina, A. Th17-cells in depression: Implication in multiple sclerosis. Front. Immunol. 2022, 13, 1010304. [Google Scholar] [CrossRef] [PubMed]
- Hon, G.M.; Hassan, M.S.; Rensburg, S.J.v.; Erasmus, R.T.; Matsha, T. The Haematological Profile of Patients with Multiple Sclerosis. Open J. Mod. Neurosurg. 2012, 2, 36–44. [Google Scholar] [CrossRef]
- Kasprzycka, W.; Nieśpiałowska, M.; Jakubowska-Solarska, B. Blood count parameters in the course of multiple sclerosis. J. Transfus. Med. 2019, 12, 117–123. [Google Scholar] [CrossRef]
- Vandoolaeghe, E.; De Vos, N.; Demedts, P.; Wauters, A.; Neels, H.; De Schouwer, P.; Maes, M. Reduced number of red blood cells, lowered hematocrit and hemoglobin, and increased number of reticulocytes in major depression as indicators of activation of the inflammatory response system: Effects of antidepressant drugs. Hum. Psychopharmacol. Clin. Exp. 1999, 14, 45–52. [Google Scholar] [CrossRef]
- Maes, M.; Van de Vyvere, J.; Vandoolaeghe, E.; Bril, T.; Demedts, P.; Wauters, A.; Neels, H. Alterations in iron metabolism and the erythron in major depression: Further evidence for a chronic inflammatory process. J. Affect Disord. 1996, 40, 23–33. [Google Scholar] [CrossRef]
- Wysokiński, A.; Szczepocka, E. Red Blood Cells Parameters in Patients with Acute Schizophrenia, Unipolar Depression and Bipolar Disorder. Psychiatr. Danub. 2018, 30, 323–330. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in development of the index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zachrisson, O.; Regland, B.; Jahreskog, M.; Kron, M.; Gottfries, C.G. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale). J. Psychosom. Res. 2002, 52, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Al-Rawi, K.F.; Maes, M.; Al-Hakeim, H.K. In schizophrenia, immune-inflammatory pathways are strongly associated with depressive and anxiety symptoms, which are part of a latent trait which comprises neurocognitive impairments and schizophrenia symptoms. J. Affect. Disord. 2021, 287, 316–326. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2022, 28, 564–578. [Google Scholar] [CrossRef]
- Al-Hadrawi, D.S.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hakeim, H.K.; Maes, M. Lowered oxygen saturation and increased body temperature in acute COVID-19 largely predict chronic fatigue syndrome and affective symptoms due to Long COVID: A precision nomothetic approach. Acta Neuropsychiatr. 2022, 35, 76–87. [Google Scholar] [CrossRef]
- Maes, M.; Rachayon, M.; Jirakran, K.; Sodsai, P.; Klinchanhom, S.; Gałecki, P.; Sughondhabirom, A.; Basta-Kaim, A. The Immune Profile of Major Dysmood Disorder: Proof of Concept and Mechanism Using the Precision Nomothetic Psychiatry Approach. Cells 2022, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Thisayakorn, P.; Thipakorn, Y.; Tantavisut, S.; Sirivichayakul, S.; Maes, M. Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry 2022, 22, 369. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Musawi, A.F.; Al-Mulla, A.; Al-Dujaili, A.H.; Debnath, M.; Maes, M. The interleukin-6/interleukin-23/T helper 17-axis as a driver of neuro-immune toxicity in the major neurocognitive psychosis or deficit schizophrenia: A precision nomothetic psychiatry analysis. PLoS ONE 2022, 17, e0275839. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Khaibullin, T.; Ivanova, V.; Martynova, E.; Cherepnev, G.; Khabirov, F.; Granatov, E.; Rizvanov, A.; Khaiboullina, S. Elevated Levels of Proinflammatory Cytokines in Cerebrospinal Fluid of Multiple Sclerosis Patients. Front. Immunol. 2017, 8, 531. [Google Scholar] [CrossRef]
- Rodríguez-Sáinz Mdel, C.; Sánchez-Ramón, S.; de Andrés, C.; Rodríguez-Mahou, M.; Muñoz-Fernández, M.A. Th1/Th2 cytokine balance and nitric oxide in cerebrospinal fluid and serum from patients with multiple sclerosis. Eur. Cytokine Netw. 2002, 13, 110–114. [Google Scholar]
- Kallaur, A.P.; Oliveira, S.R.; Colado Simão, A.N.; Delicato de Almeida, E.R.; Kaminami Morimoto, H.; Lopes, J.; de Carvalho Jennings Pereira, W.L.; Marques Andrade, R.; Muliterno Pelegrino, L.; Donizete Borelli, S.; et al. Cytokine profile in relapsing-remitting multiple sclerosis patients and the association between progression and activity of the disease. Mol. Med. Rep. 2013, 7, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Hollifield, R.D.; Harbige, L.S.; Pham-Dinh, D.; Sharief, M.K. Evidence for Cytokine Dysregulation in Multiple Sclerosis: Peripheral Blood Mononuclear Cell Production of Pro-inflammatory and Anti-inflammatory Cytokines During Relapse and Remission. Autoimmunity 2003, 36, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, P.; Albrecht, M.; Kitze, B.; Weber, T.; Tumani, H.; Broocks, A.; Lüer, W.; Poser, S. Cytokine mRNA levels in mononuclear blood cells from patients with multiple sclerosis. Neurology 1994, 44, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, P.; Albrecht, M.; Kitze, B.; Weber, T.; Tumani, H.; Broocks, A.; Lüer, W.; Helwig, A.; Poser, S. Tumor necrosis factor-alpha messenger RNA expression in patients with relapsing-remitting multiple sclerosis is associated with disease activity. Ann. Neurol. 1995, 37, 82–88. [Google Scholar] [CrossRef]
- Bertolotto, A.; Capobianco, M.; Malucchi, S.; Manzardo, E.; Audano, L.; Bergui, M.; Bradac, G.B.; Mutani, R. Transforming growth factor beta1 (TGFbeta1) mRNA level correlates with magnetic resonance imaging disease activity in multiple sclerosis patients. Neurosci. Lett. 1999, 263, 21–24. [Google Scholar] [CrossRef]
- Mokhtarian, F.; Shi, Y.; Shirazian, D.; Morgante, L.; Miller, A.; Grob, D. Defective production of anti-inflammatory cytokine, TGF-beta by T cell lines of patients with active multiple sclerosis. J. Immunol. 1994, 152, 6003–6010. [Google Scholar] [CrossRef]
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009, 132, 3329–3341. [Google Scholar] [CrossRef]
- Kebir, H.; Ifergan, I.; Alvarez, J.I.; Bernard, M.; Poirier, J.; Arbour, N.; Duquette, P.; Prat, A. Preferential recruitment of interferon-γ–expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009, 66, 390–402. [Google Scholar] [CrossRef]
- Kalra, S.; Lowndes, C.; Durant, L.; Strange, R.C.; Al-Araji, A.; Hawkins, C.P.; Curnow, S.J. Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217319899695. [Google Scholar] [CrossRef]
- Babaloo, Z.; Aliparasti, M.R.; Babaiea, F.; Almasi, S.; Baradaran, B.; Farhoudi, M. The role of Th17 cells in patients with relapsing-remitting multiple sclerosis: Interleukin-17A and interleukin-17F serum levels. Immunol. Lett. 2015, 164, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Nani, J.V.; Noto, C.; Rizzo, L.; Hayashi, M.A.F.; Brietzke, E. Impairments in Peripheral Blood T Effector and T Regulatory Lymphocytes in Bipolar Disorder Are Associated with Staging of Illness and Anti-cytomegalovirus IgG Levels. Mol. Neurobiol. 2021, 58, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, W.W.; Derada Troletti, C.; Reijerkerk, A.; Romero, I.A.; de Vries, H.E. The blood-brain barrier in multiple sclerosis: MicroRNAs as key regulators. CNS Neurol. Disord. Drug Targets 2015, 14, 157–167. [Google Scholar] [CrossRef]
- Irizar, H.; Muñoz-Culla, M.; Sepúlveda, L.; Sáenz-Cuesta, M.; Prada, Á.; Castillo-Triviño, T.; Zamora-López, G.; López de Munain, A.; Olascoaga, J.; Otaegui, D. Transcriptomic profile reveals gender-specific molecular mechanisms driving multiple sclerosis progression. PLoS ONE 2014, 9, e90482. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M.; Murdjeva, M.; Puri, B.K. Do Human Endogenous Retroviruses Contribute to Multiple Sclerosis, and if So, How? Mol. Neurobiol. 2019, 56, 2590–2605. [Google Scholar] [CrossRef]
- Morandi, E.; Tarlinton, R.E.; Gran, B. Multiple Sclerosis between Genetics and Infections: Human Endogenous Retroviruses in Monocytes and Macrophages. Front. Immunol. 2015, 6, 647. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002, 2, 37–45. [Google Scholar] [CrossRef]
- McCabe, M.P. Mood and self-esteem of persons with multiple sclerosis following an exacerbation. J. Psychosom. Res. 2005, 59, 161–166. [Google Scholar] [CrossRef]
- Jefferies, K. The neuropsychiatry of multiple sclerosis. Adv. Psychiatr. Treat. 2006, 12, 214–220. [Google Scholar] [CrossRef]
- Koutsouraki, E.; Hatzifilipou, E.; Michmizos, D.; Cotsavasiloglou, C.; Costa, V.; Baloyannis, S. Increase in interleukin-6 levels is related to depressive phenomena in the acute (relapsing) phase of multiple sclerosis. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 442–448. [Google Scholar] [CrossRef]
- Frei, K.; Fredrikson, S.; Fontana, A.; Link, H. Interleukin-6 is elevated in plasma in multiple sclerosis. J. Neuroimmunol. 1991, 31, 147–153. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Jennings Pereira, W.L.; Flauzino, T.; Alfieri, D.F.; Oliveira, S.R.; Kallaur, A.P.; Simão, A.N.C.; Lozovoy, M.A.B.; Kaimen-Maciel, D.R.; Maes, M.; Reiche, E.M.V. Immune-inflammatory, metabolic and hormonal biomarkers are associated with the clinical forms and disability progression in patients with multiple sclerosis: A follow-up study. J. Neurol. Sci. 2020, 410, 116630. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Ringel, K. Activation of cell-mediated immunity in depression: Association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 169–175. [Google Scholar] [CrossRef]
- Moylan, S.; Maes, M.; Wray, N.R.; Berk, M. The neuroprogressive nature of major depressive disorder: Pathways to disease evolution and resistance, and therapeutic implications. Mol. Psychiatry 2013, 18, 595–606. [Google Scholar] [CrossRef]
- Maes, M.; Anderson, G.; Kubera, M.; Berk, M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin. Ther. Targets 2014, 18, 495–512. [Google Scholar] [CrossRef]
- Cui, M.; Dai, W.; Kong, J.; Chen, H. Th17 Cells in Depression: Are They Crucial for the Antidepressant Effect of Ketamine? Front. Pharmacol. 2021, 12, 649144. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Fardan, A.S.; El-Sherbeeny, A.M.; Ibrahim, K.E.; Attia, S.M. IL-17A causes depression-like symptoms via NFκB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine 2017, 97, 14–24. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.-H.; Chang, K.-A. Sex Difference in Peripheral Inflammatory Biomarkers in Drug-Naïve Patients with Major Depression in Young Adulthood. Biomedicines 2021, 9, 708. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab. Brain Dis. 2013, 28, 523–540. [Google Scholar] [CrossRef]
- Vieira, M.M.; Ferreira, T.B.; Pacheco, P.A.; Barros, P.O.; Almeida, C.R.; Araújo-Lima, C.F.; Silva-Filho, R.G.; Hygino, J.; Andrade, R.M.; Linhares, U.C.; et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J. Neuroimmunol. 2010, 229, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Mikova, O.; Yakimova, R.; Bosmans, E.; Kenis, G.; Maes, M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur. Neuropsychopharmacol. 2001, 11, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Sirivichayakul, S.; Matsumoto, A.K.; Maes, A.; Michelin, A.P.; de Oliveira Semeão, L.; de Lima Pedrão, J.V.; Moreira, E.G.; Barbosa, D.S.; Geffard, M.; et al. Increased Levels of Plasma Tumor Necrosis Factor-α Mediate Schizophrenia Symptom Dimensions and Neurocognitive Impairments and Are Inversely Associated with Natural IgM Directed to Malondialdehyde and Paraoxonase 1 Activity. Mol. Neurobiol. 2020, 57, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Prajeeth, C.K.; Kronisch, J.; Khorooshi, R.; Knier, B.; Toft-Hansen, H.; Gudi, V.; Floess, S.; Huehn, J.; Owens, T.; Korn, T.; et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflammation. 2017, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, A.; Roy, P.; Lobaugh, N.; Feinstein, K.; O’connor, P.; Black, S. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology 2004, 62, 586–590. [Google Scholar] [CrossRef]
- Khatibi, A.; Moradi, N.; Rahbari, N.; Salehi, T.; Dehghani, M. Development and Validation of Fear of Relapse Scale for Relapsing-Remitting Multiple Sclerosis: Understanding Stressors in Patients. Front. Psychiatry 2020, 11, 226. [Google Scholar] [CrossRef]
- Hanken, K.; Sander, C.; Schlake, H.-P.; Kastrup, A.; Eling, P.; Hildebrandt, H. Fatigue in Multiple Sclerosis is related to relapses, autonomic dysfunctions and introversion: A quasi-experimental study. Mult. Scler. Relat. Disord. 2019, 36, 101401. [Google Scholar] [CrossRef]
- Moore, P.; Hirst, C.; Harding, K.E.; Clarkson, H.; Pickersgill, T.P.; Robertson, N.P. Multiple sclerosis relapses and depression. J. Psychosom. Res. 2012, 73, 272–276. [Google Scholar] [CrossRef]
- Šabanagić-Hajrić, S.; Suljić, E.; Kučukalić, A. Fatigue during multiple sclerosis relapse and its relationship to depression and neurological disability. Psychiatr. Danub. 2015, 27, 406–412. [Google Scholar]
- Maes, M.; Twisk, F.N.M.; Ringel, K. Inflammatory and Cell-Mediated Immune Biomarkers in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Depression: Inflammatory Markers Are Higher in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome than in Depression. Psychother. Psychosom. 2012, 81, 286–295. [Google Scholar] [CrossRef]
- Groen, K.; Maltby, V.E.; Sanders, K.A.; Scott, R.J.; Tajouri, L.; Lechner-Scott, J. Erythrocytes in multiple sclerosis—forgotten contributors to the pathophysiology? Mult. Scler. J. Exp. Transl. Clin. 2016, 2, 2055217316649981. [Google Scholar] [CrossRef]
- Peng, Y.F.; Cao, W.Y.; Zhang, Q.; Chen, D.; Zhang, Z.X. Assessment of the Relationship Between Red Cell Distribution Width and Multiple Sclerosis. Medicine 2015, 94, e1182. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Schmidt, B.R.; Wilhelmy, J.; Nguyen, V.; Abugherir, A.; Do, J.K.; Nemat-Gorgani, M.; Davis, R.W.; Ramasubramanian, A.K. Red blood cell deformability is diminished in patients with Chronic Fatigue Syndrome. Clin. Hemorheol. Microcirc. 2019, 71, 113–116. [Google Scholar] [CrossRef]
- Deng, W.; Feng, X.; Li, X.; Wang, D.; Sun, L. Hypoxia-inducible factor 1 in autoimmune diseases. Cell. Immunol. 2016, 303, 7–15. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, J.; Cui, R. Effect of Hypoxic Injury in Mood Disorder. Neural. Plast. 2017, 2017, 6986983. [Google Scholar] [CrossRef] [PubMed]
- Jóźwik, M.; Jóźwik, M.; Jóźwik, M.; Szczypka, M.; Gajewska, J.; Laskowska-Klita, T. Antioxidant defence of red blood cells and plasma in stored human blood. Clin. Chim. Acta 1997, 267, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Revin, V.V.; Gromova, N.V.; Revina, E.S.; Samonova, A.Y.; Tychkov, A.Y.; Bochkareva, S.S.; Moskovkin, A.A.; Kuzmenko, T.P. The Influence of Oxidative Stress and Natural Antioxidants on Morphometric Parameters of Red Blood Cells, the Hemoglobin Oxygen Binding Capacity, and the Activity of Antioxidant Enzymes. Biomed. Res. Int. 2019, 2019, 2109269. [Google Scholar] [CrossRef] [PubMed]
| Variables | HC (n = 30) | MS Patients (n = 63) | F/X2 | df | p-Value |
|---|---|---|---|---|---|
| Age (Years) | 31.70(5.38) | 29.76(8.40) | 1.33 | 1/91 | 0.252 |
| Sex (M/F) | 19/11 | 23/40 | 5.90 | 1 | 0.015 |
| Marital status (Married/Single) | 19/11 | 29/34 | 2.43 | 1 | 0.119 |
| Employment (Y/N) | 28/2 | 11/52 | 48.04 | 1 | <0.001 |
| Smoking (Y/N) | 5/25 | 9/54 | 0.090 | 1 | 0.764 |
| BMI (Kg/m2) | 26.57(4.53) | 24.86(3.56) | 3.92 | 1/91 | 0.051 |
| Duration of illness (years) | - | 5.51(4.72) | - | - | |
| MSSS | 0 | 2.08 (2.01) | MWU | - | <0.001 |
| EDSS | 0 | 1 (1–2.5) | MWU | - | <0.001 |
| ADL | 14.00 | 13.06(1.40) | MWU | - | <0.001 |
| PC_disabilities | −0.967(0) | 0.518(0.659) | 151.41 | 1/91 | <0.001 |
| Relapsing remission/Progressive MS | - | 55/8 | - | - | - |
| WBC (thousand/mm3) | 6.59(1.75) | 9.49(2.96) | 24.59 | 1/91 | 0.009 |
| RBC (million/mm3) * | 4.915(0.111) | 4.926(0.086) | 0.00 | 1/81 | 0.940 |
| Hct (%) * | 42.974(0.947) | 40.838(0.732) | 3.08 | 1/81 | 0.083 |
| Hb (g/dL) * | 14.702(0.337) | 14.175(0.261) | 1.48 | 1/81 | 0.227 |
| MCV (fl) * | 87.931(1.249) | 84.144(0.965) | 5.58 | 1/81 | 0.021 |
| MCH (pg) * | 30.147(0.525) | 28.269(0.405) | 6.20 | 1/81 | 0.015 |
| RDW-SD (fl) * | 36.943(0.955) | 41.322(0.738) | 12.73 | 1/81 | <0.001 |
| PC_RBCs * | 0.189(0.153) | −0.041(0.118) | 1.37 | 1/81 | 0.245 |
| PC_RBCindices * | 0.332(0.183) | −0.084(0.141) | 3.15 | 1/81 | 0.080 |
| Pure depression | −0.964(1.002) | 0.459(0.592) | 73.62 | 1/91 | <0.0013 ** |
| Pure anxiety | −0.807(0.645) | 0.384(0.907) | 41.57 | 1/91 | <0.0013 ** |
| Pure physiosomatic | −1.223(0.524) | 0.585(0.524) | 243.61 | 1/91 | <0.0013 ** |
| Chronic fatigue | −0.912(0.578) | 0.434(0.856) | 60.68 | 1/91 | <0.0013 ** |
| Autonomic symptoms | −0.944(0.518) | 0.449(0.849) | 68.49 | 1/91 | <0.0013 ** |
| Insomnia | −0.824(0.532) | 0.440(0.860) | 63.54 | 1/91 | <0.0013 ** |
| PC_psychopathology | −1.228(0.445) | 0.585(0.561) | 240.69 | 1/91 | <0.0013 ** |
| Variables | HC (n = 30) A | LOW MS (n = 34) B | HIGH MS (n = 29) C | F | dfh/dfe | p-Value |
|---|---|---|---|---|---|---|
| M1 (z scores) * | −0.347(0.079) C | −0.210(0.077) C | 0.605(0.174) A,B | 19.43 | 2/90 | 0.002 |
| Th1 (z scores) * | −0.329(0.096) C | −0.159(0.130) C | 0.528(0.119) A,B | 12.42 | 2/90 | 0.002 |
| Th2 (z scores) | −0.164(0.050) B | 0.135(0.062) A | 0.010(0.099) | 5.72 | 2/90 | 0.018 |
| zTh2-zTh1 (z scores) | −0.172(0.127) | 0.175(0.187) | −0.027(0.211) | 0.986 | 2/90 | 0.377 |
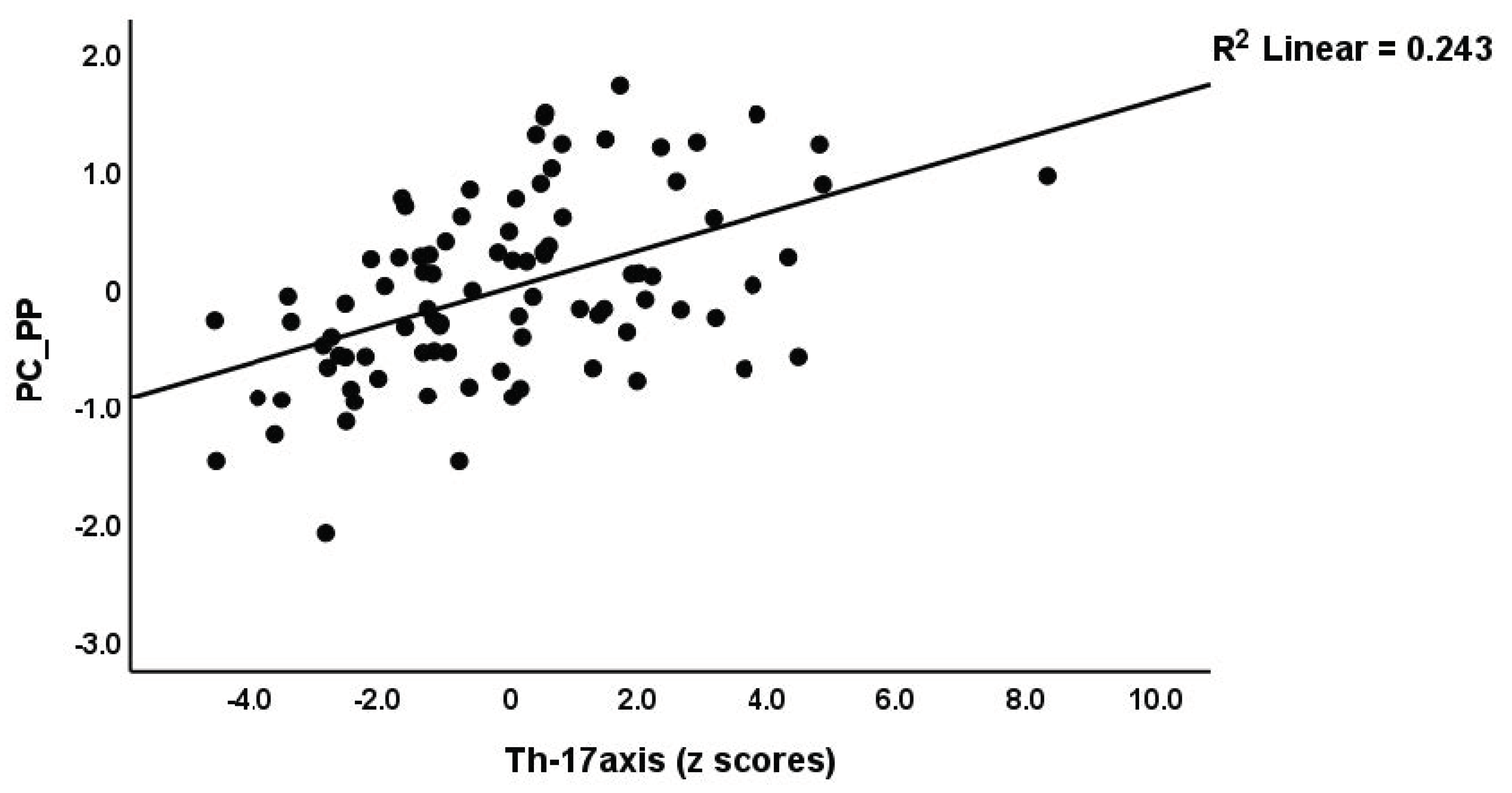
| Th17 (z scores) * | −0.304(0.092) C | −0.062(0.099) C | 0.388(0.186) A,B | 4.54 | 2/90 | 0.002 |
| Th17-axis (z scores) * | −0.909(0.218) C | −0.226(0.262) C | 1.20(0.443) A,B | 8.21 | 2/90 | 0.002 |
| IRS (z scores) * | −0.308(0.079) C | −0.212(0.090) C | 0.567(0.143) A,B | 20.35 | 2/90 | 0.002 |
| T-cell growth (z scores) * | −0.147(0.070) C | −0.216(0.076) C | 0.406(0.168) A,B | 9.08 | 2/90 | 0.002 |
| Growth factors (z scores) * | −0.333(0.068) C | −0.243(0.103) C | 0.629(0.190) A,B | 12.90 | 2/90 | 0.002 |
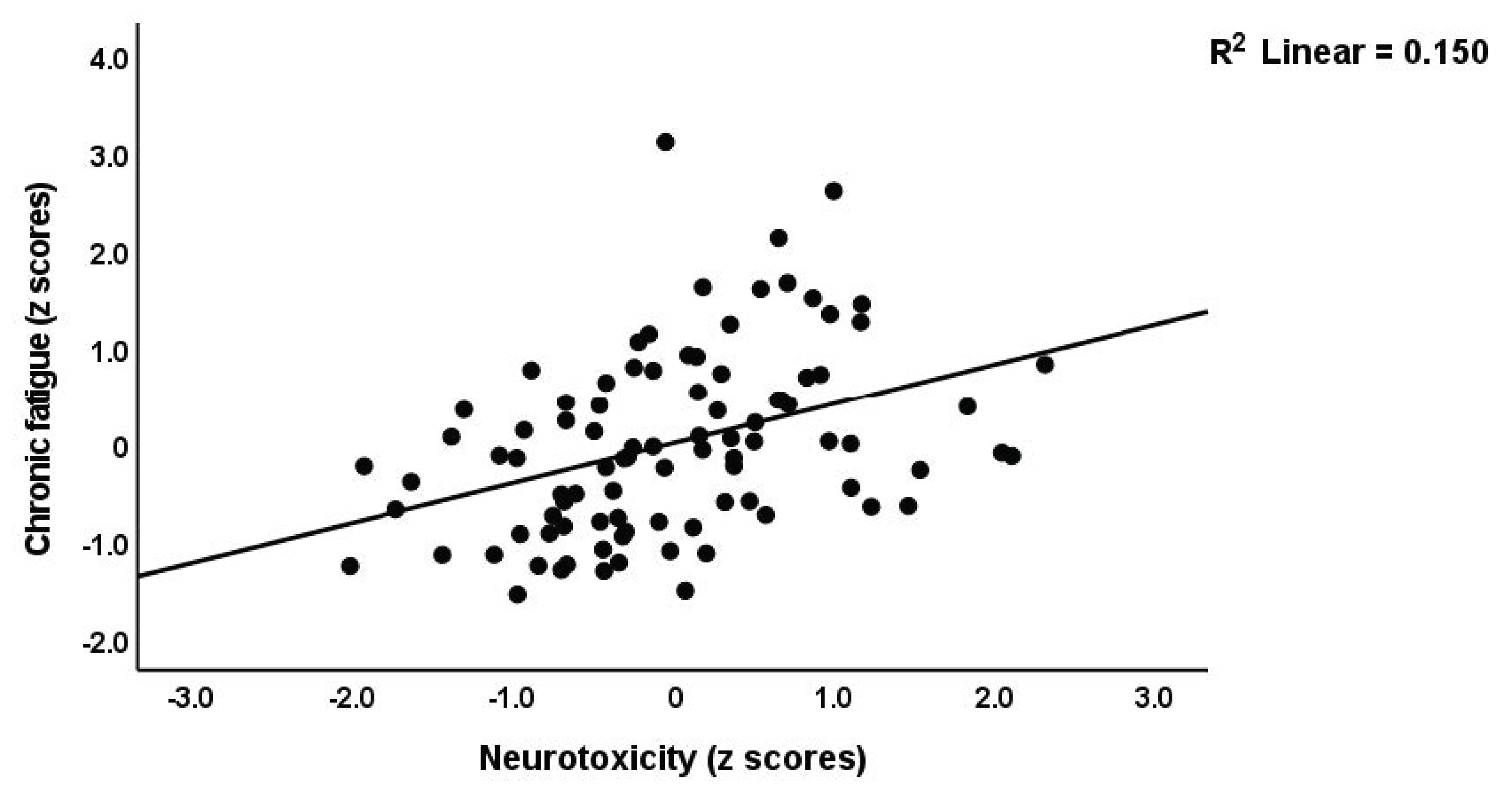
| Neurotoxicity (z scores) * | −0.330(0.086) C | −0.141(0.099) C | 0.507(0.136) A,B | 15.65 | 2/90 | 0.002 |
| CIRS (z scores) | −0.225(0.076) B | 0.210(0.103) A | −0.013(0.174) | 3.26 | 2/90 | 0.044 |
| Dependent Variables | Explanatory Variables | Coefficients of Input Variables | Model Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| β | t | p | R2 | F | df | p | ||
| In all subjects combined | ||||||||
| #1. PC_disabilities | Model | 0.424 | 21.32 | 3/87 | <0.001 | |||
| Th1 | 0.338 | 2.61 | 0.011 | |||||
| CIRS | 0.269 | 3.27 | 0.002 | |||||
| Th17-axis | 0.281 | 2.17 | 0.033 | |||||
| #2. MSSS | Model | 0.533 | 31.6 | 3/83 | <0.001 | |||
| T-cell growth | 0.252 | 1.72 | 0.089 | |||||
| CIRS | 0.386 | 5.01 | <0.001 | |||||
| Th1 | 0.445 | 3.01 | 0.004 | |||||
| In patients only | ||||||||
| #3. MSSS | Model | 0.178 | 6.06 | 2/56 | 0.004 | |||
| CIRS | 0.397 | 3.04 | 0.004 | |||||
| Th1 | 0.356 | 2.72 | 0.009 | |||||
| Dependent Variables | Explanatory Variables | Coefficients of Input Variables | Model Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| β | t | p | R2 | F | df | p | ||
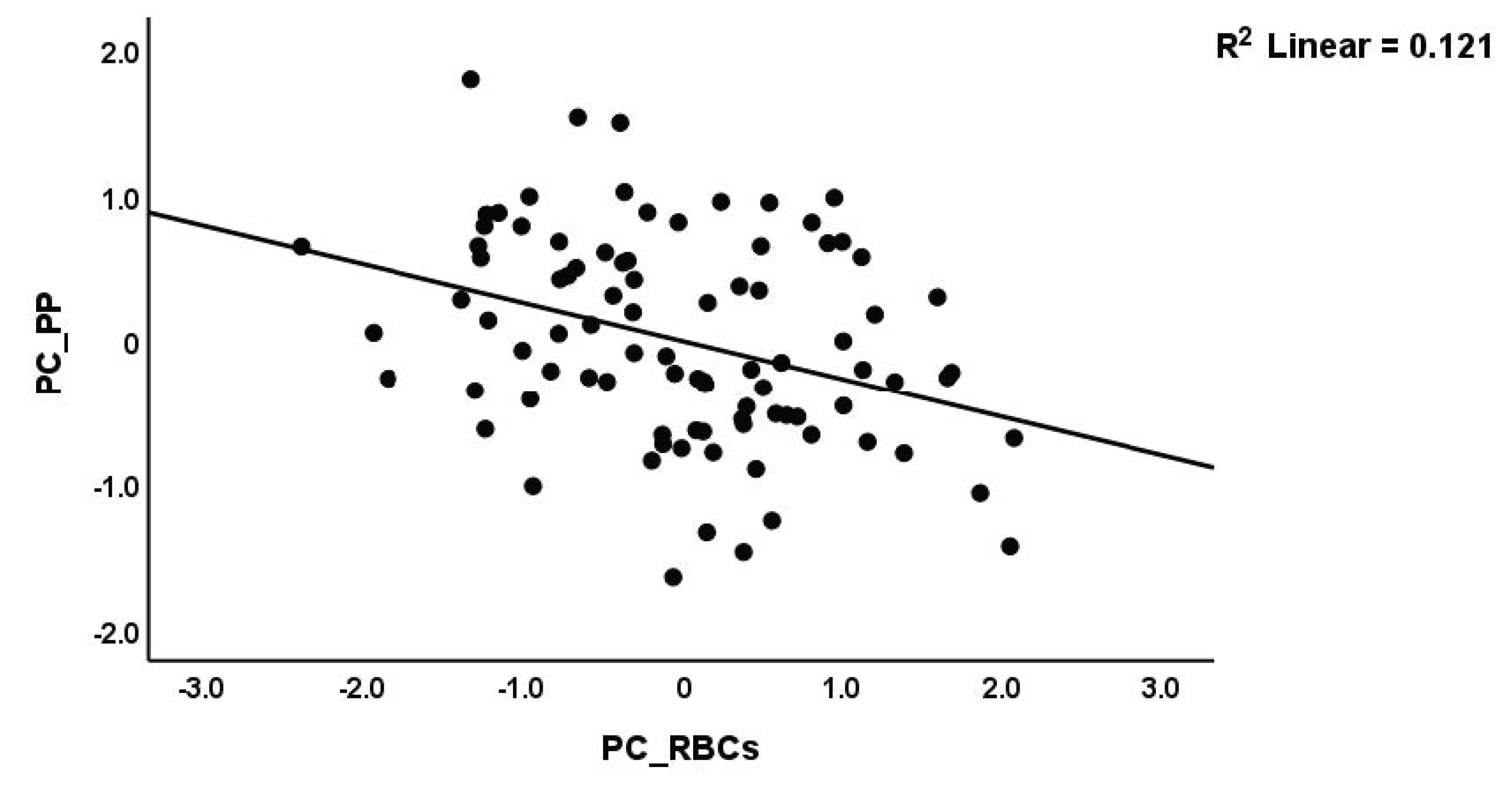
| #1. PC_PP (in all subjects) | Model | 0.506 | 21.51 | 4/84 | <0.001 | |||
| Th17-axis | 0.425 | 5.1 | <0.001 | |||||
| WBCs | 0.304 | 3.6 | <0.001 | |||||
| PC_RBCs | −0.269 | −3.41 | <0.001 | |||||
| CIRS | 0.17 | 2.15 | 0.034 | |||||
| #2. PC_PP (with forced entry of MSSS scale score) | Model | 0.563 | 27.06 | 4/84 | <0.001 | |||
| MSSS | 0.425 | 4.98 | <0.001 | |||||
| Sex | −0.228 | −3.11 | 0.003 | |||||
| WBCs | 0.247 | 3.19 | 0.002 | |||||
| T-cell growth | 0.214 | 2.56 | 0.012 | |||||
| #3. PC_PP (in patients only) | Model | 0.171 | 5.98 | 4/58 | 0.004 | |||
| PC_RBCs | −0.358 | −2.97 | 0.004 | |||||
| WBCs | 0.254 | 2.11 | 0.039 | |||||
| #4. Pure depression | Model | 0.243 | 9.08 | 3/85 | <0.001 | |||
| WBCs | 0.324 | 3.19 | 0.002 | |||||
| PC_RBCs | −0.207 | −2.15 | 0.035 | |||||
| Th17-axis | 0.218 | 2.14 | 0.036 | |||||
| #5. Pure anxiety | Model | 0.347 | 15.08 | 3/85 | <0.001 | |||
| Th17-axis | 0.429 | 4.52 | <0.001 | |||||
| WBCs | 0.219 | 2.33 | 0.022 | |||||
| PC_RBCs | −0.189 | −2.12 | 0.037 | |||||
| #6. Pure physiosomatic | Model | 0.503 | 21.21 | 4/84 | <0.001 | |||
| Th17-axis | 0.44 | 5.29 | <0.001 | |||||
| WBCs | 0.285 | 3.4 | 0.001 | |||||
| Sex | −0.225 | −2.86 | 0.005 | |||||
| CIRS | 0.174 | 2.19 | 0.032 | |||||
| #7. Fatigue | Model | 0.417 | 20.29 | 3/85 | <0.001 | |||
| Neurotoxicity | 0.45 | 5.06 | <0.001 | |||||
| CIRS | 0.301 | 3.55 | <0.001 | |||||
| WBCs | 0.186 | 2.05 | 0.043 | |||||
| #8. Autonomic | Model | 0.339 | 14.73 | 3/86 | <0.001 | |||
| Neurotoxicity | 0.364 | 3.85 | <0.001 | |||||
| WBCs | 0.301 | 3.19 | 0.002 | |||||
| PC_RBCs | −0.189 | −2.12 | 0.037 | |||||
| #9. Sleep | Model | 0.33 | 13.97 | 3/85 | <0.001 | |||
| IRS | 0.359 | 3.83 | <0.001 | |||||
| WBCs | 0.264 | 2.85 | 0.005 | |||||
| Sex | −0.225 | −2.50 | 0.014 | |||||
| Dependent Variables | Explanatory | Coefficients of Input Variables | Corrected Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Statistics | ||||||||
| β | t | p | 95%CI | Importance | F | Df | p | ||
| #1. PC_disabilities | Model | 27.01 | 3/89 | <0.001 | |||||
| IL-17 | 0.321 | 3.59 | 0.001 | 0.143; 0.498 | 0.426 | ||||
| IFN-γ | 0.324 | 3.24 | 0.002 | 0.126; 0.522 | 0.349 | ||||
| sIL-1RA | 0.27 | 2.61 | 0.011 | 0.055; 0.405 | 0.225 | ||||
| #2. PC_PP | Model | 8.2 | 11/77 | <0.001 | |||||
| WBCs | 0.328 | 3.79 | <0.001 0.001 | 0.156; 0.501 | 0.313 | ||||
| PC_RBCs | −0.291 | −3.61 | 0.011 | −0.452; −0.131 | 0.283 | ||||
| IL-10 | 0.255 | 2.62 | 0.022 | 0.061; 0.488 | 0.149 | ||||
| IL-6 | 0.262 | 2.33 | 0.038; 0.485 | 0.118 | |||||
| #3. Pure depression | Model | 3.31 | 15/74 | <0.001 | |||||
| WBCs | 0.43 | 3.98 | <0.001 | 0.215; 0.645 | 0.322 | ||||
| PC_RBCs | −0.236 | −2.44 | 0.017 | −0.428; −0.043 | 0.121 | ||||
| IL-9 | 0.548 | 2.02 | 0.047 | 0.007; 1.089 | 0.083 | ||||
| #4. Pure anxiety | Model | 5.37 | 9/79 | <0.001 | |||||
| TNF-α | 0.503 | 2.87 | 0.005 | 0.153; 0.852 | 0.429 | ||||
| #5. Pure physiosomatic | Model | 8.84 | 9/73 | <0.001 | |||||
| WBCs | 0.114 | 4.05 | <0.001 | 0.058; 0.171 | 0.475 | ||||
| PC_RBCs | −0.219 | −2.58 | 0.012 | −0.388; −0.050 | 0.193 | ||||
| IL-13 | 0.216 | 2.01 | 0.048 | 0.002; 0.431 | 0.117 | ||||
| #6. Chronic fatigue | Model | 5.94 | 13/75 | <0.001 | |||||
| IL-4 | 0.319 | 3.37 | 0.001 | 0.130; 0.508 | 0.323 | ||||
| IFN-γ | 0.401 | 2.6 | 0.011 | 0.094–0.708 | 0.193 | ||||
| #7. Autonomic | Model | 4.49 | 12/77 | <0.001 | |||||
| MIP1A | 0.438 | 2.53 | 0.014 | 0.093; 0.783 | 0.221 | ||||
| MCP1 | 0.279 | 2.38 | 0.02 | 0.045; 0.513 | 0.196 | ||||
| #8. Sleep | Model | 16.09 | 3/85 | <0.001 | |||||
| IL-13 | 0.397 | 4.77 | 0 | 0.232; 0.563 | 0.496 | ||||
| WBCs | 0.352 | 3.95 | 0 | 0.175; 0.529 | 0.34 | ||||
| PC_RBCs | −0.244 | −2.74 | 0.007 | −0.422; −0.067 | 0.164 | ||||
| #9. PC_PP (in patients only) | Model | 3.6 | 12/48 | <0.001 | |||||
| PC_RBCs | −0.284 | −3.48 | <0.001 | −0.448; −0.120 | 0.304 | ||||
| IL-10 | 0.191 | 2.85 | 0.001 | −0.056; 0.325 | 0.204 | ||||
| IL-1Ra | −0.278 | −2.51 | 0.006 | −0.501; −0.055 | 0.158 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almulla, A.F.; Abdul Jaleel, A.-K.K.; Abo Algon, A.A.; Tunvirachaisakul, C.; Hassoun, H.K.; Al-Hakeim, H.K.; Maes, M. Mood Symptoms and Chronic Fatigue Syndrome Due to Relapsing-Remitting Multiple Sclerosis Are Associated with Immune Activation and Aberrations in the Erythron. Brain Sci. 2023, 13, 1073. https://doi.org/10.3390/brainsci13071073
Almulla AF, Abdul Jaleel A-KK, Abo Algon AA, Tunvirachaisakul C, Hassoun HK, Al-Hakeim HK, Maes M. Mood Symptoms and Chronic Fatigue Syndrome Due to Relapsing-Remitting Multiple Sclerosis Are Associated with Immune Activation and Aberrations in the Erythron. Brain Sciences. 2023; 13(7):1073. https://doi.org/10.3390/brainsci13071073
Chicago/Turabian StyleAlmulla, Abbas F., Al-Karrar Kais Abdul Jaleel, Ali Abbas Abo Algon, Chavit Tunvirachaisakul, Hayder K. Hassoun, Hussein K. Al-Hakeim, and Michael Maes. 2023. "Mood Symptoms and Chronic Fatigue Syndrome Due to Relapsing-Remitting Multiple Sclerosis Are Associated with Immune Activation and Aberrations in the Erythron" Brain Sciences 13, no. 7: 1073. https://doi.org/10.3390/brainsci13071073
APA StyleAlmulla, A. F., Abdul Jaleel, A.-K. K., Abo Algon, A. A., Tunvirachaisakul, C., Hassoun, H. K., Al-Hakeim, H. K., & Maes, M. (2023). Mood Symptoms and Chronic Fatigue Syndrome Due to Relapsing-Remitting Multiple Sclerosis Are Associated with Immune Activation and Aberrations in the Erythron. Brain Sciences, 13(7), 1073. https://doi.org/10.3390/brainsci13071073









