Reminders of Mortality: Investigating the Effects of Different Mortality Saliences on Somatosensory Neural Activity †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Preliminary Questionnaires
2.3. EEG Recording, Pre-Processing, and Analysis
2.4. Somatosensory Painful Stimulation
2.5. MindSet Manipulation
2.6. Threat Manipulation
2.7. Anxiety State and Mood Measures
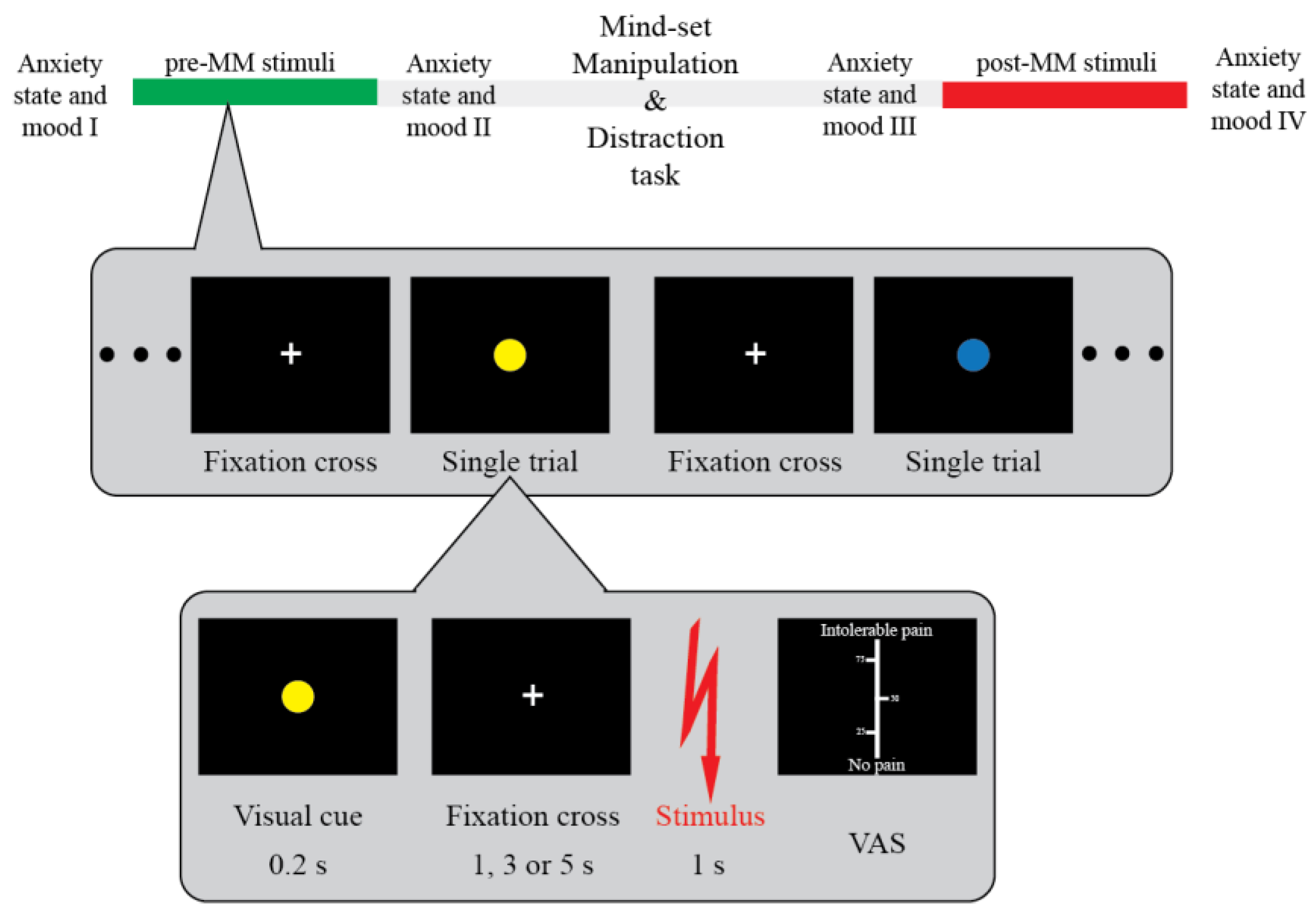
2.8. Study Design and Procedure
2.9. Data Preparation and Statistical Analysis
2.9.1. Sample Size and Statistical Power
2.9.2. Data Preparation
2.9.3. Data Analysis
3. Results
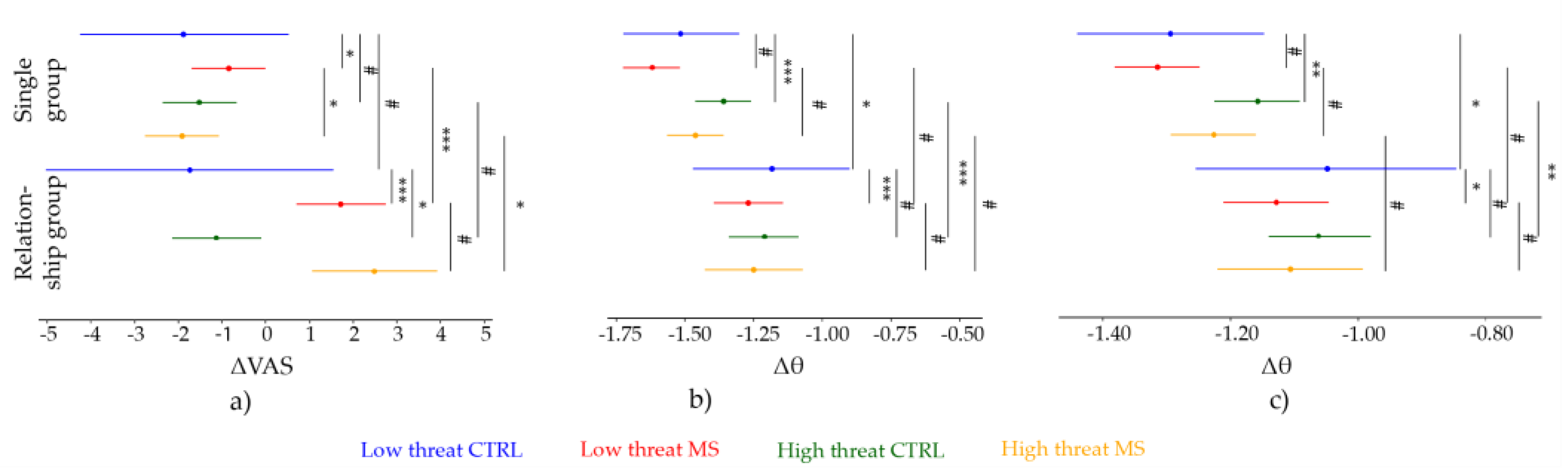
3.1. Anxiety and Mood Scores
3.2. Pain Ratings
3.2.1. Low Threat Condition
3.2.2. High Threat Condition
3.3. Brain Activtiy
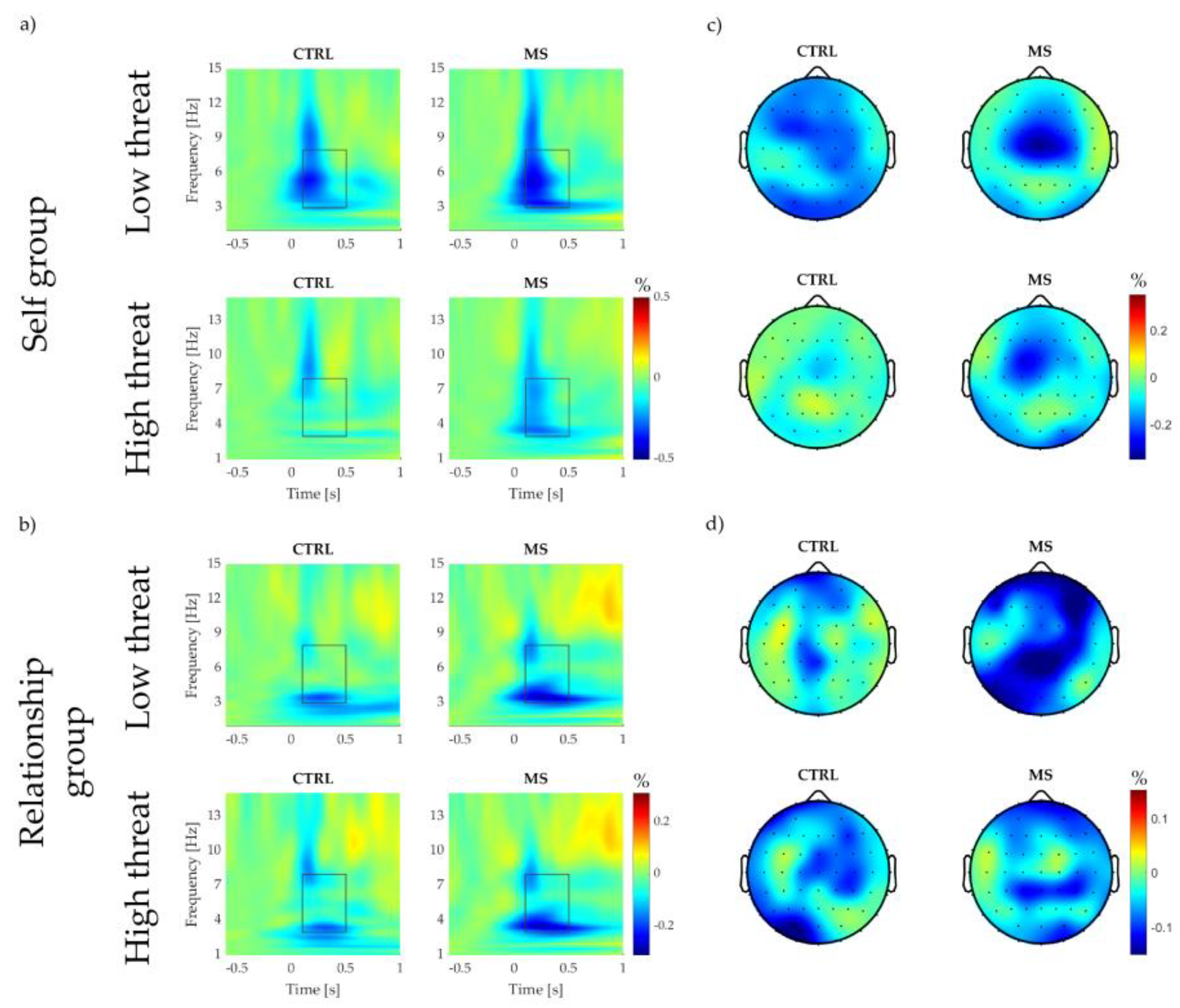
3.3.1. Event-Related Theta Power at Vertex Electrode (CZ)
Low Threat Condition
High Threat Condition
3.3.2. Event-Related Theta Power at Vertex Region (VROI)
Low Threat Condition
High Threat Condition
4. Discussion
4.1. Anxiety and Mood
4.2. Pain Ratings
4.3. Event-Related Theta Activity
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, E. Denial of Death; Simon & Schuster: New York, NY, USA, 1973; ISBN 9781416590347. [Google Scholar]
- Pyszczynski, T.; Greenberg, J.; Solomon, S.; Arndt, J.; Schimel, J. Why Do People Need Self-Esteem? A Theoretical and Empirical Review. Psychol. Bull. 2004, 130, 435–468. [Google Scholar] [CrossRef] [Green Version]
- Rosenblatt, A.; Greenberg, J.; Solomon, S.; Pyszczynski, T.; Lyon, D. Evidence for Terror Management Theory: I. The Effects of Mortality Salience on Reactions to Those Who Violate or Uphold Cultural Values. J. Pers. Soc. Psychol. 1989, 57, 681–690. [Google Scholar] [CrossRef]
- Greenberg, J.; Solomon, S.; Veeder, M.; Lyon, D.; Pyszczynski, T.; Rosenblatt, A.; Kirkland, S. Evidence for Terror Managenet Theory 2: The Effects of Mortlaity Salience on Reactions to Those Who Threaten or Bolster the Cultural Worldview. J. Pers. Soc. Psychol. 1990, 58, 308–318. [Google Scholar] [CrossRef]
- Greenberg, J.; Pyszczynski, T.; Solomon, S.; Simon, L.; Breus, M. Role of Consciousness and Accessibility of Death-Related Thoughts in Mortality Salience Effects. J. Pers. Soc. Psychol. 1994, 67, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Burke, B.L.; Martens, A.; Faucher, E.H. Two Decades of Terror Management Theory: A Meta-Analysis of Mortality Salience Research. Pers. Soc. Psychol. Rev. 2010, 14, 155–195. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Schimel, J.; Arndt, J.; Faucher, E.H. A Theoretical and Empirical Review of the Death-Thought Accessibility Concept in Terror Management Research. Psychol. Bull. 2010, 136, 699–739. [Google Scholar] [CrossRef]
- Greenberg, J.; Solomon, S.; Pyszczynski, T.; Rosenblatt, A.; Burling, J.; Lyon, D.; Simon, L.; Pinel, E. Why Do People Need Self-Esteem? Converging Evidence That Self-Esteem Serves an Anxiety-Buffering Function. J. Pers. Soc. Psychol. 1992, 63, 913–922. [Google Scholar] [CrossRef]
- Greenberg, J.; Simon, L.; Pyszczynski, T.; Solomon, S.; Chatel, D. Terror Management and Tolerance: Does Mortality Salience Always Intensify Negative Reactions to Others Who Threaten One’s Worldview? J. Pers. Soc. Psychol. 1992, 63, 212–220. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Simon, L.; Greenberg, J.; Pyszczynski, T.; Solomon, S.; McGregor, H. Terror Management Theory and Self-Esteem: Evidence That Increased Self-Esteem Reduces Mortality Salience Effects. J. Pers. Soc. Psychol. 1997, 72, 24–36. [Google Scholar] [CrossRef]
- Pyszczynski, T.; Solomon, S.; Greenberg, J. Thirty Years of Terror Management Theory: From Genesis to Revelation. Adv. Exp. Soc. Psychol. 2015, 52, 1–70. [Google Scholar] [CrossRef]
- Chatard, A.; Hirschberger, G.; Pyszczynski, T. A Word of Caution about Many Labs 4: If You Fail to Follow Your Preregistered Plan, You May Fail to Find a Real Effect. PsyArXiv, 2020; (preprint on PsyArXiv Preprints). [Google Scholar] [CrossRef] [Green Version]
- Klein, R.A.; Cook, C.L.; Ebersole, C.R.; Vitiello, C.; Nosek, B.A.; Ahn, P.; Brady, A.J.; Chartier, C.R.; Christopherson, C.D.; Clay, S.; et al. Many Labs 4: Replicating Mortality Salience with and without Original Author Involvement. Collabra Psychol. 2022, 8, 35271. [Google Scholar] [CrossRef]
- Rodríguez-Ferreiro, J.; Barberia, I.; González-Guerra, J.; Vadillo, M.A. Are We Truly Special and Unique? A Replication of Goldenberg et al. (2001). R. Soc. Open Sci. 2019, 6, 191114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Qin, J.; Ma, Y. Neurocognitive Processes of Linguistic Cues Related to Death. Neuropsychologia 2010, 48, 3436–3442. [Google Scholar] [CrossRef] [PubMed]
- Klackl, J.; Jonas, E.; Kronbichler, M. Existential Neuroscience: Neurophysiological Correlates of Proximal Defenses against Death-Related Thoughts. Soc. Cogn. Affect. Neurosci. 2013, 8, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Quirin, M.; Loktyushin, A.; Arndt, J.; Kustermann, E.; Lo, Y.-Y.; Kuhl, J.; Eggert, L. Existential Neuroscience: A Functional Magnetic Resonance Imaging Investigation of Neural Responses to Reminders of One’s Mortality. Soc. Cogn. Affect. Neurosci. 2012, 7, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Valentini, E.; Nicolardi, V.; Aglioti, S.M. Visual Reminders of Death Enhance Nociceptive-Related Cortical Responses and Event-Related Alpha Desynchronisation. Biol. Psychol. 2017, 129, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentini, E.; Gyimes, I.L. Visual Cues of Threat Elicit Greater Steady-State Electroencephalographic Responses than Visual Reminders of Death. Biol. Psychol. 2018, 139, 73–86. [Google Scholar] [CrossRef]
- Tritt, S.M.; Inzlicht, M.; Harmon-Jones, E. Toward a Biological Understanding of Mortality Salience (and Other Threat Compensation Processes). Soc. Cogn. 2012, 30, 715–733. [Google Scholar] [CrossRef] [Green Version]
- Jonas, E.; McGregor, I.; Klackl, J.; Agroskin, D.; Fritsche, I.; Holbrook, C.; Nash, K.; Proulx, T.; Quirin, M. Threat and Defense: From Anxiety to Approach. Adv. Exp. Soc. Psychol. 2014, 49, 219–286. [Google Scholar]
- Valentini, E.; Koch, K.; Nicolardi, V.; Aglioti, S.M. Mortality Salience Modulates Cortical Responses to Painful Somatosensory Stimulation: Evidence from Slow Wave and Delta Band Activity. Neuroimage 2015, 120, 12–24. [Google Scholar] [CrossRef]
- Valentini, E.; Koch, K.; Aglioti, S.M. Thoughts of Death Modulate Psychophysical and Cortical Responses to Threatening Stimuli. PLoS ONE 2014, 9, e112324. [Google Scholar] [CrossRef] [Green Version]
- Porreca, F.; Navratilova, E. Reward, Motivation, and Emotion of Pain and Its Relief. Pain 2017, 158 (Suppl. S1), S43–S49. [Google Scholar] [CrossRef] [PubMed]
- Schrooten, M.G.S.; Van Damme, S.; Crombez, G.; Peters, M.L.; Vogt, J.; Vlaeyen, J.W.S. Nonpain Goal Pursuit Inhibits Attentional Bias to Pain. Pain 2012, 153, 1180–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Damme, S.; Crombez, G.; Eccleston, C.; Goubert, L. Impaired Disengagement from Threatening Cues of Impending Pain in a Crossmodal Cueing Paradigm. Eur. J. Pain 2004, 8, 227–236. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Kashima, E.S.; Moriya, H.; Masui, K.; Furutani, K.; Nomura, M.; Yoshida, H.; Ura, M. Non-Conscious Neural Regulation against Mortality Concerns. Neurosci. Lett. 2013, 552, 35–39. [Google Scholar] [CrossRef]
- Feng, C.; Azarian, B.; Ma, Y.; Feng, X.; Wang, L.; Luo, Y.-J.; Krueger, F. Mortality Salience Reduces the Discrimination between In-Group and out-Group Interactions: A Functional MRI Investigation Using Multi-Voxel Pattern Analysis. Hum. Brain Mapp. 2017, 38, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Torta, D.M.; Legrain, V.; Mouraux, A.; Valentini, E. Attention to Pain! A Neurocognitive Perspective on Attentional Modulation of Pain in Neuroimaging Studies. Cortex 2017, 89, 120–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, J.L.; McCoy, S.K.; Pyszczynski, T.; Greenberg, J.; Solomon, S. The Body as a Source of Self-Esteem: The Effect of Mortality Salience on Identification with One’s Body, Interest in Sex, and Appearance Monitoring. J. Pers. Soc. Psychol. 2000, 79, 118–130. [Google Scholar] [CrossRef]
- McGregor, I. Offensive Defensiveness: Toward an Integrative Neuroscience of Compensatory Zeal After Mortality Salience, Personal Uncertainty, and Other Poignant Self-Threats. Psychol. Inq. 2006, 17, 299–308. [Google Scholar] [CrossRef]
- Legrain, V.; Mancini, F.; Sambo, C.F.; Torta, D.M.; Ronga, I.; Valentini, E. Cognitive Aspects of Nociception and Pain. Bridging Neurophysiology with Cognitive Psychology. Neurophysiol. Clin. Neurophysiol. 2012, 42, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Taubman-Ben-Ari, O.; Findler, L.; Mikulincer, M. The Effects of Mortality Salience on Relationship Strivings and Beliefs: The Moderating Role of Attachment Style. Br. J. Soc. Psychol. 2002, 41, 419–441. [Google Scholar] [CrossRef]
- Taubman-Ben-Ari, O.; Florian, V.; Mikulincer, M. The Impact of Mortality Salience on Reckless Driving: A Test of Terror Management Mechanisms. J. Pers. Soc. Psychol. 1999, 76, 35–45. [Google Scholar] [CrossRef]
- Buss, D.M.; Schmitt, D.P. Sexual Strategies Theory: An Evolutionary Perspective on Human Mating. Psychol. Rev. 1993, 100, 204–232. [Google Scholar] [CrossRef] [PubMed]
- Koole, S.L.; Tjew A Sin, M.; Schneider, I.K. Embodied Terror Management: Interpersonal Touch Alleviates Existential Concerns among Individuals with Low Self-Esteem. Psychol. Sci. 2014, 25, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulincer, M.; Florian, V. Exploring Individual Differences in Reactions to Mortality Salience: Does Attachment Style Regulate Terror Management Mechanisms? J. Pers. Soc. Psychol. 2000, 79, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Lin, C.S.; Brodersen, K.H.; Bingel, U.; Ploner, M.; Tracey, I. Anterior Insula Integrates Information about Salience into Perceptual Decisions about Pain. J. Neurosci. 2010, 30, 16324–16331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, K.; McNair, N.A.; Harris, J.A.; Sharpe, L.; Colagiuri, B. In Anticipation of Pain: Expectancy Modulates Corticospinal Excitability, Autonomic Response, and Pain Perception. Pain 2021, 162, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Lagos, J.P. Time-Frequency Analysis for Responses Evoked by Nociceptive and Non-Nociceptive Stimuli Based on EEG Signals. Master’s Thesis, Faculty of Engineer LTH Department of Biomedical Engineering, Lund University, Lund, Sweden; Lund University Publications—Student Papers. 23p. Available online: https://core.ac.uk/reader/289944750.
- Pereira, S.; Reay, K.; Bottell, J.; Walker, L.; Dzikiti, C. University Student Mental Health Survey 2018. Insight Netw. Dig. 2019, 35. [Google Scholar]
- Greenberg, J.; Pyszczynski, T.; Solomon, S. The Causes and Consequences of a Need for Self-Esteem: A Terror Management Theory. In Public Self and Private Self; Baumeister, R.F., Ed.; Springer: New York, NY, USA, 1986; pp. 189–212. ISBN 978-1-4613-9564-5. [Google Scholar]
- Schultz, D.M.; Arnau, R.C. Effects of a Brief Mindfulness Induction on Death-Related Anxiety. OMEGA J. Death Dying 2017, 79, 313–335. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.C.; Pyszczynski, T. Reducing Defensive Responses to Thoughts of Death: Meditation, Mindfulness, and Buddhism. J. Pers. Soc. Psychol. 2019, 116, 101–118. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Trait Anxiety Inventory. Anxiety 1987, 19, 2009. [Google Scholar]
- Crawford, J.R.; Henry, J.D. The Positive and Negative Affect Schedule (PANAS): Construct Validity, Measurement Properties and Normative Data in a Large Non-Clinical Sample. Br. J. Clin. Psychol. 2004, 43, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.; Allen, J.J.B.; Greenberg, J. Traces of Terror: Subliminal Death Primes and Facial Electromyographic Indices of Affect. Motiv. Emot. 2001, 25, 253–277. [Google Scholar] [CrossRef]
- Greenberg, J.; Martens, A.; Jonas, E.; Eisenstadt, D.; Pyszczynski, T.; Solomon, S. Psychological Defense in Anticipation of Anxiety: Eliminating the Potential for Anxiety Eliminates the Effect of Mortality Salience on Worldview Defense. Psychol. Sci. 2003, 14, 516–519. [Google Scholar] [CrossRef]
- Klackl, J.; Jonas, E. Effects of Mortality Salience on Physiological Arousal. Front. Psychol. 2019, 10, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, Y.; Luo, S.; Wu, B.; Wu, X.; Han, S. Mortality Salience Enhances Racial In-Group Bias in Empathic Neural Responses to Others’ Suffering. Neuroimage 2015, 118, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tian, J. Reminders of Mortality Alter Pain-Evoked Potentials in a Chinese Sample. Front. Psychol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dor-Ziderman, Y.; Lutz, A.; Goldstein, A. Prediction-Based Neural Mechanisms for Shielding the Self from Existential Threat. Neuroimage 2019, 202, 116080. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, Y.; Li, W.; Pfabigan, D.M.; Han, S. Neural Mechanisms of Reinforcement Learning under Mortality Threat. Soc. Neurosci. 2020, 15, 170–185. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.; Shi, Z.; Zhang, X.; Li, H.; Xu, X.; Guan, L.; Han, S.; Yang, J. Mortality Salience Impairs Self-Referential Processing: Neurophysiological and Behavioral Evidence. Curr. Psychol. 2020, 39, 782–792. [Google Scholar] [CrossRef]
- Charness, G.; Gneezy, U.; Kuhn, M.A. Experimental Methods: Between-Subject and within-Subject Design. J. Econ. Behav. Organ. 2012, 81, 1–8. [Google Scholar] [CrossRef]
- Melnik, A.; Legkov, P.; Izdebski, K.; Kärcher, S.M.; Hairston, W.D.; Ferris, D.P.; König, P. Systems, Subjects, Sessions: To What Extent Do These Factors Influence EEG Data? Front. Hum. Neurosci. 2017, 11, 150. [Google Scholar] [CrossRef] [Green Version]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; The MIT Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Mcgregor, I.; Nash, K.; Mann, N.; Phills, C.E. Anxious Uncertainty and Reactive Approach Motivation (RAM). J. Personal. Soc. Psychol. 2010, 99, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.J.; Eadeh, F.R.; Peak, S.A.; Scherer, L.D.; Schott, J.P.; Slochower, J.M. Toward a Greater Understanding of the Emotional Dynamics of the Mortality Salience Manipulation: Revisiting the “Affect-Free” Claim of Terror Management Research. J. Pers. Soc. Psychol. 2014, 106, 655–678. [Google Scholar] [CrossRef]
- Boelen, P.A.; Lenferink, L.I.M. Associations of Depressive Rumination and Positive Affect Regulation with Emotional Distress after the Death of a Loved One. Clin. Psychol. Psychother. 2020, 27, 955–964. [Google Scholar] [CrossRef]
- Hollins, M.; Harper, D.; Maixner, W. Changes in Pain from a Repetitive Thermal Stimulus: The Roles of Adaptation and Sensitization. Pain 2011, 152, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Loeser, J.D.; Melzack, R. Pain: An Overview. Lancet 1999, 353, 1607–1609. [Google Scholar] [CrossRef]
- Tracey, I. Getting the Pain You Expect: Mechanisms of Placebo, Nocebo and Reappraisal Effects in Humans. Nat. Med. 2010, 16, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Kokonyei, G.; Galambos, A.; Edes, A.E.; Kocsel, N.; Szabo, E.; Pap, D.; Kozak, L.R.; Bagdy, G.; Juhasz, G. Anticipation and Violated Expectation of Pain Are Influenced by Trait Rumination: An FMRI Study. Cogn. Affect. Behav. Neurosci. 2019, 19, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Menzies, R.E.; Sharpe, L.; Dar-Nimrod, I. The Effect of Mortality Salience on Bodily Scanning Behaviors in Anxiety-Related Disorders. J. Abnorm. Psychol. 2021, 130, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mouraux, A.; Iannetti, G.D. The Search for Pain Biomarkers in the Human Brain. Brain 2018, 141, 3290–3307. [Google Scholar] [CrossRef] [Green Version]
- Başar-Eroglu, C.; Başar, E.; Demiralp, T.; Schürmann, M. P300-Response: Possible Psychophysiological Correlates in Delta and Theta Frequency Channels. A Review. Int. J. Psychophysiol. 1992, 13, 161–179. [Google Scholar] [CrossRef]
- Huang, S.; Du, H.; Qu, C. Emotional Responses to Mortality Salience: Behavioral and ERPs Evidence. PLoS ONE 2021, 16, e0248699. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wu, B.; Fan, X.; Zhu, Y.; Wu, X.; Han, S. Thoughts of Death Affect Reward Learning by Modulating Salience Network Activity. Neuroimage 2019, 202, 116068. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.R.; Arndt, J. How Sweet It Is to Be Loved by You: The Role of Perceived Regard in the Terror Management of Close Relationships. J. Pers. Soc. Psychol. 2012, 102, 616–632. [Google Scholar] [CrossRef] [Green Version]
- Henry, E.A.; Bartholow, B.D.; Arndt, J. Death on the Brain: Effects of Mortality Salience on the Neural Correlates of Ingroup and Outgroup Categorization. Soc. Cogn. Affect. Neurosci. 2010, 5, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Han, S. Neural Responses to One’s Own Name under Mortality Threat. Neuropsychologia 2018, 108, 32–41. [Google Scholar] [CrossRef]
- McGregor, I.; Zanna, M.P.; Holmes, J.G.; Spencer, S.J. Compensatory Conviction in the Face of Personal Uncertainty: Going to Extremes and Being Oneself. J. Pers. Soc. Psychol. 2001, 80, 472–488. [Google Scholar] [CrossRef] [Green Version]
- Heine, S.J.; Proulx, T.; Vohs, K.D. The Meaning Maintenance Model: On the Coherence of Social Motivations. Personal. Soc. Psychol. Rev. 2006, 10, 88–110. [Google Scholar] [CrossRef]
- Carleton, R.N. Into the Unknown: A Review and Synthesis of Contemporary Models Involving Uncertainty. J. Anxiety Disord. 2016, 39, 30–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyimes, I.L.; Valentini, E. Reminders of Mortality: Investigating the Effects of Different Mortality Saliences on Somatosensory Neural Activity. Brain Sci. 2023, 13, 1077. https://doi.org/10.3390/brainsci13071077
Gyimes IL, Valentini E. Reminders of Mortality: Investigating the Effects of Different Mortality Saliences on Somatosensory Neural Activity. Brain Sciences. 2023; 13(7):1077. https://doi.org/10.3390/brainsci13071077
Chicago/Turabian StyleGyimes, Istvan Laszlo, and Elia Valentini. 2023. "Reminders of Mortality: Investigating the Effects of Different Mortality Saliences on Somatosensory Neural Activity" Brain Sciences 13, no. 7: 1077. https://doi.org/10.3390/brainsci13071077
APA StyleGyimes, I. L., & Valentini, E. (2023). Reminders of Mortality: Investigating the Effects of Different Mortality Saliences on Somatosensory Neural Activity. Brain Sciences, 13(7), 1077. https://doi.org/10.3390/brainsci13071077





