Refractory Hypotension in a Late-Onset Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS) Male with m.3243 A>G Mutation: A Case Report
Abstract
:1. Introduction
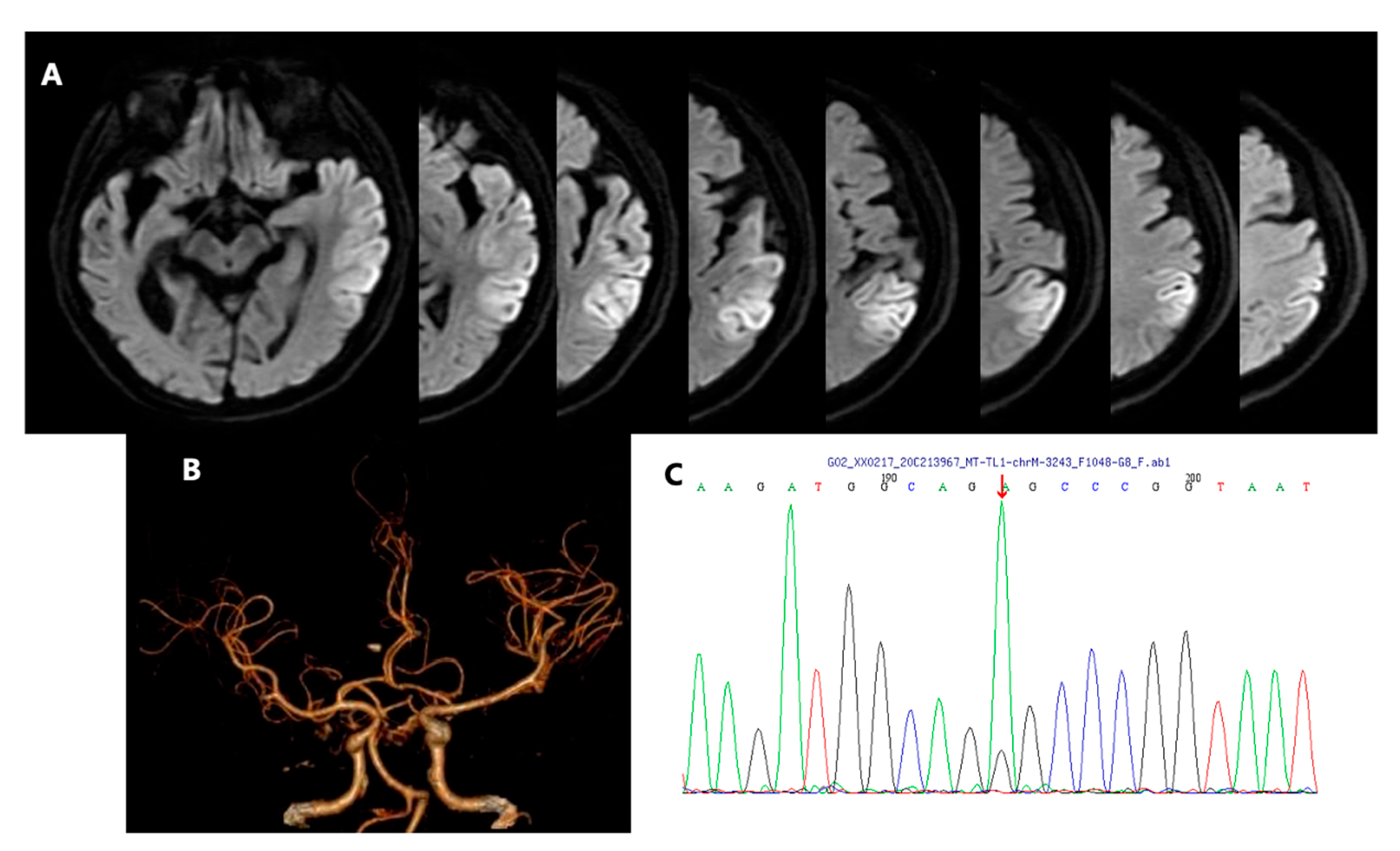
2. Case Presentation
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fan, H.C.; Lee, H.F.; Yue, C.T.; Chi, C.S. Clinical Characteristics of Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes. Life 2021, 11, 1111. [Google Scholar] [CrossRef]
- Goto, Y.; Horai, S.; Matsuoka, T.; Koga, Y.; Nihei, K.; Kobayashi, M.; Nonaka, I. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): A correlative study of the clinical features and mitochondrial DNA mutation. Neurology 1992, 42, 545–550. [Google Scholar] [CrossRef]
- Oyama, M.; Iizuka, T.; Nakahara, J.; Izawa, Y. Neuroimaging pattern and pathophysiology of cerebellar stroke-like lesions in MELAS with m.3243A>G mutation: A case report. BMC Neurol. 2020, 20, 167. [Google Scholar] [CrossRef]
- Grady, J.P.; Pickett, S.J.; Ng, Y.S.; Alston, C.L.; Blakely, E.L.; Hardy, S.A.; Feeney, C.L.; Bright, A.A.; Schaefer, A.M.; Gorman, G.S.; et al. mtDNA heteroplasmy level and copy number indicate disease burden in m.3243A>G mitochondrial disease. EMBO Mol. Med. 2018, 10, e8262. [Google Scholar] [CrossRef]
- Lax, N.Z.; Gorman, G.S.; Turnbull, D.M. Review: Central nervous system involvement in mitochondrial disease. Neuropathol. Appl. Neurobiol. 2017, 43, 102–118. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, N.; Wilson, L.; Mann, S.; Sirrs, S.; Benavente, O. Clinical Reasoning: A complicated case of MELAS. Neurology 2016, 87, e189–e195. [Google Scholar] [CrossRef] [Green Version]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, M.; Orsucci, D.; Angelini, C.; Bertini, E.; Carelli, V.; Comi, G.P.; Donati, A.; Minetti, C.; Moggio, M.; Mongini, T.; et al. The m.3243A>G mitochondrial DNA mutation and related phenotypes. A matter of gender? J. Neurol. 2014, 261, 504–510. [Google Scholar] [CrossRef]
- Kärppä, M.; Syrjälä, P.; Tolonen, U.; Majamaa, K. Peripheral neuropathy in patients with the 3243A>G mutation in mitochondrial DNA. J. Neurol. 2003, 250, 216–221. [Google Scholar] [CrossRef]
- Finsterer, J.; Zarrouk-Mahjoub, S. Mitochondrial vasculopathy. World J. Cardiol. 2016, 8, 333–339. [Google Scholar] [CrossRef]
- Iizuka, T.; Sakai, F.; Ide, T.; Miyakawa, S.; Sato, M.; Yoshii, S. Regional cerebral blood flow and cerebrovascular reactivity during chronic stage of stroke-like episodes in MELAS—Implication of neurovascular cellular mechanism. J. Neurol. Sci. 2007, 257, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Pascual, J.M.; Anziska, Y.; Gooch, C.L.; Engelstad, K.; Jhung, S.; DiMauro, S.; De Vivo, D.C. Nerve conduction abnormalities in patients with MELAS and the A3243G mutation. Arch. Neurol. 2006, 63, 746–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, G.; Opfer-Gehrking, T.; Offord, K.; Atkinson, E.; O’Brien, P.; Low, P. The Autonomic Symptom Profile: A new instrument to assess autonomic symptoms. Neurology 1999, 52, 523–528. [Google Scholar] [CrossRef]
- Parsons, T.; Weimer, L.; Engelstad, K.; Linker, A.; Battista, V.; Wei, Y.; Hirano, M.; Dimauro, S.; De Vivo, D.C.; Kaufmann, P. Autonomic symptoms in carriers of the m.3243A>G mitochondrial DNA mutation. Arch. Neurol. 2010, 67, 976–979. [Google Scholar] [CrossRef] [Green Version]
- Zelnik, N.; Axelrod, F.B.; Leshinsky, E.; Griebel, M.L.; Kolodny, E.H. Mitochondrial Encephalomyopathies Presenting with Features of Autonomic and Visceral Dysfunction. Pediatr. Neurol. 1996, 14, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A. Fluid Resuscitation for Refractory Hypotension. Front. Vet. Sci. 2021, 8, 621696. [Google Scholar] [CrossRef]
- Tetsuka, S.; Ogawa, T.; Hashimoto, R.; Kato, H. Clinical features, pathogenesis, and management of stroke-like episodes due to MELAS. Metab. Brain. Dis. 2021, 36, 2181–2193. [Google Scholar] [CrossRef]
- DiMauro, S.; Hirano, M. Mitochondrial encephalomyopathies: An update. Neuromuscul. Disord. 2005, 15, 276–286. [Google Scholar] [CrossRef]
- Koga, Y.; Povalko, N.; Nishioka, J.; Katayama, K.; Kakimoto, N.; Matsuishi, T. MELAS and L-arginine therapy: Pathophysiology of stroke-like episodes. Ann. N. Y. Acad. Sci. 2010, 1201, 104–110. [Google Scholar] [CrossRef]
- Pavlakis, S.G.; Phillips, P.C.; DiMauro, S.; De Vivo, D.C.; Rowland, L.P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: A distinctive clinical syndrome. Ann. Neurol. 1984, 16, 481–488. [Google Scholar] [CrossRef]
- Koenig, M.K.; Emrick, L.; Karaa, A.; Korson, M.; Scaglia, F.; Parikh, S.; Goldstein, A. Recommendations for the Management of Strokelike Episodes in Patients with Mitochondrial Encephalomyopathy, Lactic Acidosis, and Strokelike Episodes. JAMA Neurol. 2016, 73, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Pavlakis, S.G. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS): Current concepts. J. Child Neurol. 1994, 9, 4–13. [Google Scholar] [CrossRef]
- Stoquart-Elsankari, S.; Lehmann, P.; Périn, B.; Gondry-Jouet, C.; Godefroy, O. MRI and diffusion-weighted imaging followup of a stroke-like event in a patient with MELAS. J. Neurol. 2008, 255, 1593–1595. [Google Scholar] [CrossRef]
- Ohama, E.; Ohara, S.; Ikuta, F.; Tanaka, K.; Nishizawa, M.; Miyatake, T. Mitochondrial angiopathy in cerebral blood vessels of mitochondrial encephalomyopathy. Acta. Neuropathol. 1987, 74, 226–233. [Google Scholar] [CrossRef]
- Kishi, M.; Yamamura, Y.; Kurihara, T.; Fukuhara, N.; Tsuruta, K.; Matsukura, S.; Hayashi, T.; Nakagawa, M.; Kuriyama, M. An autopsy case of mitochondrial encephalomyopathy: Biochemical and electron microscopic studies of the brain. J. Neurol. Sci. 1988, 86, 31–40. [Google Scholar] [CrossRef]
- Iizuka, T.; Sakai, F.; Suzuki, N.; Hata, T.; Tsukahara, S.; Fukuda, M.; Takiyama, Y. Neuronal hyperexcitability in stroke-like episodes of MELAS syndrome. Neurology 2002, 59, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Lax, N.Z.; Pienaar, I.S.; Reeve, A.K.; Hepplewhite, P.D.; Jaros, E.; Taylor, R.W.; Kalaria, R.N.; Turnbull, D.M. Microangiopathy in the cerebellum of patients with mitochondrial DNA disease. Brain 2012, 135, 1736–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramegna, L.L.; Cortesi, I.; Mitolo, M.; Evangelisti, S.; Talozzi, L.; Cirillo, L.; Tonon, C.; Lodi, R. Major cerebral vessels involvement in patients with MELAS syndrome: Worth a scan? A systematic review. J. Neuroradiol. 2021, 48, 359–366. [Google Scholar] [CrossRef]
- Ng, Y.S.; Lax, N.Z.; Blain, A.P.; Erskine, D.; Baker, M.R.; Polvikoski, T.; Thomas, R.H.; Morris, C.M.; Lai, M.; Whittaker, R.G.; et al. Forecasting stroke-like episodes and outcomes in mitochondrial disease. Brain 2022, 145, 542–554. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Adesina, A.M.; Jones, J.; Scaglia, F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015, 116, 4–12. [Google Scholar] [CrossRef]
- Kaufmann, P.; Engelstad, K.; Wei, Y.; Kulikova, R.; Oskoui, M.; Battista, V.; Koenigsberger, D.Y.; Pascual, J.M.; Sano, M.; Hirano, M.; et al. Protean phenotypic features of the A3243G mitochondrial DNA mutation. Arch. Neurol. 2009, 66, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-X.; Shang, B.; Chen, W.-Z.; Lu, Y.; Wang, J. Adult-onset of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome with hypothyroidism and psychiatric disorders. eNeurologicalSci 2017, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Szczepanczyk, K.; Mirza, F.S. Late-Onset Melas with Midd: An Uncommon Age of Presentation. AACE Clin. Case Rep. 2018, 4, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Pauls, A.D.; Sandhu, V.; Young, D.; Nevay, D.L.; Yeung, D.F.; Sirrs, S.; Tsang, M.Y.; Tsang, T.S.M.; Lehman, A.; Mezei, M.M.; et al. High rate of hypertension in patients with m.3243A>G MELAS mutations and POLG variants. Mitochondrion 2020, 53, 194–202. [Google Scholar] [CrossRef]
- Chong-Nguyen, C.; Stalens, C.; Goursot, Y.; Bougouin, W.; Stojkovic, T.; Behin, A.; Mochel, F.; Berber, N.; Eymard, B.; Duboc, D.; et al. A high prevalence of arterial hypertension in patients with mitochondrial diseases. J. Inherit. Metab. Dis. 2020, 43, 478–485. [Google Scholar] [CrossRef]
- Hannah-Shmouni, F.; Sirrs, S.; Mezei, M.M.; Waters, P.J.; Mattman, A. Increased Prevalence of Hypertension in Young Adults with High Heteroplasmy Levels of the MELAS m.3243A>G Mutation. JIMD Rep. 2014, 12, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Naini, A.; Kaufmann, P.; Shanske, S.; Engelstad, K.; De Vivo, D.C.; Schon, E.A. Hypocitrullinemia in patients with MELAS: An insight into the “MELAS paradox”. J. Neurol. Sci. 2005, 229–230, 187–193. [Google Scholar] [CrossRef]
- Majamaa-Voltti, K.; Majamaa, K.; Peuhkurinen, K.; Mäkikallio, T.H.; Huikuri, H.V. Cardiovascular autonomic regulation in patients with 3243A > G mitochondrial DNA mutation. Ann. Med. 2004, 36, 225–231. [Google Scholar] [CrossRef]
- Metzler, M.; Duerr, S.; Granata, R.; Krismer, F.; Robertson, D.; Wenning, G.K. Neurogenic orthostatic hypotension: Pathophysiology, evaluation, and management. J. Neurol. 2013, 260, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Ng, Y.S.; Feeney, C.; Schaefer, A.M.; Holmes, C.E.; Hynd, P.; Alston, C.L.; Grady, J.P.; Roberts, M.; Maguire, M.; Bright, A.; et al. Pseudo-obstruction, stroke, and mitochondrial dysfunction: A lethal combination. Ann. Neurol. 2016, 80, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y.; Brentzel, S.; Eisenhofer, G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology 2003, 60, 1327–1332. [Google Scholar] [CrossRef]
- Ikawa, M.; Povalko, N.; Koga, Y. Arginine therapy in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 17–22. [Google Scholar] [CrossRef]
- Khalaf, D.; Kruger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, Y.; Akita, Y.; Nishioka, J.; Yatsuga, S.; Povalko, N.; Katayama, K.; Matsuishi, T. MELAS and L-arginine therapy. Mitochondrion 2007, 7, 133–139. [Google Scholar] [CrossRef]
- Koga, Y.; Akita, Y.; Nishioka, J.; Yatsuga, S.; Povalko, N.; Tanabe, Y.; Fujimoto, S.; Matsuishi, T. L-arginine improves the symptoms of strokelike episodes in MELAS. Neurology 2005, 64, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, R.J.; Ng, Y.S.; Errington, L.; Blain, A.P.; McFarland, R.; Gorman, G.S. l-Arginine in Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like Episodes: A Systematic Review. Neurology 2022, 98, e2318–e2328. [Google Scholar] [CrossRef]
- Duschek, S.; Hoffmann, A.; Reyes Del Paso, G.A.; Ettinger, U. Autonomic Cardiovascular Control and Executive Function in Chronic Hypotension. Ann. Behav. Med. 2017, 51, 442–453. [Google Scholar] [CrossRef]
- La Via, L.; Sanfilippo, F.; Continella, C.; Triolo, T.; Messina, A.; Robba, C.; Astuto, M.; Hernandez, G.; Noto, A. Agreement between Capillary Refill Time measured at Finger and Earlobe sites in different positions: A pilot prospective study on healthy volunteers. BMC Anesth. 2023, 23, 30. [Google Scholar] [CrossRef]
- Lewin, J.; Maconochie, I. Capillary refill time in adults. Emerg. Med. J. 2008, 25, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Kelly, A.M.; Kerr, D.; Clooney, M.; Jolley, D. Impact of patient and environmental factors on capillary refill time in adults. Am. J. Emerg. Med. 2008, 26, 62–65. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, E.; Ye, C.; Wu, B. Refractory Hypotension in a Late-Onset Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS) Male with m.3243 A>G Mutation: A Case Report. Brain Sci. 2023, 13, 1080. https://doi.org/10.3390/brainsci13071080
Wang Y, Zhang E, Ye C, Wu B. Refractory Hypotension in a Late-Onset Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS) Male with m.3243 A>G Mutation: A Case Report. Brain Sciences. 2023; 13(7):1080. https://doi.org/10.3390/brainsci13071080
Chicago/Turabian StyleWang, Youjie, Enhui Zhang, Chen Ye, and Bo Wu. 2023. "Refractory Hypotension in a Late-Onset Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS) Male with m.3243 A>G Mutation: A Case Report" Brain Sciences 13, no. 7: 1080. https://doi.org/10.3390/brainsci13071080
APA StyleWang, Y., Zhang, E., Ye, C., & Wu, B. (2023). Refractory Hypotension in a Late-Onset Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS) Male with m.3243 A>G Mutation: A Case Report. Brain Sciences, 13(7), 1080. https://doi.org/10.3390/brainsci13071080







