Biological Risk Factors Influencing Vascular Cognitive Impairments: A Review of the Evidence
Abstract
:1. Introduction
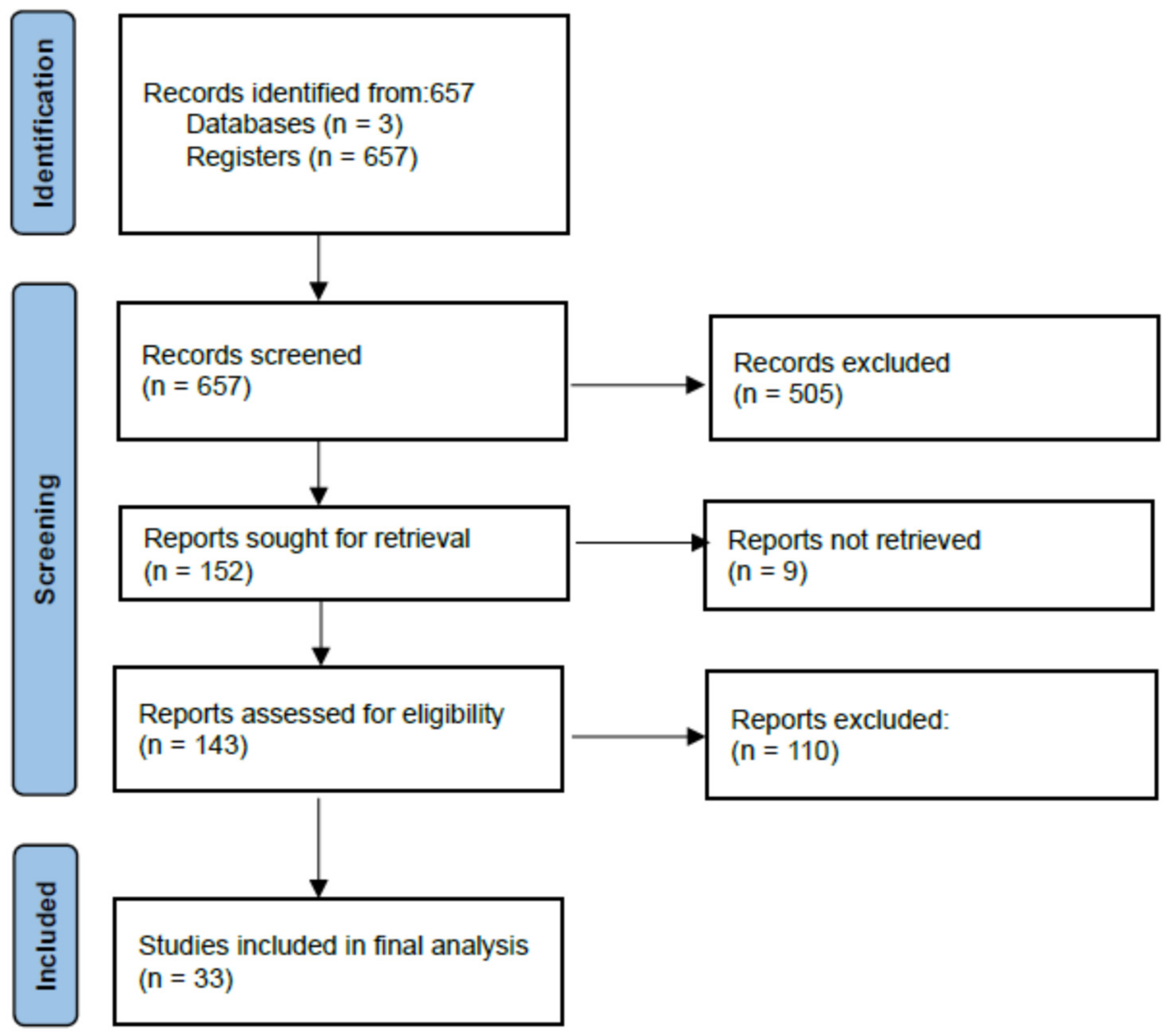
2. Materials and Methods
2.1. Search Strategies and Article Eligility
2.2. Quality Analysis and Data Extraction
3. Results
3.1. What Is the Relationship between Ageing Caused by Vascular Disease and Cognitive Reserve?
3.2. Is There an Underlying Pathophysiological Link between Blood Pressure Variability, Stroke, Diabetes, and Cognitive Impairment?
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dichgans, M.; Leys, D. Vascular Cognitive Impairment. Circ. Res. 2017, 120, 573–591. [Google Scholar] [CrossRef]
- Lilly, L.S.; Harvard Medical School (Eds.) Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016; 467p. [Google Scholar]
- Nagai, M.; Hoshide, S.; Kario, K. Role of 24-hour blood pressure management in preventing kidney disease and stroke. Contrib. Nephrol. 2013, 179, 67–80. [Google Scholar] [PubMed]
- Ropper, A.H.; Samuels, M.A.; Klein, J. Adams and Victor’s Principles of Neurology, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2019; 1653p. [Google Scholar]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F.; PRISMA-IPD Development Group. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: The PRISMA-IPD Statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Barnes, C.A.; Grady, C.; Jones, R.N.; Raz, N. Brain reserve, cognitive reserve, compensation, and maintenance: Operationalization, validity, and mechanisms of cognitive resilience. Neurobiol. Aging 2019, 83, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. J. Alzheimers Assoc. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Pinter, D.; Enzinger, C.; Fazekas, F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J. Neurol. 2015, 262, 2411–2419. [Google Scholar] [CrossRef]
- Stern, Y. How Can Cognitive Reserve Promote Cognitive and Neurobehavioral Health? Arch. Clin. Neuropsychol. 2021, 36, 1291–1295. [Google Scholar] [CrossRef]
- Colangeli, S.; Boccia, M.; Verde, P.; Guariglia, P.; Bianchini, F.; Piccardi, L. Cognitive Reserve in Healthy Aging and Alzheimer’s Disease: A Meta-Analysis of fMRI Studies. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 443–449. [Google Scholar] [CrossRef]
- Han, S.; Lee, J.Y.; Cho, S.I.; Oh, D.J.; Yoon, D.H. Risk Factors for Various Cognitive Function Decline Trajectories in Adults over 40 Years of Age: A Retrospective Cohort Study. Psychiatry Investig. 2023, 20, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Martorana, A.; Assogna, M.; DE Lucia, V.; Motta, C.; Bonomi, C.G.; Bernocchi, F.; DI Donna, M.G.; Koch, G. Cognitive reserve and Alzheimer’s biological continuum: Clues for prediction and prevention of dementia. Minerva Med. 2021, 112, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Pietzuch, M.; King, A.E.; Ward, D.D.; Vickers, J.C. The Influence of Genetic Factors and Cognitive Reserve on Structural and Functional Resting-State Brain Networks in Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.T. Cognitive Reserve and the Prevention of Dementia: The Role of Physical and Cognitive Activities. Curr. Psychiatry Rep. 2016, 18, 85. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Pettigrew, C.; Soldan, A. Defining Cognitive Reserve and Implications for Cognitive Aging. Curr. Neurol. Neurosci. Rep. 2019, 19, 1. [Google Scholar] [CrossRef]
- Ganguli, M.; Fu, B.; Snitz, B.E.; Hughes, T.F.; Chang, C.C.H. Mild cognitive impairment: Incidence and vascular risk factors in a population-based cohort. Neurology 2013, 80, 2112–2120. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, M.; Yu, L.; Wang, L.; An, C.; Xun, S.; Zhao, X.; Gao, Y.; Wang, X. Mild Cognitive Impairment: Vascular Risk Factors in Community Elderly in Four Cities of Hebei Province, China. PLoS ONE 2015, 10, e0124566. [Google Scholar] [CrossRef] [Green Version]
- Vazirinejad, R.; Mirmotalebi, M.; Bageri, M.; Kounis, N.G.; Koniari, I.; Lilley, J.M.; Gommnami, N. Age-Related Effect of Antihypertensive Treatment on Cognitive Performance: Is it Better Preventing Dementia in Older Age? Am. J. Alzheimers Dis. Other Dement. 2019, 34, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, A.M.; Ryan, C.M.; Braffett, B.H.; Gubitosi-Klug, R.A.; Lorenzi, G.M.; Luchsinger, J.A.; Trapani, V.R.; Bebu, I.; Chaytor, N.; Hitt, S.M.; et al. Cognitive performance declines in older adults with type 1 diabetes: Results from 32 years of follow-up in the DCCT and EDIC Study. Lancet Diabetes Endocrinol. 2021, 9, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Diaz, K.M.; Veerabhadrappa, P.; Kashem, M.A.; Feairheller, D.L.; Sturgeon, K.M.; Williamson, S.T.; Crabbe, D.L.; Brown, M.D. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens. Res. 2012, 35, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, I.; Hart, M.; Chen, Y.L.; Mack, W.; Novak, V.; Chui, H.; Lipsitz, L. Antihypertensive Therapy and Cerebral Hemodynamics in Executive Mild Cognitive Impairment: Results of a Pilot Randomized Clinical Trial. J. Am. Geriatr. Soc. 2013, 61, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Foster-Dingley, J.C.; van der Grond, J.; Moonen, J.E.F.; van den Berg-Huijsmans, A.A.; de Ruijter, W.; van Buchem, M.A.; de Craen, A.J.; van der Mast, R.C. Lower Blood Pressure Is Associated With Smaller Subcortical Brain Volumes in Older Persons. Am. J. Hypertens. 2015, 28, 1127–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lim, J.S.; Oh, M.S.; Yu, K.H.; Lee, J.S.; Park, J.H.; Kim, Y.J.; Rha, J.H.; Hwang, Y.H.; Heo, S.H.; et al. Blood pressure variability is related to faster cognitive decline in ischemic stroke patients: PICASSO subanalysis. Sci. Rep. 2021, 11, 5049. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; Wijsman, L.W.; Foster-Dingley, J.C.; Stott, D.J.; Ford, I.; Buckley, B.M.; Sattar, N.; Jukema, J.W.; van Osch, M.J.; van der Grond, J.; et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ 2013, 347, f4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, M.; Dote, K.; Kato, M.; Sasaki, S.; Oda, N.; Kagawa, E.; Nakano, Y.; Yamane, A.; Higashihara, T.; Miyauchi, S.; et al. Visit-to-Visit Blood Pressure Variability and Alzheimer’s Disease: Links and Risks. J. Alzheimer’s Dis. 2017, 59, 515–526. [Google Scholar] [CrossRef]
- van Middelaar, T.; Richard, E.; Moll van Charante, E.P.; van Gool, W.A.; van Dalen, J.W. Visit-to-Visit Blood Pressure Variability and Progression of White Matter Hyperintensities Among Older People With Hypertension. J. Am. Med. Dir. Assoc. 2019, 20, 1175–1177.e1. [Google Scholar] [CrossRef]
- Tully, P.J.; Debette, S.; Tzourio, C. The association between systolic blood pressure variability with depression, cognitive decline and white matter hyperintensities: The 3C Dijon MRI study. Psychol. Med. 2018, 48, 1444–1453. [Google Scholar] [CrossRef]
- Haratz, S.; Weinstein, G.; Molshazki, N.; Beeri, M.S.; Ravona-Springer, R.; Marzeliak, O.; Goldbourt, U.; Tanne, D. Impaired Cerebral Hemodynamics and Cognitive Performance in Patients with Atherothrombotic Disease. J. Alzheimer’s Dis. 2015, 46, 137–144. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yamamoto, T.; Kalaria, R.N.; Senzaki, H.; Maki, T.; Hase, Y.; Kitamura, A.; Washida, K.; Yamada, M.; Ito, H.; et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012, 123, 381–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawlor, B.; Segurado, R.; Kennelly, S.; Olde Rikkert, M.G.M.; Howard, R.; Pasquier, F.; Börjesson-Hanson, A.; Tsolaki, M.; Lucca, U.; Molloy, D.W.; et al. Nilvadipine in mild to moderate Alzheimer disease: A randomised controlled trial. PLoS Med. 2018, 15, e1002660. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, H.; Melkas, S.; Madureira, S.; Verdelho, A.; Ferro, J.M.; Fazekas, F.; Schmidt, R.; Scheltens, P.; Barkhof, F.; Wardlaw, J.M.; et al. Cognitive reserve moderates long-term cognitive and functional outcome in cerebral small vessel disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1296–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gąsecki, D.; Kwarciany, M.; Nyka, W.; Narkiewicz, K. Hypertension, Brain Damage and Cognitive Decline. Curr. Hypertens Rep. 2013, 15, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Baltes, P.B. The aging mind: Potential and limits. Gerontologist 1993, 33, 580–594. [Google Scholar] [CrossRef]
- Raz, N.; Lindenberger, U.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Williamson, A.; Dahle, C.; Gerstorf, D.; Acker, J.D. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 2005, 15, 1676–1689. [Google Scholar] [CrossRef] [Green Version]
- Tamnes, C.K.; Walhovd, K.B.; Dale, A.M.; Østby, Y.; Grydeland, H.; Richardson, G.; Westlye, L.T.; Roddey, J.C.; Hagler, D.J., Jr.; Due-Tønnessen, P.; et al. Brain development and aging: Overlapping and unique patterns of change. NeuroImage 2013, 68, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Gunning-Dixon, F.M.; Brickman, A.M.; Cheng, J.C.; Alexopoulos, G.S. Aging of cerebral white matter: A review of MRI findings. Int. J. Geriatr. Psychiatry 2009, 24, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Román, G.C. Vascular Dementia Prevention: A Risk Factor Analysis. Cerebrovasc. Dis. 2005, 20 (Suppl. 2), 91–100. [Google Scholar] [CrossRef]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild cognitive impairment: Ten years later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. European Cardiovascular Disease Statistics 4th edition 2012: EuroHeart II. Eur. Heart J. 2013, 34, 3007–3013. [Google Scholar]
- Daffner, K.R. Promoting Successful Cognitive Aging: A Comprehensive Review. J. Alzheimers Dis. 2010, 19, 1101–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dautricourt, S.; Gonneaud, J.; Landeau, B.; Calhoun, V.D.; De Flores, R.; Poisnel, G.; Bougacha, S.; Ourry, V.; Touron, E.; Kuhn, E.; et al. Dynamic functional connectivity patterns associated with dementia risk. Alzheimer’s Res. Ther. 2022, 14, 72. [Google Scholar] [CrossRef]
- Forette, F.; Seux, M.L.; Staessen, J.A.; Thijs, L.; Babarskiene, M.R.; Babeanu, S.; Bossini, A.; Fagard, R.; Gil-Extremera, B.; Laks, T.; et al. The prevention of dementia with antihypertensive treatment: New evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch. Intern. Med. 2002, 162, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Dolui, S.; Detre, J.A.; Gaussoin, S.A.; Herrick, J.S.; Wang, D.J.J.; Tamura, M.K.; Cho, M.E.; Haley, W.E.; Launer, L.J.; Punzi, H.A.; et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral Blood Flow: Secondary Analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Neurol. 2022, 79, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Winblad, B.; Fratiglioni, L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005, 4, 487–499. [Google Scholar] [CrossRef]
- Vidal, J.S.; Sigurdsson, S.; Jonsdottir, M.K.; Eiriksdottir, G.; Thorgeirsson, G.; Kjartansson, O.; Garcia, M.E.; van Buchem, M.A.; Harris, T.B.; Gudnason, V.; et al. Coronary artery calcium, brain function and structure: The AGES-Reykjavik Study. Stroke 2010, 41, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.T.; Hong, F.F.; Yang, S.L. Atherosclerosis: The Culprit and Co-victim of Vascular Dementia. Front. Neurosci. 2021, 15, 673440. [Google Scholar] [CrossRef]
- Kuller, L.H.; Lopez, O.L.; Jagust, W.J.; Becker, J.T.; DeKosky, S.T.; Lyketsos, C.; Kawas, C.; Breitner, J.C.; Fitzpatrick, A.; Dulberg, C. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 2005, 64, 1548–1552. [Google Scholar] [CrossRef]
- Rusanen, M.; Kivipelto, M.; Levälahti, E.; Laatikainen, T.; Tuomilehto, J.; Soininen, H.; Ngandu, T. Heart diseases and long-term risk of dementia and Alzheimer’s disease: A population-based CAIDE study. J. Alzheimers Dis. 2014, 42, 183–191. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, M.; Spulber, G.; Damangir, S.; Niskanen, E.; Ngandu, T.; Soininen, H.; Kivipelto, M.; Solomon, A. Midlife CAIDE Dementia Risk Score and Dementia-Related Brain Changes up to 30 Years Later on Magnetic Resonance Imaging. J. Alzheimers Dis. 2015, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.J.; Pottegård, A.; Mantel-Teeuwisse, A.K.; Forslund, T.; Hjemdahl, P.; Wettermark, B.; Hallas, J.; Olesen, M.; Bennie, M.; Mueller, T.; et al. Oral anticoagulants in patients with atrial fibrillation at low stroke risk: A multicentre observational study. Eur. Heart J. 2022, 43, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
| Research Questions (RQs) | Search Strategy |
|---|---|
| RQ 1: What is the relationship between ageing caused by vascular disease and cognitive reserve? | cognitive reserve AND cognitive ageing |
| RQ 2: Is there an underlying pathophysiological link between blood pressure variability, stroke, diabetes, and cognitive impairment? | cognitive reserve AND cognitive ageing and adults and blood pressure cognitive reserve AND cognitive ageing and adults and stroke cognitive reserve AND cognitive ageing and adults and diabetes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iluț, S.; Vesa, Ş.C.; Văcăraș, V.; Brăiță, L.; Dăscălescu, V.-C.; Fantu, I.; Mureșanu, D.-F. Biological Risk Factors Influencing Vascular Cognitive Impairments: A Review of the Evidence. Brain Sci. 2023, 13, 1094. https://doi.org/10.3390/brainsci13071094
Iluț S, Vesa ŞC, Văcăraș V, Brăiță L, Dăscălescu V-C, Fantu I, Mureșanu D-F. Biological Risk Factors Influencing Vascular Cognitive Impairments: A Review of the Evidence. Brain Sciences. 2023; 13(7):1094. https://doi.org/10.3390/brainsci13071094
Chicago/Turabian StyleIluț, Silvina, Ştefan Cristian Vesa, Vitalie Văcăraș, Lavinia Brăiță, Vlad-Constantin Dăscălescu, Ioana Fantu, and Dafin-Fior Mureșanu. 2023. "Biological Risk Factors Influencing Vascular Cognitive Impairments: A Review of the Evidence" Brain Sciences 13, no. 7: 1094. https://doi.org/10.3390/brainsci13071094
APA StyleIluț, S., Vesa, Ş. C., Văcăraș, V., Brăiță, L., Dăscălescu, V.-C., Fantu, I., & Mureșanu, D.-F. (2023). Biological Risk Factors Influencing Vascular Cognitive Impairments: A Review of the Evidence. Brain Sciences, 13(7), 1094. https://doi.org/10.3390/brainsci13071094









