Neural Correlates of Impaired Grasp Function in Children with Unilateral Spastic Cerebral Palsy
Abstract
:1. Introduction
2. Typically Developing Grasp
2.1. Neural Mechanisms Underlying Grasp
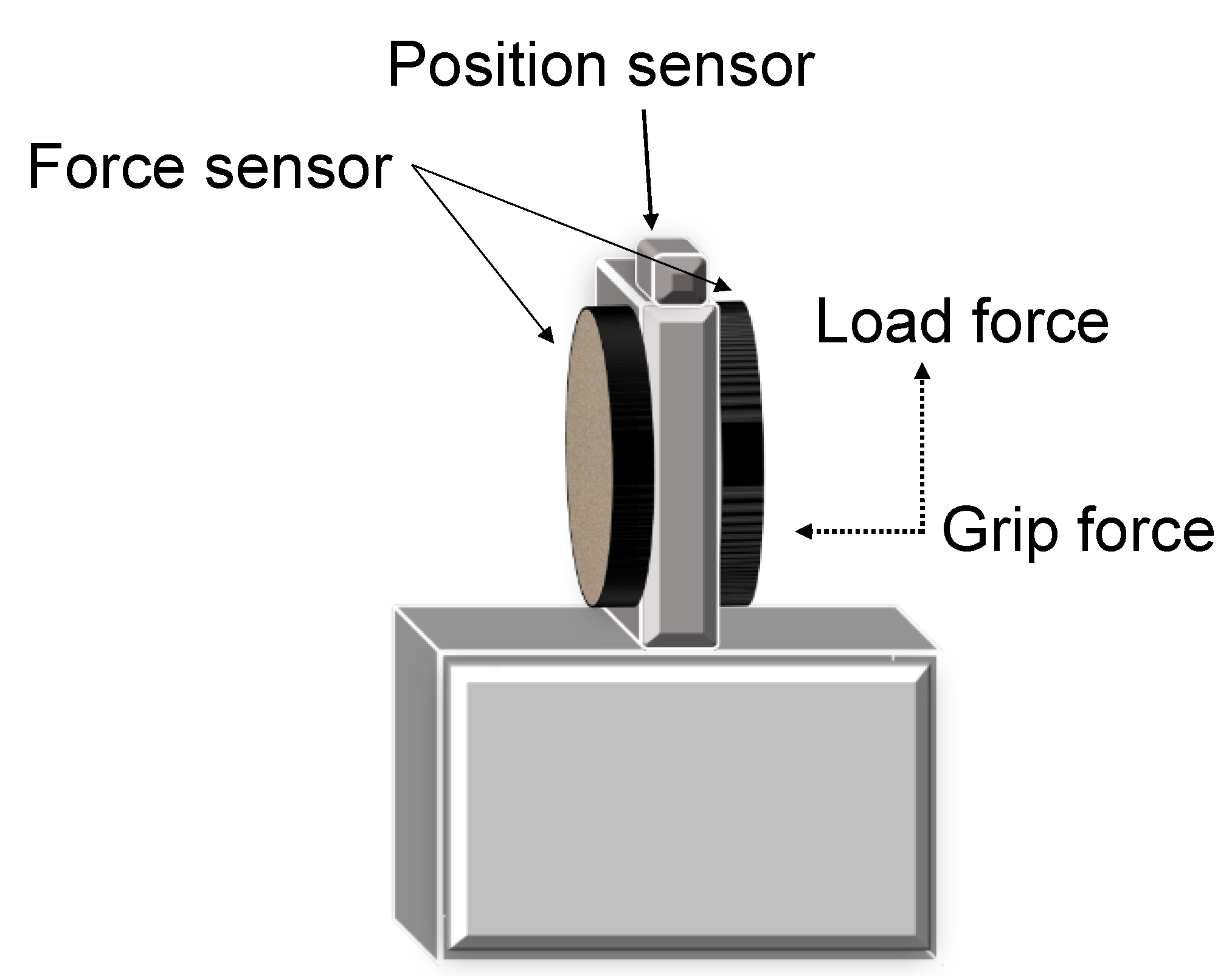
2.2. Grasping in Typically Developed Adults
3. Development of the CST and Grasp in Typically Developing Children
4. Grasp Impairments in Children with USCP
4.1. Precision Grip Impairments in Children with USCP
4.1.1. Sensory Adaptation Impairments
4.1.2. Anticipatory Control Impairments
4.1.3. Sensorimotor Integration Impairments
5. Neural Basis of Grasp Impairments in Children with USCP
5.1. CST Development in Children with USCP
5.2. Damage to the CST in Relation to Grasp Impairments in Children with USCP
5.3. Lesion Type and CST Organization in Relationship to Grasp Impairments
5.4. Development of Sensory Tract in Children with USCP
5.5. Sensory Tract Integrity in Relation to Grasp Impairments in Children with USCP
5.6. Relationship of Sensory and Motor Tracts on Grasp Impairments
5.7. Relationship between Sensory and Motor Information and the Corpus Callosum in Relationship to Grasp in Children with USCP
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Himmelmann, K.; Hagberg, G.; Beckung, E.; Hagberg, B.; Uvebrant, P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr. 2005, 94, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Klingels, K.; Demeyere, I.; Jaspers, E.; de Cock, P.; Molenaers, G.; Boyd, R.N.; Feys, H. Upper limb impairments and their impact on activity measures in children with unilateral cerebral palsy. Eur. J. Paediatr. Neurol. 2012, 16, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C.; Gordon, A.M.; Forssberg, H. Basic co-ordination of manipulative forces of children with cerebral palsy. Dev. Med. Child Neurol. 1991, 33, 661–670. [Google Scholar] [CrossRef]
- Gordon, A.M.; Duff, S.V. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. I: Anticipatory scaling. Dev. Med. Child Neurol. 1999, 41, 166–175. [Google Scholar] [CrossRef]
- Gordon, A.M.; Duff, S.V. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 1999, 41, 586–591. [Google Scholar] [CrossRef]
- Johansson, R.; Westling, G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp. Brain Res. 1987, 66, 141–154. [Google Scholar] [CrossRef]
- Johansson, R.S.; Westling, G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp. Brain Res. 1984, 56, 550–564. [Google Scholar] [CrossRef]
- Johansson, R.S.; Westling, G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp. Brain Res. 1988, 71, 59–71. [Google Scholar] [CrossRef]
- Westling, G.; Johansson, R.S. Factors influencing the force control during precision grip. Exp. Brain Res. 1984, 53, 277–284. [Google Scholar] [CrossRef]
- Bleyenheuft, Y.; Thonnard, J.-L. Predictive and Reactive Control of Precision Grip in Children with Congenital Hemiplegia. Neurorehabilit. Neural Repair 2010, 24, 318–327. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Gordon, A.M.; Forssberg, H. Impaired anticipatory control of isometric forces during grasping by children with cerebral palsy. Dev. Med. Child Neurol. 1992, 34, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.C.; Gordon, A.M.; Forssberg, H. Tactile control of isometric fingertip forces during grasping in children with cerebral palsy. Dev. Med. Child Neurol. 1995, 37, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Charles, J.; Steenbergen, B. Fingertip force planning during grasp is disrupted by impaired sensorimotor integration in children with hemiplegic cerebral palsy. Pediatr. Res. 2006, 60, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutterman, J.; Lee-Miller, T.; Friel, K.M.; Dimitropoulou, K.; Gordon, A.M. Anticipatory Motor Planning and Control of Grasp in Children with Unilateral Spastic Cerebral Palsy. Brain Sci. 2021, 11, 1161. [Google Scholar] [CrossRef]
- Duque, J.; Thonnard, J.-L.; Vandermeeren, Y.; Sébire, G.; Cosnard, G.; Olivier, E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain 2003, 126, 732–747. [Google Scholar] [CrossRef] [Green Version]
- Bleyenheuft, Y.; Gordon, A.M. Precision grip control, sensory impairments and their interactions in children with hemiplegic cerebral palsy: A systematic review. Res. Dev. Disabil. 2013, 34, 3014–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzetta, A.; Bonanni, P.; Biagi, L.; Tosetti, M.; Montanaro, D.; Guerrini, R.; Cioni, G. Reorganisation of the somatosensory system after early brain damage. Clin. Neurophysiol. 2007, 118, 1110–1121. [Google Scholar] [CrossRef]
- Staudt, M.; Braun, C.; Gerloff, C.; Erb, M.; Grodd, W.; Krägeloh-Mann, I. Developing somatosensory projections bypass periventricular brain lesions. Neurology 2006, 67, 522–525. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Byrnes, M.L.; Archer, S.A.; Nagarajan, L.; Mastaglia, F.L. Differences in sensory and motor cortical organization following brain injury early in life. Ann. Neurol. 2001, 49, 320–327. [Google Scholar] [CrossRef]
- Wilke, M.; Staudt, M.; Juenger, H.; Grodd, W.; Braun, C.; Krägeloh-Mann, I. Somatosensory system in two types of motor reorganization in congenital hemiparesis: Topography and function. Hum. Brain Mapp. 2009, 30, 776–788. [Google Scholar] [CrossRef]
- Gazzaniga, M.S.; Bogen, J.E.; Sperry, R.W. Laterality effects in somesthesis following cerebral commissurotomy in man. Neuropsychologia 1963, 1, 209–215. [Google Scholar] [CrossRef]
- Gordon, A.M.; Forssberg, H.; Iwasaki, N. Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia 1994, 32, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.T.; Gutterman, J.; Ferre, C.L.; Chin, K.; Brandao, M.B.; Gordon, A.M.; Friel, K. Corpus Callosum Integrity Relates to Improvement of Upper-Extremity Function Following Intensive Rehabilitation in Children with Unilateral Spastic Cerebral Palsy. Neurorehabilit. Neural Repair 2021, 35, 534–544. [Google Scholar] [CrossRef]
- Weinstein, M.; Green, D.; Geva, R.; Schertz, M.; Fattal-Valevski, A.; Artzi, M.; Myers, V.; Shiran, S.; Gordon, A.M.; Gross-Tsur, V.; et al. Interhemispheric and intrahemispheric connectivity and manual skills in children with unilateral cerebral palsy. Brain Struct. Funct. 2014, 219, 1025–1040. [Google Scholar] [CrossRef]
- Borra, E.; Gerbella, M.; Rozzi, S.; Luppino, G. The macaque lateral grasping network: A neural substrate for generating purposeful hand actions. Neurosci. Biobehav. Rev. 2017, 75, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Arissian, K. Experiments on functional role of peripheral input to motor cortex during voluntary movements in the monkey. J. Neurophysiol. 1984, 52, 212–227. [Google Scholar] [CrossRef]
- Friel, K.M.; Barbay, S.; Frost, S.B.; Plautz, E.J.; Hutchinson, D.M.; Stowe, A.M.; Dancause, N.; Zoubina, E.V.; Quaney, B.M.; Nudo, R.J. Dissociation of Sensorimotor Deficits after Rostral Versus Caudal Lesions in the Primary Motor Cortex Hand Representation. J. Neurophysiol. 2005, 94, 1312–1324. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.G.; Kuypers, H.G. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 1968, 91, 1–14. [Google Scholar] [CrossRef]
- Lemon, R.N.; Griffiths, J. Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2005, 32, 261–279. [Google Scholar] [CrossRef]
- Martin, J.H. The Corticospinal System: From Development to Motor Control. Neuroscientist 2005, 11, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Muir, R.B.; Lemon, R.N. Corticospinal neurons with a special role in precision grip. Brain Res. 1983, 261, 312–316. [Google Scholar] [CrossRef]
- Kitai, S.; Weinberg, J. Tactile discrimination study of the dorsal column-medial lemniscal system and spino-cervico-thalamic tract in cat. Exp. Brain Res. 1968, 6, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Salimi, I.; Brochier, T.; Smith, A.M. Neuronal Activity in Somatosensory Cortex of Monkeys Using a Precision Grip. I. Receptive Fields and Discharge Patterns. J. Neurophysiol. 1999, 81, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Salimi, I.; Brochier, T.; Smith, A.M. Neuronal activity in somatosensory cortex of monkeys using a precision grip. II. Responses To object texture and weights. J. Neurophysiol. 1999, 81, 835–844. [Google Scholar] [CrossRef] [Green Version]
- Lemon, R.N.; Johansson, R.S.; Westling, G. Corticospinal control during reach, grasp, and precision lift in man. J. Neurosci. 1995, 15, 6145–6156. [Google Scholar] [CrossRef]
- Raghavan, P.; Krakauer, J.W.; Gordon, A.M. Impaired anticipatory control of fingertip forces in patients with a pure motor or sensorimotor lacunar syndrome. Brain 2006, 129, 1415–1425. [Google Scholar] [CrossRef]
- Wenzelburger, R.; Kopper, F.; Frenzel, A.; Stolze, H.; Klebe, S.; Brossmann, A.; Kuhtz-Buschbeck, J.; Gölge, M.; Illert, M.; Deuschl, G. Hand coordination following capsular stroke. Brain 2005, 128, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Moberg, E. Criticism and studvy of methods for examining sensibility in the hand. Neurology 1962, 12, 8. [Google Scholar] [CrossRef]
- Cole, K.J.; Abbs, J.H. Grip force adjustments evoked by load force perturbations of a grasped object. J. Neurophysiol. 1988, 60, 1513–1522. [Google Scholar] [CrossRef]
- Monzée, J.; Lamarre, Y.; Smith, A.M. The Effects of Digital Anesthesia on Force Control Using a Precision Grip. J. Neurophysiol. 2003, 89, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Westling, G.; Cole, K.J.; Johansson, R.S. Memory representations underlying motor commands used during manipulation of common and novel objects. J. Neurophysiol. 1993, 69, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Jenmalm, P.; Johansson, R.S. Visual and Somatosensory Information about Object Shape Control Manipulative Fingertip Forces. J. Neurosci. 1997, 17, 4486. [Google Scholar] [CrossRef] [Green Version]
- Johansson, R.S.; Cole, K.J. Sensory-motor coordination during grasping and manipulative actions. Curr. Opin. Neurobiol. 1992, 2, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Cole, K.J. Grasp stability during manipulative actions. Can. J. Physiol. Pharmacol. 1994, 72, 511–524. [Google Scholar] [CrossRef]
- Staudt, M. Brain plasticity following early life brain injury: Insights from neuroimaging. Semin. Perinatol. 2010, 34, 87–92. [Google Scholar] [CrossRef]
- Eyre, J.A. Corticospinal tract development and its plasticity after perinatal injury. Neurosci. Biobehav. Rev. 2007, 31, 1136–1149. [Google Scholar] [CrossRef]
- Eyre, J.A.; Taylor, J.P.; Villagra, F.; Smith, M.; Miller, S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology 2001, 57, 1543. [Google Scholar] [CrossRef] [Green Version]
- Eyre, J.A.; Smith, M.; Dabydeen, L.; Clowry, G.J.; Petacchi, E.; Battini, R.; Guzzetta, A.; Cioni, G. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann. Neurol. 2007, 62, 493–503. [Google Scholar] [CrossRef]
- Gordon, A.M. Chapter 3 Development of the Reach to Grasp Movement. In Advances in Psychology; Elsevier, North-Holland: Amsterdam, The Netherlands, 1994; Volume 105, pp. 37–56. [Google Scholar]
- Forssberg, H.; Eliasson, A.C.; Kinoshita, H.; Johansson, R.S.; Westling, G. Development of human precision grip. I: Basic coordination of force. Exp. Brain Res. 1991, 85, 451–457. [Google Scholar] [CrossRef]
- Forssberg, H.; Kinoshita, H.; Eliasson, A.C.; Johansson, R.S.; Westling, G.; Gordon, A.M. Development of human precision grip. Exp. Brain Res. 1992, 90, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Forssberg, H.; Eliasson, A.C.; Kinoshita, H.; Westling, G.; Johansson, R.S. Development of human precision grip. Exp. Brain Res. 1995, 104, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Auld, M.L.; Boyd, R.N.; Moseley, G.L.; Ware, R.S.; Johnston, L.M. Impact of Tactile Dysfunction on Upper-Limb Motor Performance in Children with Unilateral Cerebral Palsy. Arch. Phys. Med. Rehabil. 2012, 93, 696–702. [Google Scholar] [CrossRef]
- Poitras, I.; Martinie, O.; Robert, M.T.; Campeau-Lecours, A.; Mercier, C. Impact of Sensory Deficits on Upper Limb Motor Performance in Individuals with Cerebral Palsy: A Systematic Review. Brain Sci. 2021, 11, 744. [Google Scholar] [CrossRef]
- Wingert, J.R.; Burton, H.; Sinclair, R.J.; Brunstrom, J.E.; Damiano, D.L. Tactile sensory abilities in cerebral palsy: Deficits in roughness and object discrimination. Dev. Med. Child Neurol. 2008, 50, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Mailleux, L.; Klingels, K.; Fiori, S.; Simon-Martinez, C.; Demaerel, P.; Locus, M.; Fosseprez, E.; Boyd, R.N.; Guzzetta, A.; Ortibus, E.; et al. How does the interaction of presumed timing, location and extent of the underlying brain lesion relate to upper limb function in children with unilateral cerebral palsy? Eur. J. Paediatr. Neurol. 2017, 21, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Mailleux, L.; Simon-Martinez, C.; Radwan, A.; Blommaert, J.; Gooijers, J.; Wenderoth, N.; Klingels, K.; Ortibus, E.; Sunaert, S.; Feys, H. White matter characteristics of motor, sensory and interhemispheric tracts underlying impaired upper limb function in children with unilateral cerebral palsy. Brain Struct. Funct. 2020, 225, 1495–1509. [Google Scholar] [CrossRef]
- Simon-Martinez, C.; Jaspers, E.; Mailleux, L.; Ortibus, E.; Klingels, K.; Wenderoth, N.; Feys, H. Corticospinal Tract Wiring and Brain Lesion Characteristics in Unilateral Cerebral Palsy: Determinants of Upper Limb Motor and Sensory Function. Neural Plast. 2018, 2018, 2671613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliasson, A.-C.; Gordon, A.M. Impaired force coordination during object release in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 2000, 42, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Jebsen, R.H.; Taylor, N.; Trieschmann, R.B.; Trotter, M.J.; Howard, L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar] [PubMed]
- Mathiowetz, V.; Federman, S.; Wiemer, D. Box and Block Test of Manual Dexterity: Norms for 6–19 Year Olds. Can. J. Occup. Ther. 1985, 52, 241–245. [Google Scholar] [CrossRef]
- Bleyenheuft, Y.; Cols, C.; Arnould, C.; Thonnard, J.-L. Age-related changes in tactile spatial resolution from 6 to 16 years old. Somatosens. Mot. Res. 2006, 23, 83–87. [Google Scholar] [CrossRef]
- Boven, R.W.V.; Johnson, K.O. The limit of tactile spatial resolution in humans. Grating Orientat. Discrim. Lip Tongue Finger 1994, 44, 2361. [Google Scholar] [CrossRef]
- Cooper, J.; Majnemer, A.; Rosenblatt, B.; Birnbaum, R. The determination of sensory deficits in children with hemiplegic cerebral palsy. J. Child Neurol. 1995, 10, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Klingels, K.; De Cock, P.; Molenaers, G.; Desloovere, K.; Huenaerts, C.; Jaspers, E.; Feys, H. Upper limb motor and sensory impairments in children with hemiplegic cerebral palsy. Can they be measured reliably? Disabil. Rehabil. 2010, 32, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Moberg, E. Two-point discrimination test. A valuable part of hand surgical rehabilitation, e.g., in tetraplegia. J. Rehabil. Med. 1990, 22, 127–134. [Google Scholar] [CrossRef]
- Gordon, A.M.; Charles, J.; Duff, S.V. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. II: Bilateral coordination. Dev. Med. Child Neurol. 1999, 41, 176–185. [Google Scholar] [CrossRef]
- Holmström, L.; Vollmer, B.; Tedroff, K.; Islam, M.; Persson, J.K.; Kits, A.; Forssberg, H.; Eliasson, A.C. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev. Med. Child Neurol. 2010, 52, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Bleyenheuft, Y.; Thonnard, J.L. Tactile spatial resolution in unilateral brain lesions and its correlation with digital dexterity. J. Rehabil. Med. 2011, 43, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.-C.; Gordon, A.M.; Henrionnet, A.; Hautfenne, S.; Friel, K.M.; Bleyenheuft, Y. The effects of intensive bimanual training with and without tactile training on tactile function in children with unilateral spastic cerebral palsy: A pilot study. Res. Dev. Disabil. 2016, 49–50, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Saussez, G.; Van Laethem, M.; Bleyenheuft, Y. Changes in tactile function during intensive bimanual training in children with unilateral spastic cerebral palsy. J. Child Neurol. 2018, 33, 260–268. [Google Scholar] [CrossRef]
- Steenbergen, B.; Jongbloed-Pereboom, M.; Spruijt, S.; Gordon, A.M. Impaired motor planning and motor imagery in children with unilateral spastic cerebral palsy: Challenges for the future of pediatric rehabilitation. Dev. Med. Child Neurol. 2013, 55 (Suppl. S4), 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crajé, C.; Aarts, P.; Nijhuis-van der Sanden, M.; Steenbergen, B. Action planning in typically and atypically developing children (unilateral cerebral palsy). Res. Dev. Disabil. 2010, 31, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Ebner-Karestinos, D.; Flament, B.; Arnould, C.; Thonnard, J.L.; Bleyenheuft, Y. Precision grip control while walking down a step in children with unilateral cerebral palsy. PLoS ONE 2018, 13, e0191684. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.B.; Diermayr, G.; Gysin, P.; Gordon, A.M. Coordination of fingertip forces in object transport during gait in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 865–869. [Google Scholar] [CrossRef]
- Duff, S.V.; Gordon, A.M. Learning of grasp control in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 2003, 45, 746–757. [Google Scholar] [CrossRef]
- Blank, R.; Hermsdörfer, J. Basic motor capacity in relation to object manipulation and general manual ability in young children with spastic cerebral palsy. Neurosci. Lett. 2009, 450, 65–69. [Google Scholar] [CrossRef]
- Jaspers, E.; Byblow, W.D.; Feys, H.; Wenderoth, N. The Corticospinal Tract: A Biomarker to Categorize Upper Limb Functional Potential in Unilateral Cerebral Palsy. Front. Pediatr. 2015, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Staudt, M.; Gerloff, C.; Grodd, W.; Holthausen, H.; Niemann, G.; Krägeloh-Mann, I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann. Neurol. 2004, 56, 854–863. [Google Scholar] [CrossRef]
- Ferre, C.L.; Carmel, J.B.; Flamand, V.H.; Gordon, A.M.; Friel, K.M. Anatomical and Functional Characterization in Children with Unilateral Cerebral Palsy: An Atlas-Based Analysis. Neurorehabilit. Neural Repair 2020, 34, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Quinn, L.; Reilmann, R.; Marder, K. Coordination of Prehensile Forces during Precision Grip in Huntington’s Disease. Exp. Neurol. 2000, 163, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Prodoehl, J.; Corcos, D.M.; Vaillancourt, D.E. Basal ganglia mechanisms underlying precision grip force control. Neurosci. Biobehav. Rev. 2009, 33, 900–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, F.; Dichgans, J. Dyscoordination of pinch and lift forces during grasp in patients with cerebellar lesions. Exp. Brain Res. 1994, 101, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Eyre, J.A. Development and plasticity of the corticospinal system in man. Neural Plast. 2003, 10, 93–106. [Google Scholar] [CrossRef]
- Friel, K.M.; Chakrabarty, S.; Martin, J.H. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Dev. Med. Child Neurol. 2013, 55, 27–31. [Google Scholar] [CrossRef]
- Bleyenheuft, Y.; Grandin, C.B.; Cosnard, G.; Olivier, E.; Thonnard, J.L. Corticospinal dysgenesis and upper-limb deficits in congenital hemiplegia: A diffusion tensor imaging study. Pediatrics 2007, 120, e1502–e1511. [Google Scholar] [CrossRef]
- Friel, K.M.; Kuo, H.-C.; Carmel, J.B.; Rowny, S.B.; Gordon, A.M. Improvements in hand function after intensive bimanual training are not associated with corticospinal tract dysgenesis in children with unilateral cerebral palsy. Exp. Brain Res. 2014, 232, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Holmström, L.; Lennartsson, F.; Eliasson, A.C.; Flodmark, O.; Clark, C.; Tedroff, K.; Forssberg, H.; Vollmer, B. Diffusion MRI in corticofugal fibers correlates with hand function in unilateral cerebral palsy. Neurology 2011, 77, 775–783. [Google Scholar] [CrossRef]
- Kuczynski, A.M.; Dukelow, S.P.; Hodge, J.A.; Carlson, H.L.; Lebel, C.; Semrau, J.A.; Kirton, A. Corticospinal tract diffusion properties and robotic visually guided reaching in children with hemiparetic cerebral palsy. Hum. Brain Mapp. 2018, 39, 1130–1144. [Google Scholar] [CrossRef] [Green Version]
- Hodge, J.; Goodyear, B.; Carlson, H.; Wei, X.C.; Kirton, A. Segmental Diffusion Properties of the Corticospinal Tract and Motor Outcome in Hemiparetic Children with Perinatal Stroke. J. Child Neurol. 2017, 32, 550–559. [Google Scholar] [CrossRef]
- Marneweck, M.; Kuo, H.-C.; Smorenburg, A.R.P.; Ferre, C.L.; Flamand, V.H.; Gupta, D.; Carmel, J.B.; Bleyenheuft, Y.; Gordon, A.M.; Friel, K.M. The Relationship between Hand Function and Overlapping Motor Representations of the Hands in the Contralesional Hemisphere in Unilateral Spastic Cerebral Palsy. Neurorehabilit. Neural Repair 2018, 32, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forssberg, H.; Eliasson, A.C.; Redon-Zouitenn, C.; Mercuri, E.; Dubowitz, L. Impaired grip-lift synergy in children with unilateral brain lesions. Brain 1999, 122 Pt 6, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Barachant, A.; Gordon, A.M.; Ferre, C.; Kuo, H.-C.; Carmel, J.B.; Friel, K.M. Effect of sensory and motor connectivity on hand function in pediatric hemiplegia. Ann. Neurol. 2017, 82, 766–780. [Google Scholar] [CrossRef]
- Staudt, M.; Grodd, W.; Gerloff, C.; Erb, M.; Stitz, J.; Krägeloh-Mann, I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain 2002, 125, 2222–2237. [Google Scholar] [CrossRef] [Green Version]
- Friel, K.M.; Kuo, H.-C.; Fuller, J.; Ferre, C.L.; Brandão, M.; Carmel, J.B.; Bleyenheuft, Y.; Gowatsky, J.L.; Stanford, A.D.; Rowny, S.B.; et al. Skilled Bimanual Training Drives Motor Cortex Plasticity in Children with Unilateral Cerebral Palsy. Neurorehabilit. Neural Repair 2016, 30, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juenger, H.; de Haan, B.; Krägeloh-Mann, I.; Staudt, M.; Karnath, H.-O. Early Determination of Somatosensory Cortex in the Human Brain. Cereb. Cortex 2011, 21, 1827–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, S.; Guzzetta, A.; Pannek, K.; Boyd, R. MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connect 2011, 1, 309–316. [Google Scholar] [CrossRef]
- Tsao, H.; Pannek, K.; Boyd, R.N.; Rose, S.E. Changes in the integrity of thalamocortical connections are associated with sensorimotor deficits in children with congenital hemiplegia. Brain Struct. Funct. 2015, 220, 307–318. [Google Scholar] [CrossRef]
- Papadelis, C.; Butler, E.E.; Rubenstein, M.; Sun, L.; Zollei, L.; Nimec, D.; Snyder, B.; Grant, P.E. Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts. NeuroImage Clin. 2018, 17, 198–212. [Google Scholar] [CrossRef]
- Groeschel, S.; Hertz-Pannier, L.; Delion, M.; Loustau, S.; Husson, B.; Kossorotoff, M.; Renaud, C.; Nguyen The Tich, S.; Chabrier, S.; Dinomais, M. Association of transcallosal motor fibres with function of both hands after unilateral neonatal arterial ischemic stroke. Dev. Med. Child Neurol. 2017, 59, 1042–1048. [Google Scholar] [CrossRef] [Green Version]
- Lemon, R.N. Functional properties of monkey motor cortex neurones receiving afferent input from the hand and fingers. J. Physiol. 1981, 311, 497–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutterman, J.; Gordon, A.M. Neural Correlates of Impaired Grasp Function in Children with Unilateral Spastic Cerebral Palsy. Brain Sci. 2023, 13, 1102. https://doi.org/10.3390/brainsci13071102
Gutterman J, Gordon AM. Neural Correlates of Impaired Grasp Function in Children with Unilateral Spastic Cerebral Palsy. Brain Sciences. 2023; 13(7):1102. https://doi.org/10.3390/brainsci13071102
Chicago/Turabian StyleGutterman, Jennifer, and Andrew M. Gordon. 2023. "Neural Correlates of Impaired Grasp Function in Children with Unilateral Spastic Cerebral Palsy" Brain Sciences 13, no. 7: 1102. https://doi.org/10.3390/brainsci13071102



