Another Way to Confuse Motor Control: Manual Technique Supposed to Shorten Muscle Spindles Reduces the Muscular Holding Stability in the Sense of Adaptive Force in Male Soccer Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
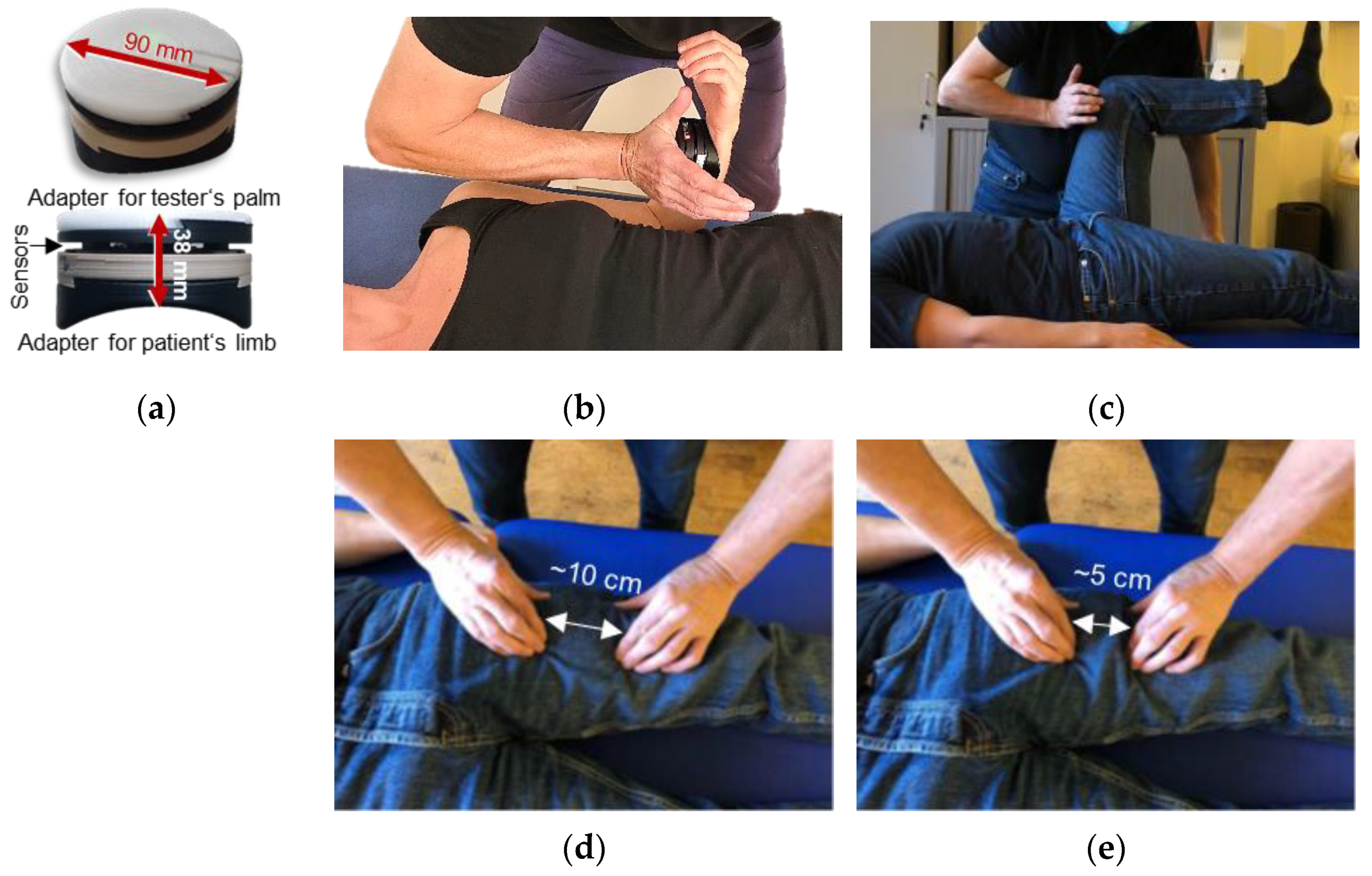
2.2. Technical Equipment
2.3. Setting and Manual Muscle Tests
2.4. Manual Spindle Technique: Manipulation of Muscle Spindles
2.5. Procedure
2.6. Data Processing and Statistical Analyses
- MVIC: peak value of the two MVIC tests.
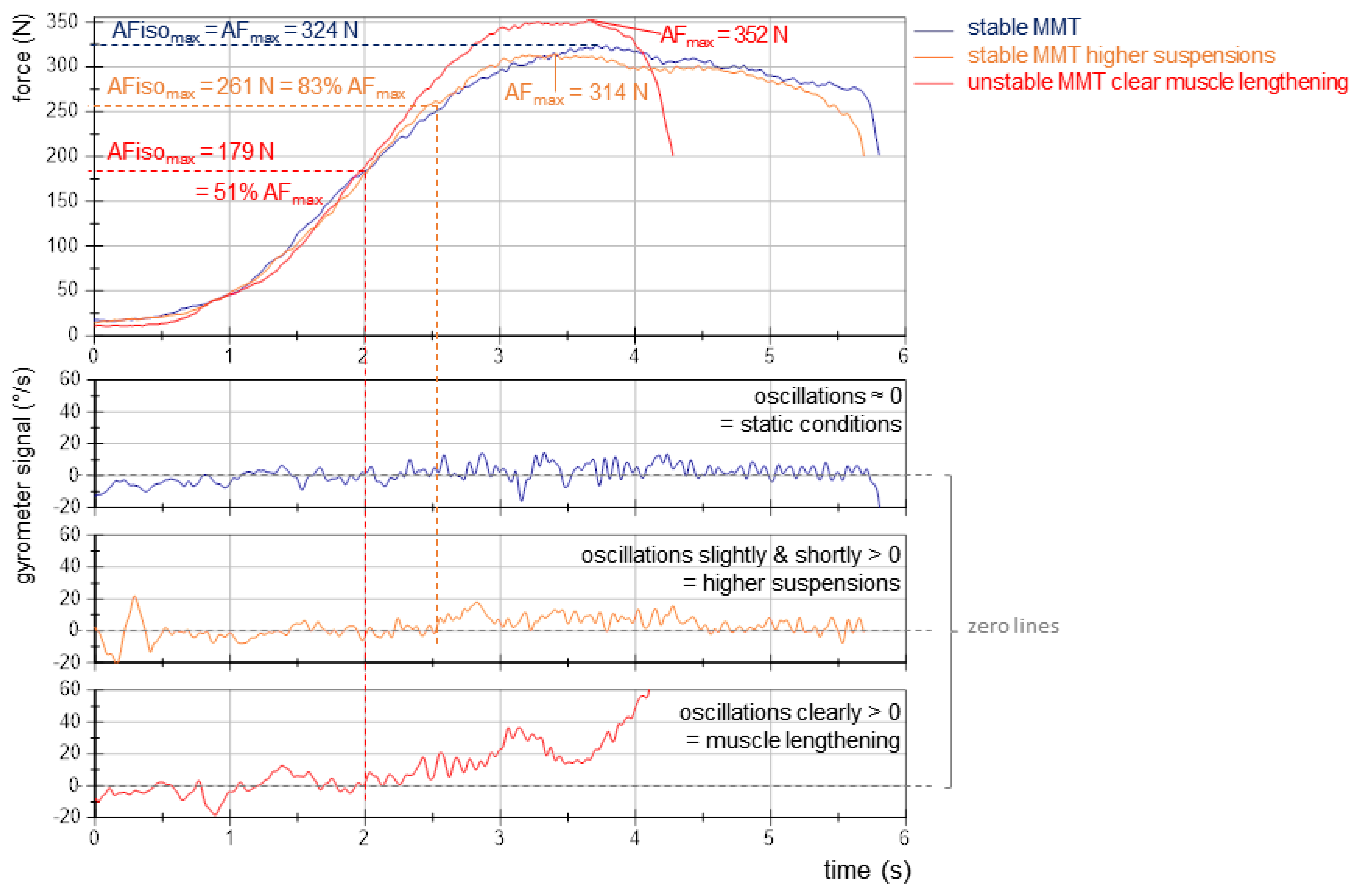
- AFmax: peak value of each MMT trial irrespective of the occurred muscle action (could either be reached under static conditions (stable MMT) or during muscle lengthening (unstable MMTs)). For the former, AFmax = AFisomax. For the latter, AFmax = AFeccmax > AFisomax (Figure 2).
- AFisomax: highest force value under static conditions, thus, during the isometric holding phase of an MMT. Since this does not necessarily refer to a peak value, the gyrometer signal was used to detect a potential breaking point (limb movement in the direction of joint extension). If the angular velocity increased consistently above zero (indicating yielding), the force value at the last zero crossing was referred to as AFisomax. If static conditions were present until the peak value was reached, AFisomax = AFmax. (Figure 2; for a detailed description, see [9,10,11,12,14]).
3. Results
3.1. Parameters of Adaptive Force Comparing Regular vs. Spindle Procedure
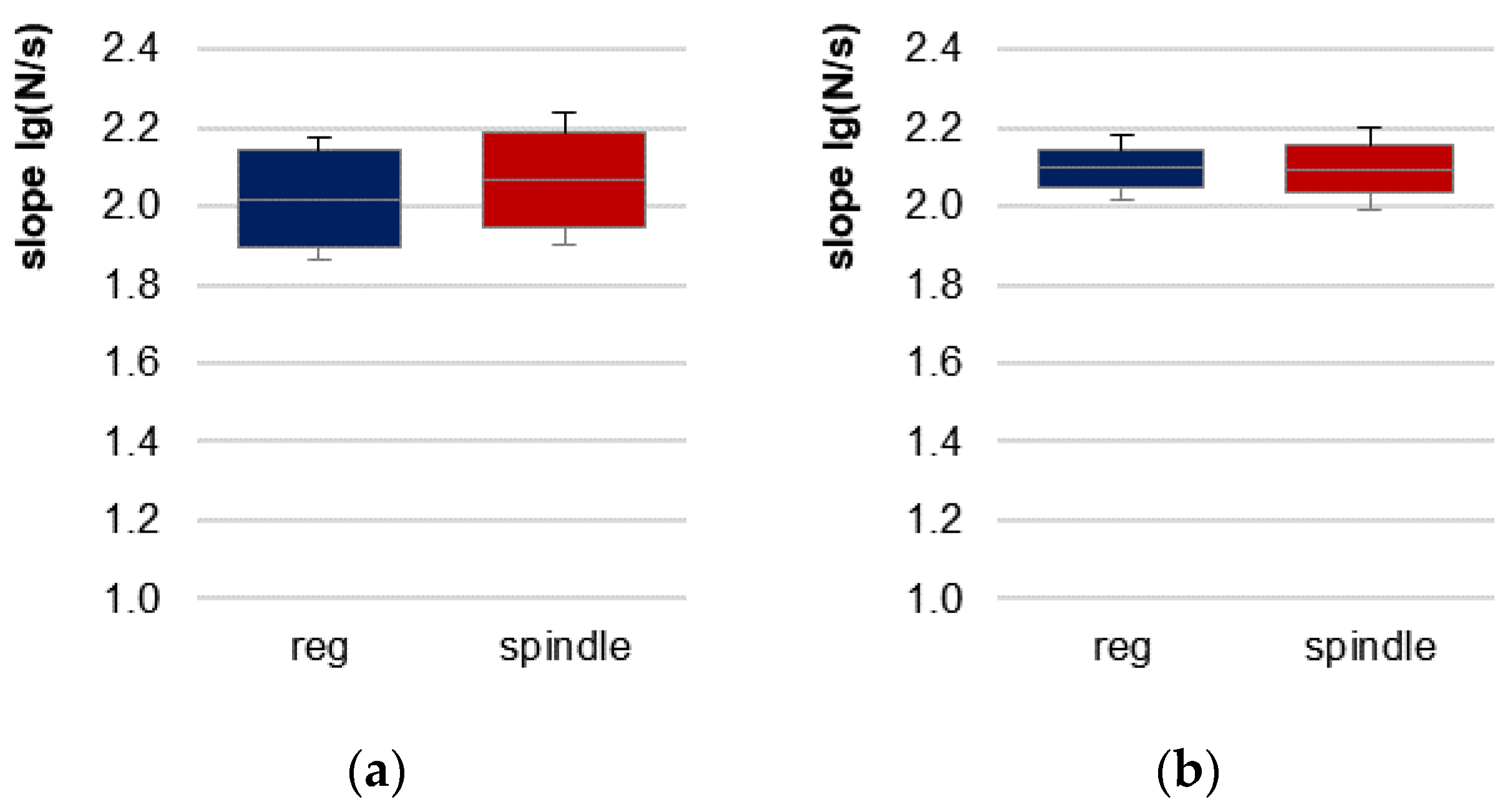
3.2. Onset of Oscillations Comparing Regular vs. Spindle Procedure
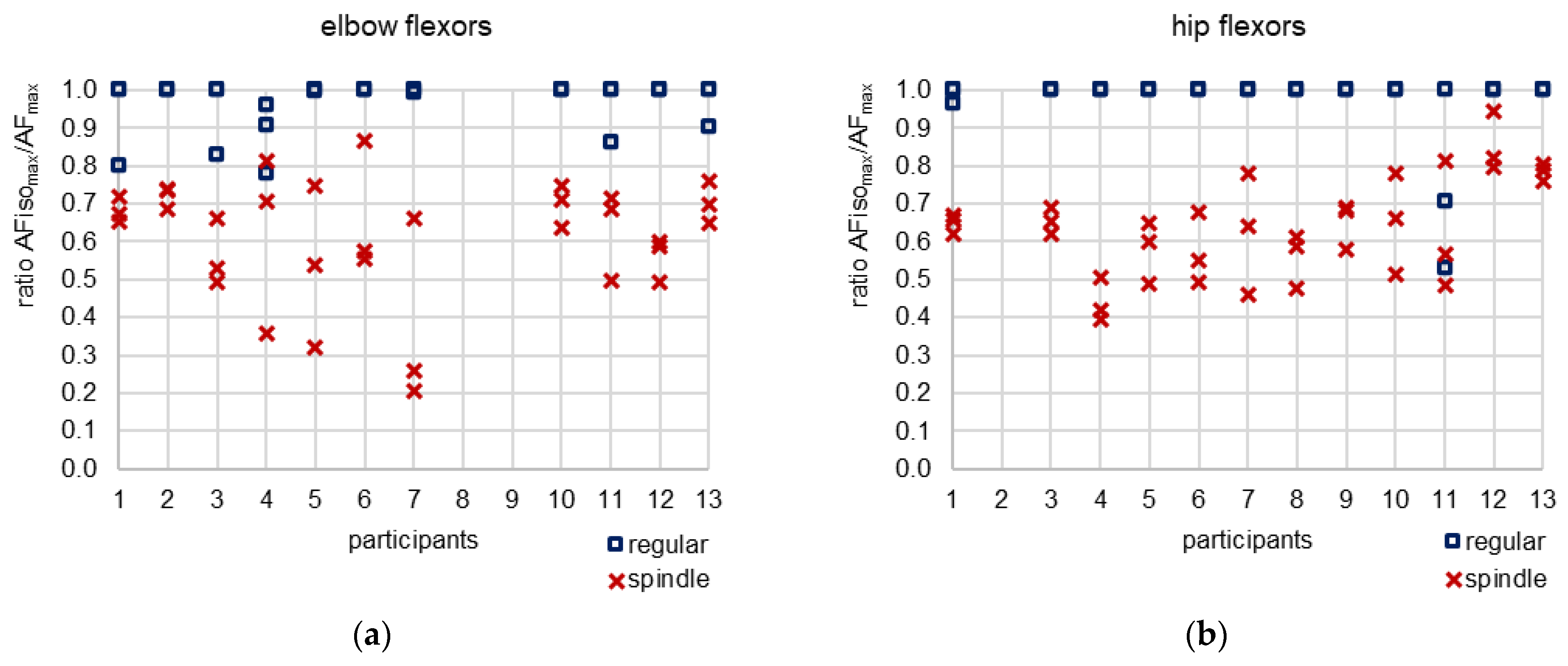
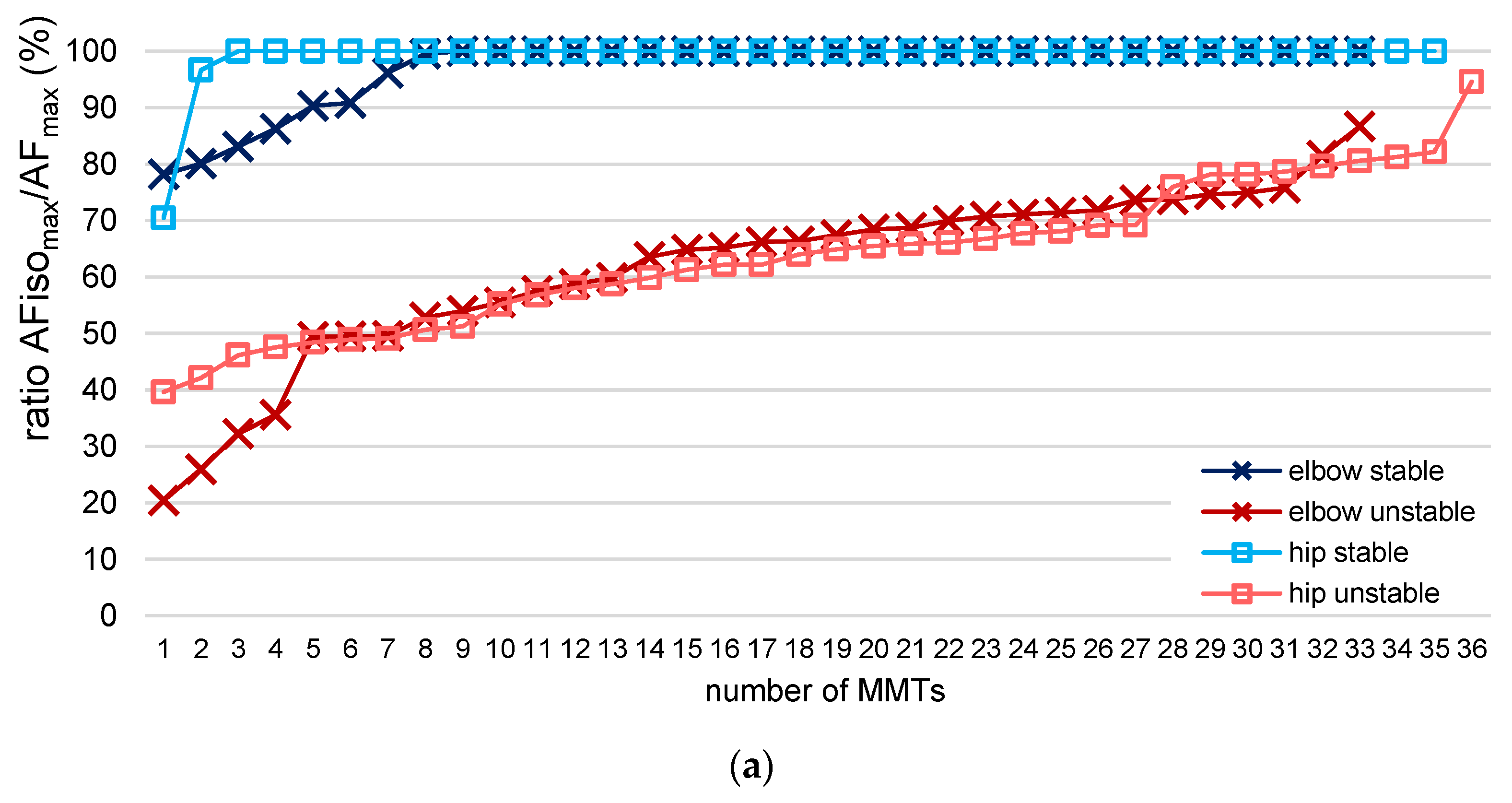
3.3. AF Data in Relation to the Tester’s Ratings of Manual Muscle Tests
4. Discussion
4.1. Methodological Considerations Comparing Regular vs. Spindle Procedure
4.2. Comparison of Tester’s Ratings of Manual Muscle Test and AF Data
4.3. Confusing or Impairing Sensorimotor Control: Neurophysiological Considerations and Practical Implications with Respect to the Adaptive Holding Capacity
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myer, G.D.; Brent, J.L.; Ford, K.R.; Hewett, T.E. Real-Time Assessment and Neuromuscular Training Feedback Techniques to Prevent Anterior Cruciate Ligament Injury in Female Athletes. Strength Cond. J. 2011, 33, 21–35. [Google Scholar] [CrossRef]
- Garrett, W.E. Muscle Strain Injuries: Clinical and Basic Aspects. Med. Sci. Sports Exerc. 1990, 22, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Fridén, J.; Lieber, R.L. Eccentric Exercise-Induced Injuries to Contractile and Cytoskeletal Muscle Fibre Components: Exercise-Induced Muscle Injury. Acta Physiol. Scand. 2001, 171, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kieb, M.; Lorbach, O.; Engelhardt, M. Muskelverletzungen: Diagnostik und Behandlungen. Orthopäde 2010, 39, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Hoff, M.; Bittmann, F. Measuring System and Method of Determining the Adaptive Force. Eur. J. Transl. Myol. 2017, 27, 6479. [Google Scholar] [CrossRef] [PubMed]
- Dech, S.; Bittmann, F.N.; Schaefer, L.V. Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria. Diagnostics 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Carnarius, F.; Dech, S.; Bittmann, F.N. Repeated Measurements of Adaptive Force: Maximal Holding Capacity Differs from Other Maximal Strength Parameters and Preliminary Characteristics for Non-Professional Strength vs. Endurance Athletes. Front. Physiol. 2023, 14, 1020954. [Google Scholar] [CrossRef]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual Muscle Testing—Force Profiles and Their Reproducibility. Diagnostics 2020, 10, 996. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Wolff, L.L.; Bittmann, F.N. Emotional Imagery Influences the Adaptive Force in Young Women: Unpleasant Imagery Reduces Instantaneously the Muscular Holding Capacity. Brain Sci. 2022, 12, 1318. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Bittmann, F.N. Adaptive Force and Emotionally Related Imaginations—Preliminary Results Suggest a Reduction of the Maximal Holding Capacity as Reaction to Disgusting Food Imagination. Heliyon 2021, 7, e07827. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Aehle, M.; Bittmann, F.N. Disgusting Odours Affect the Characteristics of the Adaptive Force in Contrast to Neutral and Pleasant Odours. Sci. Rep. 2021, 11, 16410. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. The Adaptive Force as a Potential Biomechanical Parameter in the Recovery Process of Patients with Long COVID. Diagnostics 2023, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Case Report: Individualized Pulsed Electromagnetic Field Therapy in a Long COVID Patient Using the Adaptive Force as Biomarker. Front. Med. 2023, 9, 879971. [Google Scholar] [CrossRef]
- Bittmann, F.N.; Dech, S.; Schaefer, L.V. How to Confuse Motor Control: Passive Muscle Shortening after Contraction in Lengthened Position Reduces the Muscular Holding Stability in the Sense of Adaptive Force. Life 2023, 13, 911. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Giuriati, W.; Ravara, B.; Porzionato, A.; Albertin, G.; Stecco, C.; Macchi, V.; De Caro, R.; Martinello, T.; Gomiero, C.; Patruno, M.; et al. Muscle Spindles of the Rat Sternomastoid Muscle. Eur. J. Transl. Myol. 2018, 28, 7904. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G.; Knellwolf, T.P. Functional Properties of Human Muscle Spindles. J. Neurophysiol. 2018, 120, 452–467. [Google Scholar] [CrossRef]
- Lakie, M.; Campbell, K.S. Muscle Thixotropy—Where Are We Now? J. Appl. Physiol. 2019, 126, 1790–1799. [Google Scholar] [CrossRef]
- Stubbs, P.W.; Walsh, L.D.; D’Souza, A.; Héroux, M.E.; Bolsterlee, B.; Gandevia, S.C.; Herbert, R.D. History-Dependence of Muscle Slack Length Following Contraction and Stretch in the Human Vastus Lateralis: History-Dependence of Muscle Slack Length. J. Physiol. 2018, 596, 2121–2129. [Google Scholar] [CrossRef]
- Proske, U.; Tsay, A.; Allen, T. Muscle Thixotropy as a Tool in the Study of Proprioception. Exp. Brain Res. 2014, 232, 3397–3412. [Google Scholar] [CrossRef]
- Gregory, J.E.; Wise, A.K.; Wood, S.A.; Prochazka, A.; Proske, U. Muscle History, Fusimotor Activity and the Human Stretch Reflex. J. Physiol. 1998, 513, 927–934. [Google Scholar] [CrossRef]
- Banks, R.W.; Ellaway, P.H.; Prochazka, A.; Proske, U. Secondary Endings of Muscle Spindles: Structure, Reflex Action, Role in Motor Control and Proprioception. Exp. Physiol. 2021, 106, 2339–2366. [Google Scholar] [CrossRef] [PubMed]
- Budini, F.; Tilp, M. Changes in H-Reflex Amplitude to Muscle Stretch and Lengthening in Humans. Rev. Neurosci. 2016, 27, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Héroux, M.E.; Anderman, I.; Nykvist Vouis, S.; Diong, J.; Stubbs, P.W.; Herbert, R.D. History-Dependence of Muscle Slack Length in Humans: Effects of Contraction Intensity, Stretch Amplitude, and Time. J. Appl. Physiol. 2020, 129, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.S. Applied Kinesiology: Synopsis; Systems D C: Pueblo, CO, USA, 1988; ISBN 978-0-929721-00-2. [Google Scholar]
- Garten, H. Lehrbuch Applied Kinesiology: Muskelfunktion—Dysfunktion—Therapie, 2nd ed.; Elsevier, Urban & Fischer: München, Germany, 2012; ISBN 978-3-437-56851-0. [Google Scholar]
- Conable, K.M.; Rosner, A.L. A Narrative Review of Manual Muscle Testing and Implications for Muscle Testing Research. J. Chiropr. Med. 2011, 10, 157–165. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Are There Two Forms of Isometric Muscle Action? Results of the Experimental Study Support a Distinction between a Holding and a Pushing Isometric Muscle Function. BMC Sports Sci. Med. Rehabil. 2017, 9, 11. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Chapman, D.; Newton, M.; Nosaka, K. Eccentric Torque-Velocity Relationship of the Elbow Flexors. Isokinet. Exerc. Sci. 2005, 13, 139–145. [Google Scholar] [CrossRef]
- Doss, W.S.; Karpovich, P.V. A Comparison of Concentric, Eccentric, and Isometric Strength of Elbow Flexors. J. Appl. Physiol. 1965, 20, 351–353. [Google Scholar] [CrossRef]
- Griffin, J.W. Differences in Elbow Flexion Torque Measured Concentrically, Eccentrically, and Isometrically. Phys. Ther. 1987, 67, 1205–1208. [Google Scholar] [CrossRef]
- Seliger, V.; Dolejš, L.; Karas, V. A Dynamometric Comparison of Maximum Eccentric, Concentric, and Isometric Contractions Using EMG and Energy Expenditure Measurements. Eur. J. Appl. Physiol. 1980, 45, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Amiridis, I.G.; Martin, A.; Morlon, B.; Martin, L.; Cometti, G.; Pousson, M.; Van Hoecke, J. Co-Activation and Tension-Regulating Phenomena during Isokinetic Knee Extension in Sedentary and Highly Skilled Humans. Eur. J. Appl. Physiol. 1996, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Morecraft, R.J.; Tanjii, J. Cingulofrontal Interactions and the Cingulate Motor Areas. In Cingulate Neurobiology and Disease; Oxford University Press: New York, NY, USA, 2009; ISBN 978-0-19-856696-0. [Google Scholar]
- Galizia, C.G.; Lledo, P.-M. (Eds.) Neurosciences—From Molecule to Behavior: A University Textbook; Springer Spektrum: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Huggenberger, S.; Moser, N.; Schröder, H.; Cozzi, B.; Granato, A.; Merighi, A. Neuroanatomie des Menschen: Mit 202 Größtenteils Farbigen Abbildungen; Springer-Lehrbuch; Springer: Berlin, Germany, 2019; ISBN 978-3-662-56461-5. [Google Scholar]
- Caligiore, D.; Pezzulo, G.; Baldassarre, G.; Bostan, A.C.; Strick, P.L.; Doya, K.; Helmich, R.C.; Dirkx, M.; Houk, J.; Jörntell, H.; et al. Consensus Paper: Towards a Systems-Level View of Cerebellar Function: The Interplay Between Cerebellum, Basal Ganglia, and Cortex. Cerebellum 2017, 16, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.A. Human Motor Control; Elsevier Academic Press: Amsterdam, The Netherlands, 2010; ISBN 978-0-12-374226-1. [Google Scholar]
- Rothwell, J.C. Control of Human Voluntary Movement; Aspen Publishers: Rockville, MD, USA, 1987; ISBN 978-0-87189-311-6. [Google Scholar]
- Albus, J.S. A Theory of Cerebellar Function. Math. Biosci. 1971, 10, 25–61. [Google Scholar] [CrossRef]
- Ashe, J.; Bushara, K. The Olivo-Cerebellar System as a Neural Clock. In Neurobiology of Interval Timing; Merchant, H., de Lafuente, V., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 829, pp. 155–165. ISBN 978-1-4939-1781-5. [Google Scholar]
- Lawrenson, C.; Bares, M.; Kamondi, A.; Kovács, A.; Lumb, B.; Apps, R.; Filip, P.; Manto, M. The Mystery of the Cerebellum: Clues from Experimental and Clinical Observations. Cerebellum Ataxias 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef]
- Ivry, R.B. Exploring the Role of the Cerebellum in Sensory Anticipation and Timing: Commentary on Tesche and Karhu. Hum. Brain Mapp. 2000, 9, 115–118. [Google Scholar] [CrossRef]
- Bengtsson, F.; Ekerot, C.-F.; Jörntell, H. In Vivo Analysis of Inhibitory Synaptic Inputs and Rebounds in Deep Cerebellar Nuclear Neurons. PLoS ONE 2011, 6, e18822. [Google Scholar] [CrossRef]
- Lang, E.J.; Apps, R.; Bengtsson, F.; Cerminara, N.L.; De Zeeuw, C.I.; Ebner, T.J.; Heck, D.H.; Jaeger, D.; Jörntell, H.; Kawato, M.; et al. The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum 2017, 16, 230–252. [Google Scholar] [CrossRef]
- Groenewegen, H.J. The Basal Ganglia and Motor Control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef]
- Jörntell, H. Cerebellar Physiology: Links between Microcircuitry Properties and Sensorimotor Functions: Cerebellar Physiology. J. Physiol. 2017, 595, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Finch, D.M.; Olson, C.R. Functional Heterogeneity in Cingulate Cortex: The Anterior Executive and Posterior Evaluative Regions. Cereb. Cortex 1992, 2, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Nimchinsky, E.A.; Vogt, L.J.; Hof, P.R. Human Cingulate Cortex: Surface Features, Flat Maps, and Cytoarchitecture. J. Comp. Neurol. 1995, 359, 490–506. [Google Scholar] [CrossRef]
- Doya, K. Complementary Roles of Basal Ganglia and Cerebellum in Learning and Motor Control. Curr. Opin. Neurobiol. 2000, 10, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. The Cerebellum in Parkinson’s Disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.A.; Hintzen, A.; Goldau, M.; von Cramon, D.Y.; Timmermann, L.; Tittgemeyer, M. Cerebellar Networks with Basal Ganglia: Feasibility for Tracking Cerebello-Pallidal and Subthalamo-Cerebellar Projections in the Human Brain. Eur. J. Neurosci. 2013, 38, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Wilson, C.J. Chapter II The Basal Ganglia. In Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 1996; Volume 12, pp. 371–468. ISBN 978-0-444-82451-6. [Google Scholar]
- Wise, S.P.; Murray, E.A.; Gerfen, C.R. The Frontal Cortex-Basal Ganglia System in Primates. Crit. Rev. Neurobiol. 1996, 10, 317–356. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, C.J.; Kinsella, G.J.; Storey, E. The Cerebellum and Neuropsychological Functioning: A Critical Review. J. Clin. Exp. Neuropsychol. 2012, 34, 35–56. [Google Scholar] [CrossRef]
- Schlerf, J.E.; Galea, J.M.; Bastian, A.J.; Celnik, P.A. Dynamic Modulation of Cerebellar Excitability for Abrupt, But Not Gradual, Visuomotor Adaptation. J. Neurosci. 2012, 32, 11610–11617. [Google Scholar] [CrossRef]
- Jueptner, M.; Ottinger, S.; Fellows, S.J.; Adamschewski, J.; Flerich, L.; Müller, S.P.; Diener, H.C.; Thilmann, A.F.; Weiller, C. The Relevance of Sensory Input for the Cerebellar Control of Movements. NeuroImage 1997, 5, 41–48. [Google Scholar] [CrossRef]
- Welsh, J.P.; Lang, E.J.; Suglhara, I.; Llinás, R. Dynamic Organization of Motor Control within the Olivocerebellar System. Nature 1995, 374, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Vitek, J.L.; Ashe, J.; DeLong, M.R.; Alexander, G.E. Physiologic Properties and Somatotopic Organization of the Primate Motor Thalamus. J. Neurophysiol. 1994, 71, 1498–1513. [Google Scholar] [CrossRef]
- Sherman, S. Thalamus. Scholarpedia 2006, 1, 1583. [Google Scholar] [CrossRef]
- Haber, S.N.; Calzavara, R. The Cortico-Basal Ganglia Integrative Network: The Role of the Thalamus. Brain Res. Bull. 2009, 78, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.A. The Role of the Thalamus in Motor Control. Curr. Opin. Neurobiol. 2003, 13, 663–670. [Google Scholar] [CrossRef]
- Pruszynski, J.A.; Scott, S.H. Optimal Feedback Control and the Long-Latency Stretch Response. Exp. Brain Res. 2012, 218, 341–359. [Google Scholar] [CrossRef]
- Dickenson, A.H. Editorial I. Br. J. Anaesth. 2002, 88, 755–757. [Google Scholar] [CrossRef]
- Bueti, D.; Walsh, V.; Frith, C.; Rees, G. Different Brain Circuits Underlie Motor and Perceptual Representations of Temporal Intervals. J. Cogn. Neurosci. 2008, 20, 204–214. [Google Scholar] [CrossRef]
- D’Angelo, E. Physiology of the Cerebellum. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 154, pp. 85–108. ISBN 978-0-444-63956-1. [Google Scholar]
- Rao, S.M.; Harrington, D.L.; Haaland, K.Y.; Bobholz, J.A.; Cox, R.W.; Binder, J.R. Distributed Neural Systems Underlying the Timing of Movements. J. Neurosci. 1997, 17, 5528–5535. [Google Scholar] [CrossRef]
- Lang, E.J.; Sugihara, I.; Welsh, J.P.; Llinás, R. Patterns of Spontaneous Purkinje Cell Complex Spike Activity in the Awake Rat. J. Neurosci. 1999, 19, 2728–2739. [Google Scholar] [CrossRef]
- Manning, C.D.; Tolhurst, S.A.; Bawa, P. Proprioceptive Reaction Times and Long-Latency Reflexes in Humans. Exp. Brain Res. 2012, 221, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Timmermann, L.; Kujala, J.; Dirks, M.; Schmitz, F.; Salmelin, R.; Schnitzler, A. The Neural Basis of Intermittent Motor Control in Humans. Proc. Natl. Acad. Sci. USA 2002, 99, 2299–2302. [Google Scholar] [CrossRef] [PubMed]
- Bays, P.M.; Wolpert, D.M. Computational Principles of Sensorimotor Control That Minimize Uncertainty and Variability: Computational Principles of Sensorimotor Control. J. Physiol. 2007, 578, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S. How Is Grasping Modified by Somatosensory Input? In Motor Control: Concepts and Issues; John Wiley: London, UK, 1991; ISBN 978-0-471-92919-2. [Google Scholar]
- Todorov, E. Optimality Principles in Sensorimotor Control. Nat. Neurosci. 2004, 7, 907–915. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Procedure | M | SD | t or z * | df or n * | p | dz or r * |
|---|---|---|---|---|---|---|---|
| Elbow flexors | |||||||
| MVIC (N) | - | 313.793 | 40.513 | - | - | - | - |
| AFmax (N) | regular | 294.316 | 29.268 | −3.213 | 10 | 0.009 | 0.969 |
| spindle | 318.040 | 41.515 | |||||
| AFisomax (N) | regular | 286.012 | 32.241 | 9.325 | 10 | <0.001 | 2.812 |
| spindle | 197.228 | 47.738 | |||||
| AFosc (N) | regular | 246.187 | 33.040 | −8.950 | 10 | <0.001 | 2.698 |
| spindle | 295.412 | 38.131 | |||||
| ratio AFisomax/AFmax | regular | 0.971 | 0.039 | 2.934 * | 11 * | 0.003 * | 0.885 * |
| spindle | 0.614 | 0.101 | |||||
| ratio AFisomax/MVIC | regular | 0.919 | 0.103 | 7.340 | 10 | <0.001 | 2.213 |
| spindle | 0.626 | 0.123 | |||||
| ratio AFosc/AFmax | regular | 0.834 | 0.043 | −7.455 | 10 | <0.001 | 2.248 |
| spindle | 0.930 | 0.045 | |||||
| ratio AFosc/AFisomax | regular | 0.864 | 0.065 | −2.943 * | 11 * | 0.003 * | 0.885 * |
| spindle | 1.692 | 0.599 | |||||
| slope (lg(N/s)) | regular | 2.020 | 0.207 | −1.511 * | 11 * | 0.131 | - |
| spindle | 2.069 | 0.205 | |||||
| Hip flexors | |||||||
| MVIC (N) | - | 311.525 | 44.415 | - | - | - | - |
| AFmax (N) | regular | 295.816 | 38.929 | −5.150 | 11 | <0.001 | 1.487 |
| spindle | 327.257 | 52.307 | |||||
| AFisomax (N) | regular | 289.512 | 46.788 | 10.028 | 11 | <0.001 | 2.895 |
| spindle | 209.156 | 50.657 | |||||
| AFosc (N) | regular | 230.325 | 34.084 | −9.700 | 11 | <0.001 | 2.800 |
| spindle | 275.344 | 25.778 | |||||
| ratio AFisomax/AFmax | regular | 0.978 | 0.074 | 3.059 * | 12 * | 0.002 * | 0.883 * |
| spindle | 0.637 | 0.105 | |||||
| ratio AFisomax/MVIC | regular | 0.932 | 0.104 | 9.231 | 11 | <0.001 | 2.478 |
| spindle | 0.670 | 0.128 | |||||
| ratio AFosc/AFmax | regular | 0.779 | 0.044 | −3.039 | 11 | 0.011 | 0.877 |
| spindle | 0.851 | 0.063 | |||||
| ratio AFosc/AFisomax | regular | 0.805 | 0.084 | −3.059 * | 12 * | 0.002 * | 0.883 * |
| spindle | 1.398 | 0.292 | |||||
| slope [lg(N/s)] | regular | 2.097 | 0.083 | 0.038 | 11 | 0.970 | - |
| spindle | 2.096 | 0.108 | |||||
| Muscle | Rating Tester | Number MMTs | AFisomax/AFmax | |
|---|---|---|---|---|
| n | M | CVintra 1 | ||
| Elbow flexors | stable | 33 | 0.97 | 0.05 |
| unstable | 33 | 0.61 | 0.24 | |
| unclear | - | - | - | |
| Hip flexors | stable | 35 | 0.99 | 0.02 |
| unstable | 36 | 0.64 | 0.13 | |
| unclear | 1 | 0.53 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bittmann, F.N.; Dech, S.; Schaefer, L.V. Another Way to Confuse Motor Control: Manual Technique Supposed to Shorten Muscle Spindles Reduces the Muscular Holding Stability in the Sense of Adaptive Force in Male Soccer Players. Brain Sci. 2023, 13, 1105. https://doi.org/10.3390/brainsci13071105
Bittmann FN, Dech S, Schaefer LV. Another Way to Confuse Motor Control: Manual Technique Supposed to Shorten Muscle Spindles Reduces the Muscular Holding Stability in the Sense of Adaptive Force in Male Soccer Players. Brain Sciences. 2023; 13(7):1105. https://doi.org/10.3390/brainsci13071105
Chicago/Turabian StyleBittmann, Frank N., Silas Dech, and Laura V. Schaefer. 2023. "Another Way to Confuse Motor Control: Manual Technique Supposed to Shorten Muscle Spindles Reduces the Muscular Holding Stability in the Sense of Adaptive Force in Male Soccer Players" Brain Sciences 13, no. 7: 1105. https://doi.org/10.3390/brainsci13071105
APA StyleBittmann, F. N., Dech, S., & Schaefer, L. V. (2023). Another Way to Confuse Motor Control: Manual Technique Supposed to Shorten Muscle Spindles Reduces the Muscular Holding Stability in the Sense of Adaptive Force in Male Soccer Players. Brain Sciences, 13(7), 1105. https://doi.org/10.3390/brainsci13071105






