A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. The Treatment of Aspirin and Bromodeoxyuridine
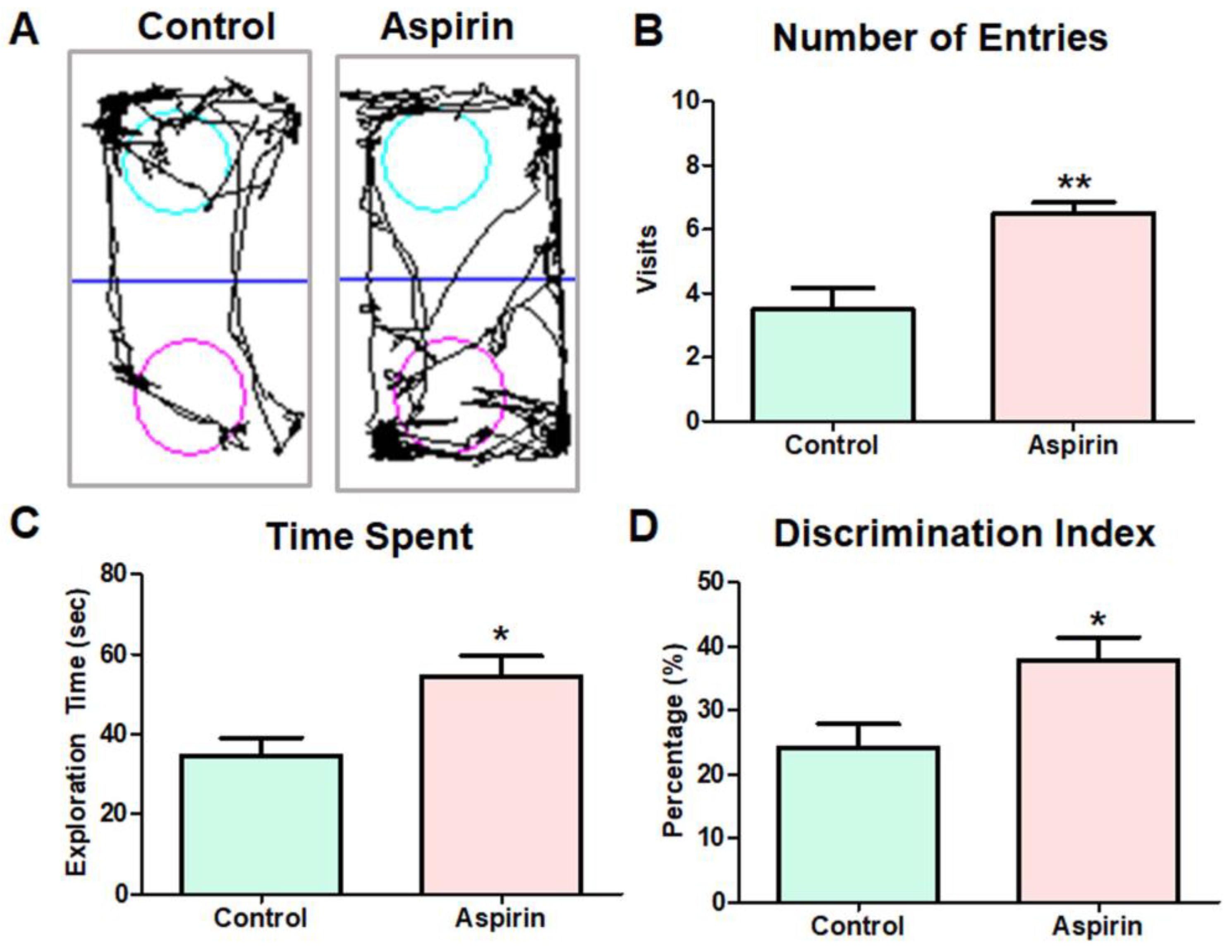
2.3. Novel Object Recognition Test
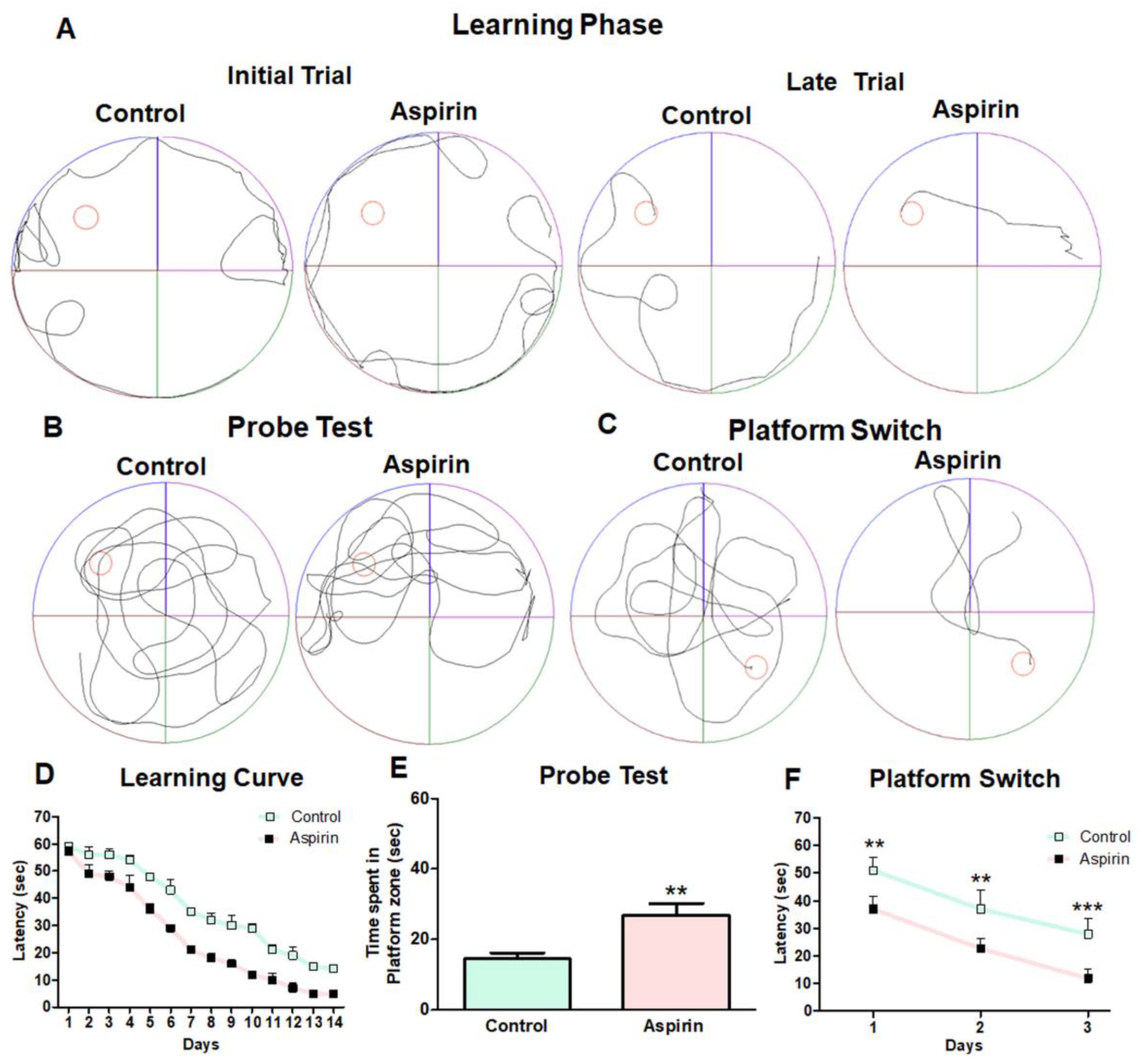
2.4. Morris Water Maze
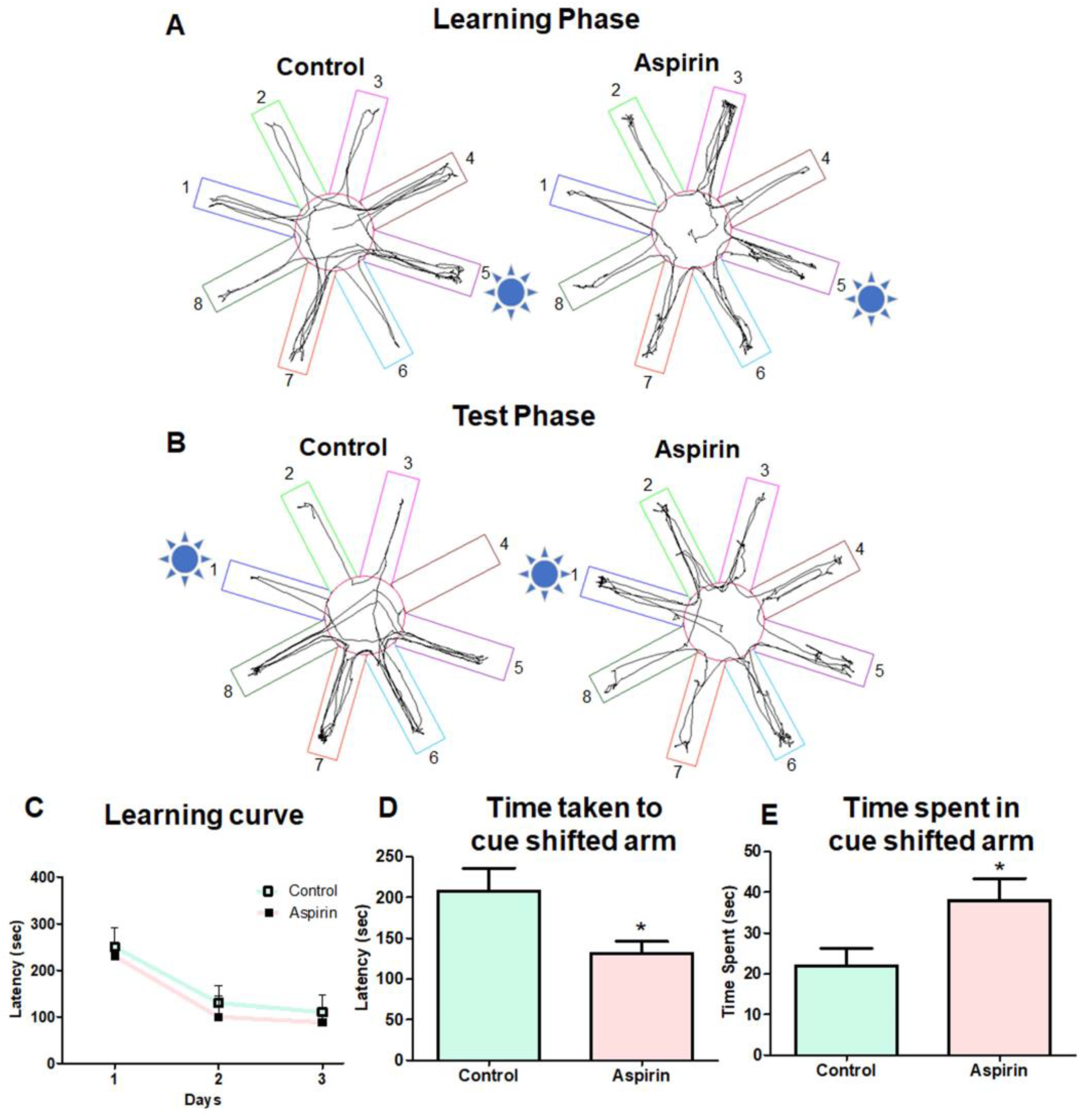
2.5. Cued Radial Arm Maze Paradigm
2.6. Animal Perfusion and Cryosection of the Brains
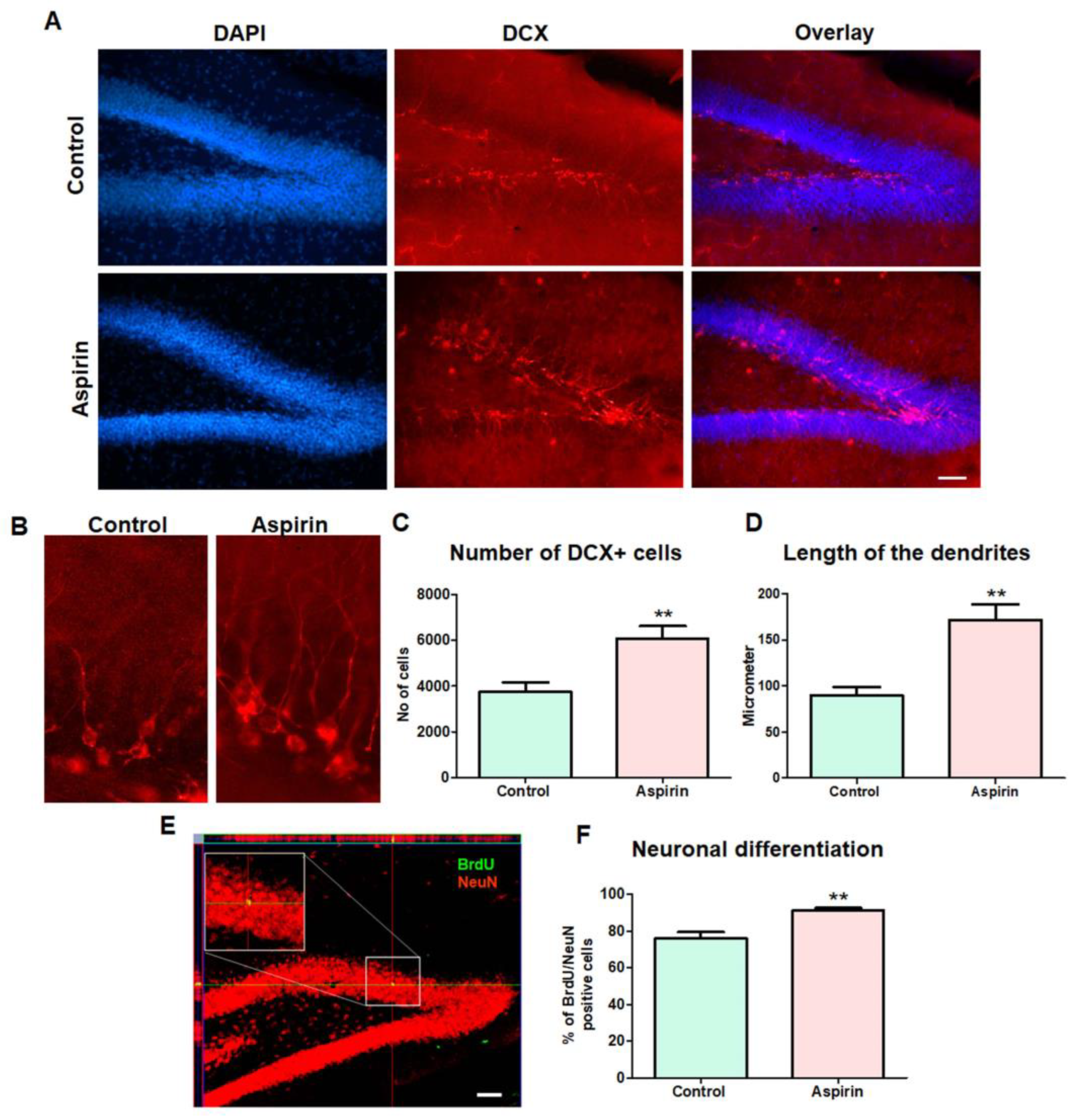
2.7. Immunohistochemistry and Assessment of Neurogenesis
2.8. Estimation of Acetylcholinesterase Activity in the Blood
2.9. Statistical Analyses
3. Results
3.1. Aspirin Treatment Improved Novel Object Recognition in Experimental Mice
3.2. Aspirin Treatment Enhanced Spatial Learning and Working Memory in the Morris Water Maze Task
3.3. Aspirin Treatment Improved Working Memory in Cued RAM
3.4. Aspirin Treatment Increased the Hippocampal Neurogenesis
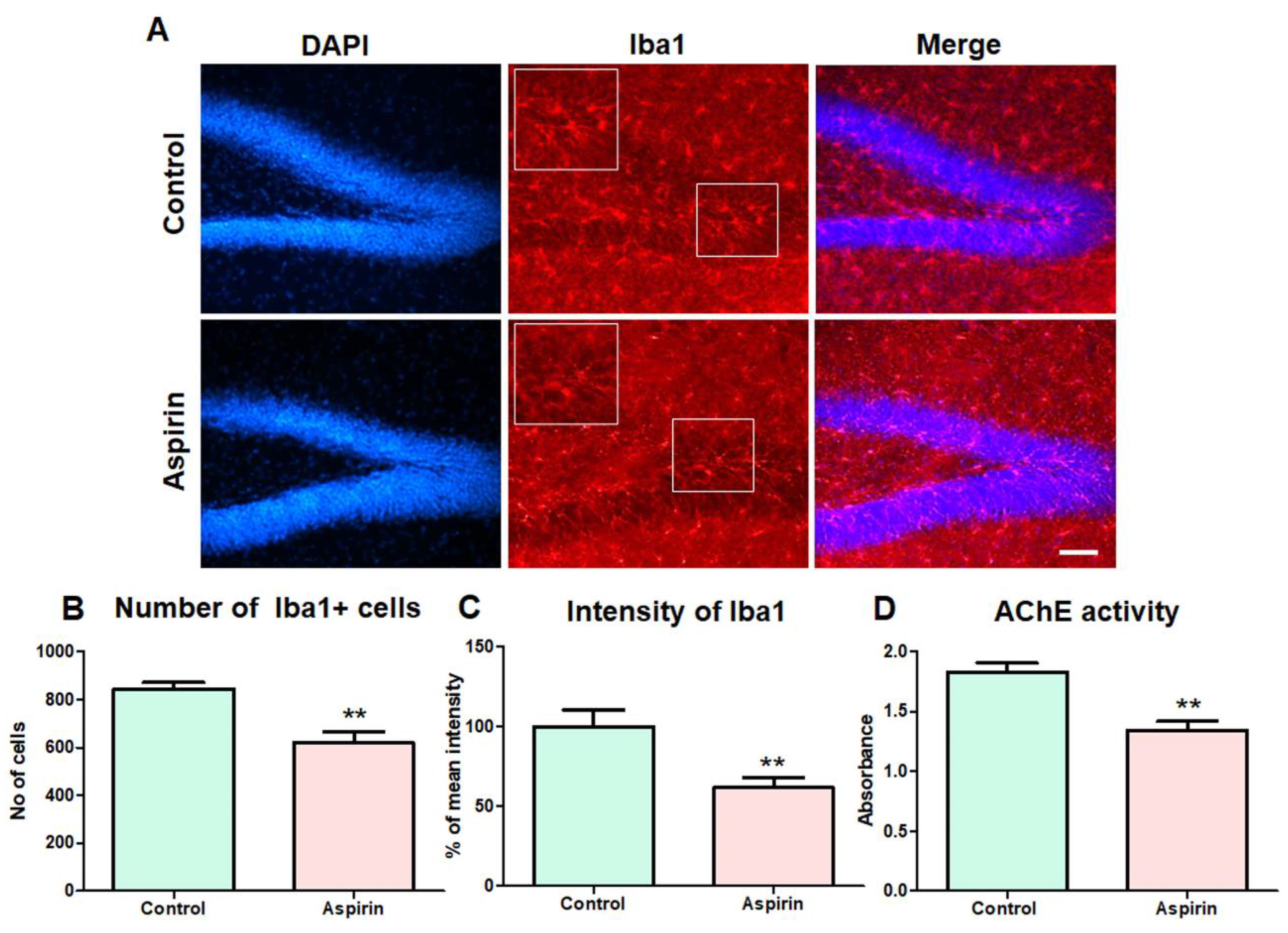
3.5. Aspirin Treatment Decreased the Abnormal Number of Microglial Cells in the Hippocampus and Reduced the Activity of AChE in the Blood of the Experimental Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Meek, I.L.; Van de Laar, M.A.; Vonkeman, H.E. Non-Steroidal Anti-Inflammatory Drugs: An Overview of Cardiovascular Risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef]
- Ittaman, S.V.; VanWormer, J.J.; Rezkalla, S.H. The Role of Aspirin in the Prevention of Cardiovascular Disease. Clin. Med. Res. 2014, 12, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Aïd, S.; Bosetti, F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie 2011, 93, 46–51. [Google Scholar] [CrossRef]
- Minghetti, L. Role of COX-2 in Inflammatory and Degenerative Brain Diseases. In Inflammation in the Pathogenesis of Chronic Diseases; Springer: Dordrecht, The Netherlands, 2007; Volume 42, pp. 127–141. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhang, X.; Ma, X.-C.; Zhang, R.-W. Aspirin Use on Incident Dementia and Mild Cognitive Decline: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 12, 578071. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Zhao, G.; Weng, L.; Guan, J.; Initiative, F.T.A.D.N. Aspirin using was associated with slower cognitive decline in patients with Alzheimer’s disease. PLoS ONE 2021, 16, e0252969. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Roy, A.; Kundu, M.; Jana, M.; Luan, C.-H.; Gonzalez, F.J.; Pahan, K. Aspirin binds to PPARα to stimulate hippocampal plasticity and protect memory. Proc. Natl. Acad. Sci. USA 2018, 115, E7408–E7417. [Google Scholar] [CrossRef]
- Gorenflo, M.P.; Davis, P.B.; Kendall, E.K.; Olaker, V.R.; Kaelber, D.C.; Xu, R. Association of Aspirin Use with Reduced Risk of Developing Alzheimer’s Disease in Elderly Ischemic Stroke Patients: A Retrospective Cohort Study. J. Alzheimer’s Dis. 2023, 91, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Hou, Y.; Huang, S.; Pei, G. Alzheimer’s Amyloid-β Accelerates Human Neuronal Cell Senescence Which Could Be Rescued by Sirtuin-1 and Aspirin. Front. Cell. Neurosci. 2022, 16, 906270. [Google Scholar] [CrossRef]
- Kandasamy, M.; Anusuyadevi, M.; Aigner, K.M.; Unger, M.S.; Kniewallner, K.M.; de Sousa, D.M.B.; Altendorfer, B.; Mrowetz, H.; Bogdahn, U.; Aigner, L. TGF-β Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia? Aging Dis. 2020, 11, 828–850. [Google Scholar] [CrossRef]
- Surya, K.; Manickam, N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 1–17, preprint. [Google Scholar] [CrossRef] [PubMed]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, D.; Cryan, J.F.; Nolan, Y.M. Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell 2019, 18, e13007. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Hollands, C. Hippocampal neurogenesis: Learning to remember. Prog. Neurobiol. 2016, 138–140, 1–18. [Google Scholar] [CrossRef]
- Schouten, M.; Buijink, M.R.; Lucassen, P.J.; Fitzsimons, C.P. New Neurons in Aging Brains: Molecular Control by Small Non-Coding RNAs. Front. Neurosci. 2012, 6, 25. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef]
- Chan, S.; Brophy, M.; Nishimura, N.; Schaffer, C.B. Aspirin treatment does not increase microhemorrhage size in young or aged mice. PLoS ONE 2019, 14, e0204295. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Yesudhas, A.; Roshan, S.A.; Radhakrishnan, R.K.; Abirami, G.P.P.; Manickam, N.; Selvaraj, K.; Elumalai, G.; Shanmugaapriya, S.; Anusuyadevi, M.; Kandasamy, M. Intramuscular Injection of BOTOX® Boosts Learning and Memory in Adult Mice in Association with Enriched Circulation of Platelets and Enhanced Density of Pyramidal Neurons in the Hippocampus. Neurochem. Res. 2020, 45, 2856–2867. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Assessing Spatial Learning and Memory in Rodents. ILAR J. 2014, 55, 310–332. [Google Scholar] [CrossRef]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Stafstrom, C.E. Behavioral and Cognitive Testing Procedures in Animal Models of Epilepsy. In Models of Sei-zures and Epilepsy; Pitkänen, A., Schwartzkroin, P.A., Moshé, S.L., Eds.; Academic Press: Burlington, NJ, USA, 2006; Chapter 49; pp. 613–628. ISBN 978-0-12-088554-1. [Google Scholar]
- Kandasamy, M.; Couillard-Despres, S.; Raber, K.A.; Stephan, M.; Lehner, B.; Winner, B.; Kohl, Z.; Rivera, F.J.; Nguyen, H.P.; Riess, O.; et al. Stem Cell Quiescence in the Hippocampal Neurogenic Niche Is Associated with Elevated Transforming Growth Factor-β Signaling in an Animal Model of Huntington Disease. J. Neuropathol. Exp. Neurol. 2010, 69, 717–728. [Google Scholar] [CrossRef]
- Selvaraj, D.B.; Vergil Andrews, J.F.; Anusuyadevi, M.; Kandasamy, M. Ranitidine Alleviates Anxiety-like Behaviors and Improves the Density of Pyramidal Neurons upon Deactivation of Microglia in the CA3 Region of the Hippocampus in a Cysteamine HCl-Induced Mouse Model of Gastrointestinal Disorder. Brain Sci. 2023, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Woitke, F.; Blank, A.; Fleischer, A.-L.; Zhang, S.; Lehmann, G.-M.; Broesske, J.; Haase, M.; Redecker, C.; Schmeer, C.W.; Keiner, S. Post-Stroke Environmental Enrichment Improves Neurogenesis and Cognitive Function and Reduces the Generation of Aberrant Neurons in the Mouse Hippocampus. Cells 2023, 12, 652. [Google Scholar] [CrossRef]
- Pei, P.; Cui, S.; Zhang, S.; Hu, S.; Wang, L.; Yang, W. Effect of Electroacupuncture at Fengchi on Facial Allodynia, Microglial Activation, and Microglia–Neuron Interaction in a Rat Model of Migraine. Brain Sci. 2022, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Wang, X.; Cui, Y.; Cao, Y.; Zhao, Z.; Zhang, J. Metabolic reprogramming mediates hippocampal microglial M1 polarization in response to surgical trauma causing perioperative neurocognitive disorders. J. Neuroinflammation 2021, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Lehner, B.; Kraus, S.; Sander, P.R.; Marschallinger, J.; Rivera, F.J.; Trümbach, D.; Ueberham, U.; Reitsamer, H.A.; Strauss, O.; et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med. 2014, 18, 1444–1459. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.-G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in Aging and Alzheimer’s Disease: A Comparative Species Review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a Biomarker in Environmental and Occupational Medicine: New Insights and Future Perspectives. BioMed Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef]
- Kandasamy, M.; Radhakrishnan, R.K.; Poornimai Abirami, G.P.; Roshan, S.A.; Yesudhas, A.; Balamuthu, K.; Prahalathan, C.; Shanmugaapriya, S.; Moorthy, A.; Essa, M.M.; et al. Possible Existence of the Hypothalamic-Pituitary-Hippocampal (HPH) Axis: A Reciprocal Relationship Between Hippocampal Specific Neuroestradiol Synthesis and Neuroblastosis in Ageing Brains with Special Reference to Menopause and Neurocognitive Disorders. Neurochem. Res. 2019, 44, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.K.; Kandasamy, M. SARS-CoV-2-Mediated Neuropathogenesis, Deterioration of Hippocampal Neurogenesis and Dementia. Am. J. Alzheimer’s Dis. Other Dementiasr 2022, 37. [Google Scholar] [CrossRef] [PubMed]
- Roshan, S.A.; Elangovan, G.; Gunaseelan, D.; Jayachandran, S.K.; Kandasamy, M.; Anusuyadevi, M. Pathogenomic Signature and Aberrant Neurogenic Events in Experimental Cerebral Ischemic Stroke: A Neurotranscriptomic-Based Implication for Dementia. J. Alzheimer’s Dis. 2023, 1–20, preprint. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, L.; Ai, G.; Spitale, R.C.; Bhat, G.J. Molecular targets of aspirin and cancer prevention. Br. J. Cancer 2014, 111, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Alsop, R.J.; Toppozini, L.; Marquardt, D.; Kučerka, N.; Harroun, T.A.; Rheinstädter, M.C. Aspirin inhibits formation of cholesterol rafts in fluid lipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 805–812. [Google Scholar] [CrossRef]
- Mo, C.; Sun, G.; Lu, M.-L.; Zhang, L.; Wang, Y.-Z.; Sun, X.; Yang, Y.-S. Proton pump inhibitors in prevention of low-dose aspirin-associated upper gastrointestinal injuries. World J. Gastroenterol. 2015, 21, 5382–5392. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Binzen, U.; Mörz, H.; Bugert, P.; Schedel, A.; Treede, R.-D.; Greffrath, W. Acetylsalicylic acid enhances tachyphylaxis of repetitive capsaicin responses in TRPV1-GFP expressing HEK293 cells. Neurosci. Lett. 2014, 563, 101–106. [Google Scholar] [CrossRef]
- Peng, B.-G.; Chen, S.; Lin, X. Aspirin selectively augmented N-methyl-d-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci. Lett. 2003, 343, 21–24. [Google Scholar] [CrossRef]
- Fujikawa, I.; Ando, T.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ochiai, T.; Suzuki-Karasaki, Y. Aspirin Induces Mitochondrial Ca2+ Remodeling in Tumor Cells via ROS–Depolarization–Voltage-Gated Ca2+ Entry. Int. J. Mol. Sci. 2020, 21, 4771. [Google Scholar] [CrossRef]
- Patel, D.; Roy, A.; Pahan, K. PPARα serves as a new receptor of aspirin for neuroprotection. J. Neurosci. Res. 2019, 98, 626–631. [Google Scholar] [CrossRef]
- Wang, M.; Yu, H.; Li, Z.; Gong, D.; Liu, X. Benefits and Risks Associated with Low-Dose Aspirin Use for the Primary Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Control Trials and Trial Sequential Analysis. Am. J. Cardiovasc. Drugs 2022, 22, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Gelbenegger, G.; Postula, M.; Pecen, L.; Halvorsen, S.; Lesiak, M.; Schoergenhofer, C.; Jilma, B.; Hengstenberg, C.; Siller-Matula, J.M. Aspirin for primary prevention of cardiovascular disease: A meta-analysis with a particular focus on subgroups. BMC Med. 2019, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Alberts, M.J.; Bergman, D.L.; Molner, E.; Jovanovic, B.D.; Ushiwata, I.; Teruya, J. Antiplatelet Effect of Aspirin in Patients with Cerebrovascular Disease. Stroke 2004, 35, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Dean, O.; Drexhage, H.; McNeil, J.J.; Moylan, S.; O’Neil, A.; Davey, C.G.; Sanna, L.; Maes, M. Aspirin: A review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 2013, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Storey, E.; Murray, A.M.; Woods, R.L.; Wolfe, R.; Reid, C.M.; Nelson, M.R.; Chong, T.T.; Williamson, J.D.; Ward, S.A.; et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 2020, 95, e320–e331. [Google Scholar] [CrossRef]
- Price, J.F.; Stewart, M.C.; Deary, I.J.; Murray, G.D.; Sandercock, P.; Butcher, I.; Fowkes, F.G.R. Low dose aspirin and cognitive function in middle aged to elderly adults: Randomised controlled trial. BMJ 2008, 337, a1198. [Google Scholar] [CrossRef]
- Chang, A.; Chung, N.-C.; Lawther, A.J.; Ziegler, A.I.; Shackleford, D.M.; Sloan, E.K.; Walker, A.K. The Anti-Inflammatory Drug Aspirin Does Not Protect Against Chemotherapy-Induced Memory Impairment by Paclitaxel in Mice. Front. Oncol. 2020, 10, 564965. [Google Scholar] [CrossRef]
- Smith, J.W.; Al-Khamees, O.; Costall, B.; Naylor, R.J.; Smythe, J.W. Chronic aspirin ingestion improves spatial learning in adult and aged rats. Pharmacol. Biochem. Behav. 2002, 71, 233–238. [Google Scholar] [CrossRef]
- Rizwan, S.; Idrees, A.; Ashraf, M.; Ahmed, T. Memory-enhancing effect of aspirin is mediated through opioid system modulation in an AlCl3-induced neurotoxicity mouse model. Exp. Ther. Med. 2016, 11, 1961–1970. [Google Scholar] [CrossRef]
- Rivi, V.; Batabyal, A.; Benatti, C.; Tascedda, F.; Blom, J.M.C.; Lukowiak, K. Aspirin reverts lipopolysaccharide-induced learning and memory impairment: First evidence from an invertebrate model system. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.-P.; Ding, W.-Y.; Wang, P. Molecular Mechanism of Acetylsalicylic Acid in Improving Learning and Memory Im-pairment in APP/PS1 Transgenic Mice by Inhibiting the Abnormal Cell Cycle Re-Entry of Neurons. Front. Mol. Neurosci. 2022, 15, 1006216. [Google Scholar]
- Willis, C.M.; Nicaise, A.M.; Krzak, G.; Ionescu, R.-B.; Pappa, V.; D’Angelo, A.; Agarwal, R.; Repollés-De-Dalmau, M.; Peruzzotti-Jametti, L.; Pluchino, S. Soluble factors influencing the neural stem cell niche in brain physiology, inflammation, and aging. Exp. Neurol. 2022, 355, 114124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Wu, Y.; Li, L.-Y.; Zheng, J.; Liu, R.-G.; Zhou, J.-P.; Yuan, S.-Y.; Shang, Y.; Yao, S.-L. Aspirin-triggered lipoxin A4attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J. Neuroinflammation 2011, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Romano, M. Lipoxin and Aspirin-Triggered Lipoxins. Sci. World J. 2010, 10, 1048–1064. [Google Scholar] [CrossRef]
- Svensson, C.I.; Zattoni, M.; Serhan, C.N. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 2007, 204, 245–252. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Xie, J.; Jiang, X.; Xiao, B.; Hu, X.; Xiang, J. Aspirin attenuates liver fibrosis by suppressing TGF-β1/Smad signaling. Mol. Med. Rep. 2022, 25, 181. [Google Scholar] [CrossRef]
- Redondo, S.; Santos-Gallego, C.G.; Ganado, P.; García, M.; Rico, L.; Del Rio, M.; Tejerina, T. Acetylsalicylic Acid Inhibits Cell Proliferation by Involving Transforming Growth Factor-β. Circulation 2003, 107, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Pozzoli, G.; Petrucci, G.; Navarra, P.; Marei, H.E.; Cenciarelli, C. Aspirin inhibits proliferation and promotes differentiation of neuroblastoma cells via p21 Waf1 protein up-regulation and Rb1 pathway modulation. J. Cell. Mol. Med. 2019, 23, 7078–7087. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Li, Z.; Guo, J.; Li, M. Aspirin is Involved in the Cell Cycle Arrest, Apoptosis, Cell Migration, and Invasion of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2029. [Google Scholar] [CrossRef]
- Wu, L.; Luo, Z.; Liu, Y.; Jia, L.; Jiang, Y.; Du, J.; Guo, L.; Bai, Y.; Liu, Y. Aspirin inhibits RANKL-induced osteoclast differentiation in dendritic cells by suppressing NF-κB and NFATc1 activation. Stem Cell Res. Ther. 2019, 10, 375. [Google Scholar] [CrossRef]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Dioli, C.; Patrício, P.; Sousa, N.; Kokras, N.; Dalla, C.; Guerreiro, S.; Santos-Silva, M.A.; Rego, A.C.; Pinto, L.; Ferreiro, E.; et al. Chronic stress triggers divergent dendritic alterations in immature neurons of the adult hippocampus, depending on their ultimate terminal fields. Transl. Psychiatry 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.-Y.; Li, A.; So, K.-F. Involvement of Adult Hippocampal Neurogenesis in Learning and Forgetting. Neural Plast. 2015, 2015, 717958. [Google Scholar] [CrossRef] [PubMed]
- Thong, E.H.; Lee, E.C.Y.; Yun, C.-Y.; Li, T.Y.W.; Sia, C.-H. Aspirin Therapy, Cognitive Impairment, and Dementia—A Review. Futur. Pharmacol. 2023, 3, 144–161. [Google Scholar] [CrossRef]
- Davis, K.A.S.; Bishara, D.; Molokhia, M.; Mueller, C.; Perera, G.; Stewart, R.J. Aspirin in people with dementia, long-term benefits, and harms: A systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 943–954. [Google Scholar] [CrossRef]
- Persegani, C.; Russo, P.; Lugaresi, E.; Nicolini, M.; Torlini, M. Neuroprotective effects of low-doses of aspirin. Hum. Psychopharmacol. Clin. Exp. 2001, 16, 193–194. [Google Scholar] [CrossRef]
- Ulubaş, B.; Çımen, M.Y.B.; Apa, D.D.; Saritaş, E.; Muşlu, N.; Çımen, B. The Protective Effects of Acetylsalicylic Acid on Free Radical Production in Cisplatin Induced Nephrotoxicity: An Experimental Rat Model. Drug Chem. Toxicol. 2003, 26, 259–270. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Hao, Z.; Liu, A.; Li, X.; Li, H.; Xia, N.; Wang, Z.; Zhang, Z.; Bai, J.; et al. Aspirin ameliorates the cognition impairment in mice following benzo[a]pyrene treatment via down-regulating BDNF IV methylation. Neurotoxicology 2022, 89, 20–30. [Google Scholar] [CrossRef]
- Grilli, M.; Pizzi, M.; Memo, M.; Spano, P. Neuroprotection by Aspirin and Sodium Salicylate through Blockade of NF-κB Activation. Science 1996, 274, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Chen, D.; Chen, T.; Sun, X. Aspirin Exerts Neuroprotective Effects by Reversing Lipopolysaccharide-Induced Secondary Brain Injury and Inhibiting Matrix Metalloproteinase-3 Gene Expression. Dis. Markers 2021, 2021, 3682034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.M.; Chen, L.-J.; Trares, K.; Stocker, H.; Holleczek, B.; Beyreuther, K.; Brenner, H.; Schöttker, B. Long-term low-dose acetylsalicylic use shows protective potential for the development of both vascular dementia and Alzheimer’s disease in patients with coronary heart disease but not in other individuals from the general population: Results from two large cohort studies. Alzheimer’s Res. Ther. 2022, 14, 75. [Google Scholar] [CrossRef]
- Chandra, S.; Jana, M.; Pahan, K. Aspirin Induces Lysosomal Biogenesis and Attenuates Amyloid Plaque Pathology in a Mouse Model of Alzheimer’s Disease via PPARα. J. Neurosci. 2018, 38, 6682–6699. [Google Scholar] [CrossRef]
- Evin, G.; Egrave, G.; Evin, Q.-X.L.V. Platelets and Alzheimer’s disease: Potential of APP as a biomarker. World J. Psychiatry 2012, 2, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, L.Y.; Dávila-Rodríguez, J.; Rivera-Aponte, D.E.; Zueva, L.V.; Washington, A.V.; Sanabria, P.; Inyushin, M.Y. Platelets are responsible for the accumulation of β-amyloid in blood clots inside and around blood vessels in mouse brain after thrombosis. Brain Res. Bull. 2016, 128, 98–105. [Google Scholar] [CrossRef]
- E Hasselmo, M. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Chang, Q.; Gold, P.E. Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiol. Learn. Mem. 2008, 89, 167–177. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Bawaskar, H.S.; Bawaskar, P.H.; Bawaskar, P.H. RBC acetyl cholinesterase: A poor man’s early diagnostic biomarker for familial alzheimer’s and Parkinson’s disease dementia. J. Neurosci. Rural. Pract. 2015, 6, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease: Getting On and Staying On. Curr. Ther. Res. 2003, 64, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Enos, J.; Muñoz-Núñez, E.; Gutiérrez, M.; Quiroz-Carreño, S.; Pastene-Navarrete, E.; Céspedes Acuña, C. Dyhidro-β-agarofurans natural and synthetic as acetylcholinesterase and COX inhibitors: Interaction with the peripheral anionic site (AChE-PAS), and anti-inflammatory potentials. J. Enzym. Inhib. Med. Chem. 2022, 37, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, F.H.; Han, B.; Zhang, L.M. Long-term but Not Short-term Aspirin Treatment Attenuates Diabetes-associated Learning and Memory Decline in Mice. Exp. Clin. Endocrinol. Diabetes 2010, 119, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, A.; David, D.C. Molecular modeling and docking analysis of aspirin with pde7b in the Context of Neuro-Inflammation. Bioinformation 2020, 16, 183–188. [Google Scholar] [CrossRef]
- Kwon, K.J.; Kim, M.K.; Lee, E.J.; Kim, J.N.; Choi, B.-R.; Kim, S.Y.; Cho, K.S.; Han, J.-S.; Kim, H.Y.; Shin, C.Y.; et al. Effects of donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a rat model of vascular dementia. J. Neurol. Sci. 2014, 347, 66–77. [Google Scholar] [CrossRef]
- Yerman, T.; Gan, W.Q.; Sin, D.D. The influence of gender on the effects of aspirin in preventing myocardial infarction. BMC Med. 2007, 5, 29. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergil Andrews, J.F.; Selvaraj, D.B.; Kumar, A.; Roshan, S.A.; Anusuyadevi, M.; Kandasamy, M. A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice. Brain Sci. 2023, 13, 1108. https://doi.org/10.3390/brainsci13071108
Vergil Andrews JF, Selvaraj DB, Kumar A, Roshan SA, Anusuyadevi M, Kandasamy M. A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice. Brain Sciences. 2023; 13(7):1108. https://doi.org/10.3390/brainsci13071108
Chicago/Turabian StyleVergil Andrews, Jemi Feiona, Divya Bharathi Selvaraj, Akshay Kumar, Syed Aasish Roshan, Muthuswamy Anusuyadevi, and Mahesh Kandasamy. 2023. "A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice" Brain Sciences 13, no. 7: 1108. https://doi.org/10.3390/brainsci13071108
APA StyleVergil Andrews, J. F., Selvaraj, D. B., Kumar, A., Roshan, S. A., Anusuyadevi, M., & Kandasamy, M. (2023). A Mild Dose of Aspirin Promotes Hippocampal Neurogenesis and Working Memory in Experimental Ageing Mice. Brain Sciences, 13(7), 1108. https://doi.org/10.3390/brainsci13071108






