Remote Assessment of Parkinson’s Disease Patients Amidst the COVID-19 Lockdown in Mexico
Abstract
1. Introduction
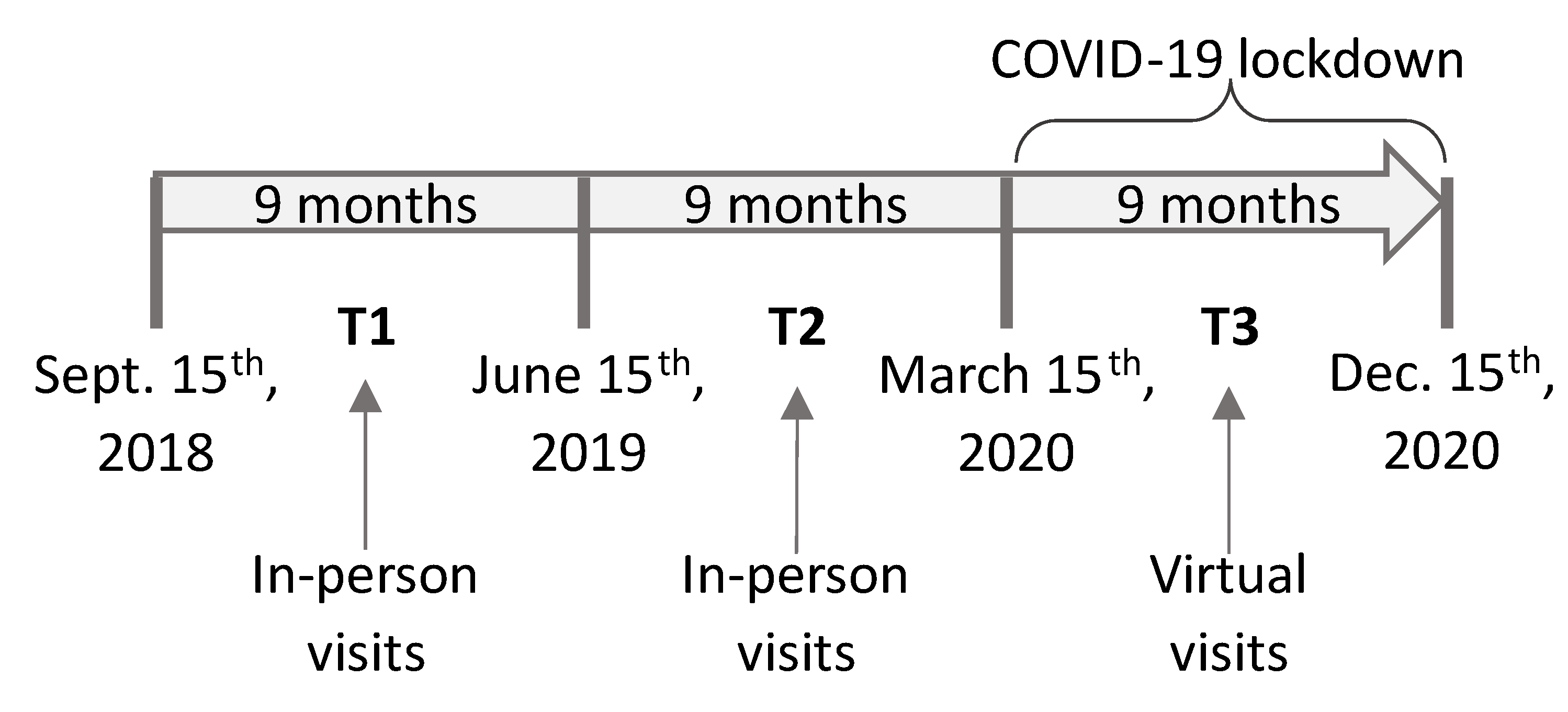
2. Materials and Methods
2.1. Participants
2.2. Virtual Visits
2.3. Feasibility and Acceptability Survey
2.4. Assessment of Motor and Non-Motor Symptoms
2.5. Statistical Analyses
3. Results
3.1. Feasibility and Acceptability of Online Consultations
3.2. Personal Connection and Time Savings
3.3. Impact of COVID-19 Lockdown on PD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Listings of WHO’s Response to COVID-19. Available online: https://www.who.int/news/item/29-06-2020-covidtimeline (accessed on 12 January 2023).
- Suárez, V.; Quezada, M.S.; Ruiz, S.O.; De Jesús, E.R. Epidemiología de COVID-19 en México: Del 27 de febrero al 30 de abril de 2020. Rev. Clin. Esp. 2020, 220, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.M.; Brundin, P.; Fung, V.S.; Kang, U.J.; Burn, D.J.; Colosimo, C.; Chiang, H.-L.; Alcalay, R.N.; Trenkwalder, C.; Committee, M.-S.I. Impact of the COVID-19 pandemic on Parkinson’s disease and movement disorders. Mov. Disord. 2020, 6, 357. [Google Scholar] [CrossRef] [PubMed]
- Bhidayasiri, R.; Virameteekul, S.; Kim, J.-M.; Pal, P.K.; Chung, S.-J. COVID-19: An early review of its global impact and considerations for Parkinson’s disease patient care. J. Mov. Disord. 2020, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Helmich, R.C.; Bloem, B.R. The impact of the COVID-19 pandemic on Parkinson’s disease: Hidden sorrows and emerging opportunities. J. Park. Dis. 2020, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Shalash, A.; Roushdy, T.; Essam, M.; Fathy, M.; Dawood, N.L.; Abushady, E.M.; Elrassas, H.; Helmi, A.; Hamid, E. Mental Health, Physical Activity, and Quality of Life in Parkinson's Disease During COVID-19 Pandemic. Mov. Disord. 2020, 35, 1097–1099. [Google Scholar] [CrossRef]
- Miele, G.; Straccia, G.; Moccia, M.; Leocani, L.; Tedeschi, G.; Bonavita, S.; Lavorgna, L.; Digital Technologies, Web and Social Media Study Group of the Italian Society of Neurology. Telemedicine in Parkinson’s disease: How to ensure patient needs and continuity of care at the time of COVID-19 pandemic. Telemed. E-Health 2020, 26, 1533–1536. [Google Scholar] [CrossRef]
- Wilkinson, J.R.; Spindler, M.; Wood, S.M.; Marcus, S.C.; Weintraub, D.; Morley, J.F.; Stineman, M.G.; Duda, J.E. High patient satisfaction with telehealth in Parkinson disease: A randomized controlled study. Neurol. Clin. Pract. 2016, 6, 241–251. [Google Scholar] [CrossRef]
- Beck, C.A.; Beran, D.B.; Biglan, K.M.; Boyd, C.M.; Dorsey, E.R.; Schmidt, P.N.; Simone, R.; Willis, A.W.; Galifianakis, N.B.; Katz, M. National randomized controlled trial of virtual house calls for Parkinson disease. Neurology 2017, 89, 1152–1161. [Google Scholar] [CrossRef]
- Korn, R.E.; Shukla, A.W.; Katz, M.; Keenan, H.T.; Goldenthal, S.; Auinger, P.; Zhu, W.; Dodge, M.; Rizer, K.; Achey, M.A. Virtual visits for Parkinson disease: A multicenter noncontrolled cohort. Neurol. Clin. Pract. 2017, 7, 283–295. [Google Scholar] [CrossRef]
- Ben-Pazi, H.; Browne, P.; Chan, P.; Cubo, E.; Guttman, M.; Hassan, A.; Hatcher-Martin, J.; Mari, Z.; Moukheiber, E.; Okubadejo, N. The promise of telemedicine for movement disorders: An interdisciplinary approach. Curr. Neurol. Neurosci. Rep. 2018, 18, 26. [Google Scholar] [CrossRef]
- Spear, K.L.; Auinger, P.; Simone, R.; Dorsey, E.; Francis, J. Patient views on telemedicine for Parkinson disease. J. Park. Dis. 2019, 9, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ahn, J.H.; Choi, I.; Mun, J.K.; Cho, J.W.; Youn, J. The changes of exercise pattern and clinical symptoms in patients with Parkinson's disease in the era of COVID-19 pandemic. Park. Relat. Disord. 2020, 80, 148–151. [Google Scholar] [CrossRef]
- van der Heide, A.; Meinders, M.J.; Bloem, B.R.; Helmich, R.C. The impact of the COVID-19 pandemic on psychological distress, physical activity, and symptom severity in Parkinson’s disease. J. Park. Dis. 2020, 10, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Baschi, R.; Luca, A.; Nicoletti, A.; Caccamo, M.; Cicero, C.E.; D’Agate, C.; Di Giorgi, L.; La Bianca, G.; Castro, T.L.; Zappia, M. Changes in Motor, Cognitive, and Behavioral Symptoms in Parkinson’s Disease and Mild Cognitive Impairment During the COVID-19 Lockdown. Front. Psychiatry 2020, 11, 590134. [Google Scholar] [CrossRef] [PubMed]
- HØrmann Thomsen, T.; Wallerstedt, S.M.; Winge, K.; Bergquist, F. Life with Parkinson’s Disease During the COVID-19 Pandemic: The Pressure Is “OFF”. J. Park. Dis. 2021, 11, 491–495. [Google Scholar] [CrossRef]
- Kitani-Morii, F.; Kasai, T.; Horiguchi, G.; Teramukai, S.; Ohmichi, T.; Shinomoto, M.; Fujino, Y.; Mizuno, T. Risk factors for neuropsychiatric symptoms in patients with Parkinson’s disease during COVID-19 pandemic in Japan. PLoS ONE 2021, 16, e0245864. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E. MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- International Parkinson and Movement Disorder Society. Telemedicine in Your Movement Disorders Practice: A Step-by-Step Guide. Available online: https://www.movementdisorders.org/MDS/About/Committees--Other-Groups/Telemedicine-in-Your-Movement-Disorders-Practice-A-Step-by-Step-Guide.htm (accessed on 12 January 2023).
- Cubo, E.; Trejo Gabriel-Galán, J.M.; Seco Martínez, J.; Rioja Alcubilla, C.; Yang, C.; Fernández Arconada, O.; Mariscal Pérez, N. Comparison of office-based versus home web-based clinical assessments for Parkinson's disease. Mov. Disord. 2012, 27, 308–311. [Google Scholar] [CrossRef]
- Hanson, R.E.; Truesdell, M.; Stebbins, G.T.; Weathers, A.L.; Goetz, C.G. Telemedicine vs office visits in a movement disorders clinic: Comparative satisfaction of physicians and patients. Mov. Disord. Clin. Pract. 2019, 6, 65–69. [Google Scholar] [CrossRef]
- Shalash, A.; Fathy, M.; Dawood, N.L.; Hamid, E. Adopting Virtual Visits for Parkinson's Disease Patients During the COVID-19 Pandemic in a Developing Country. Front. Neurol. 2020, 11, 1338. [Google Scholar] [CrossRef]
- Abdolahi, A.; Scoglio, N.; Killoran, A.; Dorsey, E.R.; Biglan, K.M. Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Park. Relat. Disord. 2013, 19, 218–221. [Google Scholar] [CrossRef]
- Falla, M.; Dodich, A.; Papagno, C.; Gober, A.; Narduzzi, P.; Pierotti, E.; Falk, M.; Zappini, F.; Colosimo, C.; Turella, L. Lockdown effects on Parkinson’s disease during COVID-19 pandemic: A pilot study. Acta Neurol. Belg. 2021, 121, 1191–1198. [Google Scholar] [CrossRef]
- Shalash, A.; Helmy, A.; Salama, M.; Gaber, A.; El-Belkimy, M.; Hamid, E. A 6-month longitudinal study on worsening of Parkinson’s disease during the COVID-19 pandemic. NPJ Park. Dis. 2022, 8, 111. [Google Scholar] [CrossRef]
- Boccia, S.; Ricciardi, W.; Ioannidis, J.P.A. What Other Countries Can Learn From Italy During the COVID-19 Pandemic. JAMA Intern. Med. 2020, 180, 927–928. [Google Scholar] [CrossRef]
- Horváth, K.; Aschermann, Z.; Ács, P.; Deli, G.; Janszky, J.; Komoly, S.; Balázs, É.; Takács, K.; Karádi, K.; Kovács, N. Minimal clinically important difference on the Motor Examination part of MDS-UPDRS. Park. Relat. Disord. 2015, 21, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Dodel, R.; Spottke, A.; Bornschein, B.; Siebert, U.; Quinn, N.P. Rate of clinical progression in Parkinson's disease. A prospective study. Mov. Disord. 2007, 22, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.D.; Cavanaugh, J.T.; Earhart, G.M.; Ford, M.P.; Foreman, K.B.; Thackeray, A.; Thiese, M.S.; Dibble, L.E. Identifying clinical measures that most accurately reflect the progression of disability in Parkinson disease. Park. Relat. Disord. 2016, 25, 65–71. [Google Scholar] [CrossRef]
- Bugalho, P.; Ladeira, F.; Barbosa, R.; Marto, J.P.; Borbinha, C.; da Conceição, L.; Salavisa, M.; Saraiva, M.; Meira, B.; Fernandes, M. Progression in parkinson’s disease: Variation in motor and non-motor symptoms severity and predictors of decline in cognition, motor function, disability, and health-related quality of life as assessed by two different methods. Mov. Disord. Clin. Pract. 2021, 8, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Luis-Martínez, R.; Di Marco, R.; Weis, L.; Cianci, V.; Pistonesi, F.; Baba, A.; Carecchio, M.; Biundo, R.; Tedesco, C.; Masiero, S.; et al. Impact of social and mobility restrictions in Parkinson’s disease during COVID-19 lockdown. BMC Neurol. 2021, 21, 332. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, C.; Baumann-Vogel, H.; Sitzler, M.; Waldvogel, D.; Baumann, C.R. Worsened Parkinson’s disease progression: Impact of the COVID-19 pandemic. J. Park. Dis. 2021, 11, 1579–1583. [Google Scholar] [CrossRef]
- Silva-Batista, C.; Coelho, D.B.; Júnior, R.C.F.; Almeida, L.R.; Guimarães, A.; Nóbrega, K.C.C.; Machado Sanchez, H.; Lindquist, A.R.R.; Israel, V.L.; Kanegusuku, H.; et al. Multidimensional Factors Can Explain the Clinical Worsening in People With Parkinson's Disease During the COVID-19 Pandemic: A Multicenter Cross-Sectional Trial. Front. Neurol. 2021, 12, 708433. [Google Scholar] [CrossRef] [PubMed]
- Capecci, M.; Baldini, N.; Campignoli, F.; Lombardo, L.P.; Andrenelli, E.; Ceravolo, M.G. Clinical and Functional Evolution in Subjects with Parkinson’s Disease during SARS-CoV-2 Pandemic. Appl. Sci. 2023, 13, 1126. [Google Scholar] [CrossRef]
- Fabbri, M.; Leung, C.; Baille, G.; Béreau, M.; Courbon, C.B.; Castelnovo, G.; Carriere, N.; Damier, P.; Defebvre, L.; de Maindreville, A.D. A French survey on the lockdown consequences of COVID-19 pandemic in Parkinson’s disease. The ERCOPARK study. Park. Relat. Disord. 2021, 89, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.G.; Chahine, L.M.; Goldman, S.M.; Korell, M.; Mann, E.; Kinel, D.R.; Arnedo, V.; Marek, K.L.; Tanner, C.M. The effect of the COVID-19 pandemic on people with Parkinson’s disease. J. Park. Dis. 2020, 10, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Balci, B.; Aktar, B.; Buran, S.; Tas, M.; Donmez Colakoglu, B. Impact of the COVID-19 pandemic on physical activity, anxiety, and depression in patients with Parkinson’s disease. Int. J. Rehabil. Res. 2021, 44, 173–176. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development. OECD Better Life Index. Available online: http://www.oecdbetterlifeindex.org/ (accessed on 12 January 2023).
- Pauly, C.; Glaab, E.; Hansen, M.; Martin-Gallausiaux, C.; Ledda, M.; Marques, T.M.; Wilmes, P.; Krüger, R.; Diederich, N.J.; Consortium, N.P. Disease Progression, Resilience, and Inflammation Markers During the Coronavirus Disease 2019 Pandemic in Parkinson’s Disease. Mov. Disord. 2022, 37, 2315–2317. [Google Scholar] [CrossRef]
- Schirinzi, T.; Di Lazzaro, G.; Salimei, C.; Cerroni, R.; Liguori, C.; Scalise, S.; Alwardat, M.; Mercuri, N.B.; Pierantozzi, M.; Stefani, A. Physical activity changes and correlate effects in patients with Parkinson’s disease during COVID-19 lockdown. Mov. Disord. Clin. Pract. 2020, 7, 797–802. [Google Scholar] [CrossRef]
- Brooks, S.K.; Weston, D.; Greenberg, N. Social and psychological impact of the COVID-19 pandemic on people with Parkinson’s disease: A scoping review. Public Health 2021, 199, 77–86. [Google Scholar] [CrossRef]
- Domingos, J.; Família, C.; Fernandes, J.B.; Dean, J.; Godinho, C. Is Being Physically Active Enough or Do People with Parkinson’s Disease Need Structured Supervised Exercise? Lessons Learned from COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 2396. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Chaudhuri, K.; Rojo-Abuin, J.; Rodriguez-Blazquez, C.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O. Assessing the non-motor symptoms of Parkinson’s disease: MDS-UPDRS and NMS Scale. Eur. J. Neurol. 2015, 22, 37–43. [Google Scholar] [CrossRef]


| Mean/N°/Median | SD/Frequency | Range | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 69.96 | 6.61 | 58–83 |
| Sex (female/male) | 12/12 | 50%/50% | |
| Age of onset (years) | 60.42 | 8.94 | 47–75 |
| Duration of illness (years) | 9.54 | 5.36 | 2–20 |
| MDS-UPDRS 1 | 59.50 | 24.13 | 34.5–106 |
| Hoehn and Yahr scale 1 | 2.65 | 0.77 | 1.5–4 |
| Use of dopaminergic medication 1 | 24 | 100.0% | |
| Use of Levodopa 1 | 21 | 87.5% | |
| Highest level of education | |||
| Illiterate | 2 | 8.3% | |
| Elementary school | 7 | 29.2% | |
| Middle school | 3 | 12.5% | |
| High school | 1 | 4.2% | |
| University | 11 | 45.8% | |
| Outcomes | |||
| Number of virtual visits (median Q1) | 2 | 1–3 | |
| Satisfaction (median Q6, 7, 8, 14, 15, 17, 18, 19) | 9.5 | 6.5–10 | |
| Setup/preparation (median Q3) | 10 | 8–10 | |
| Quality of service (median Q9, 10, 11, 12) | 10 | 10–10 | |
| Setup time for virtual visits (minutes, Q4) | 16.10 | 11.63 | 2–45 |
| Saved time for patients (minutes, Q4, 5) | 123.65 | 83.02 | 15–400 |
| Device used for the virtual visit | |||
| Desktop | 3 | 15.0% | |
| Smartphone | 7 | 35.0% | |
| Laptop | 10 | 50.0% | |
| Tablet | 0 | 0.0% | |
| Number of evaluations in the period (median) | |||
| T1 | 2 | 0–3 | |
| T2 | 3 | 1–5 | |
| T3 | 2 | 1–3 | |
| Needed dopaminergic medication adjustment | 12 | 50.0% | |
| Needed medication adjustment for non-motor symptoms | 14 | 58.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-García, R.; Ortega-Robles, E.; Arias-Carrión, O. Remote Assessment of Parkinson’s Disease Patients Amidst the COVID-19 Lockdown in Mexico. Brain Sci. 2023, 13, 1114. https://doi.org/10.3390/brainsci13071114
León-García R, Ortega-Robles E, Arias-Carrión O. Remote Assessment of Parkinson’s Disease Patients Amidst the COVID-19 Lockdown in Mexico. Brain Sciences. 2023; 13(7):1114. https://doi.org/10.3390/brainsci13071114
Chicago/Turabian StyleLeón-García, Rodrigo, Emmanuel Ortega-Robles, and Oscar Arias-Carrión. 2023. "Remote Assessment of Parkinson’s Disease Patients Amidst the COVID-19 Lockdown in Mexico" Brain Sciences 13, no. 7: 1114. https://doi.org/10.3390/brainsci13071114
APA StyleLeón-García, R., Ortega-Robles, E., & Arias-Carrión, O. (2023). Remote Assessment of Parkinson’s Disease Patients Amidst the COVID-19 Lockdown in Mexico. Brain Sciences, 13(7), 1114. https://doi.org/10.3390/brainsci13071114








