Transcutaneous Vagus Nerve Stimulation for Insomnia in People Living in Places or Cities with High Altitudes: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Interventions
2.4. Sample Size Calculation
2.5. Randomisation and Blinding
2.6. Measures
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gudbjartsson, T.; Sigurdsson, E.; Gottfredsson, M.; Bjornsson, O.M.; Gudmundsson, G. High altitude illness and related diseases—A review. Laeknabladid 2019, 105, 499–507. [Google Scholar]
- Windsor, J.S.; Rodway, G.W. Sleep disturbance at altitude. Curr. Opin. Pulm. Med. 2012, 18, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, T.A.; Gore, C.J.; Hahn, A.G.; Hopkins, W.G.; Hawley, J.A.; McKenna, M.J.; Chow, C.M. Sleep in athletes undertaking protocols of exposure to nocturnal simulated altitude at 2650 m. J. Sci. Med. Sport 2005, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.; Schmidt, W.F.; Aughey, R.J.; Bourdon, P.C.; Soria, R.; Claros, J.C.; Roach, G.D. The impact of altitude on the sleep of young elite soccer players (ISA3600). Br. J. Sports Med. 2013, 47, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, G.D.; Schmidt, W.F.; Aughey, R.J.; Bourdon, P.C.; Soria, R.; Claros, J.C.; Sargent, C. The sleep of elite athletes at sea level and high altitude: A comparison of sea-level natives and high-altitude natives (ISA3600). Br. J. Sports Med. 2013, 47, 114–120. [Google Scholar] [CrossRef]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Perlis, M.L.; Posner, D.; Riemann, D.; Bastien, C.H.; Teel, J.; Thase, M. Insomnia. Lancet 2022, 400, 1047–1060. [Google Scholar] [CrossRef]
- Birling, Y.; Wang, J.; Li, G.; Wu, E.; Yu, Z.; Feng, Y.; Wu, Y. Culturally Adapted CBTI for Chinese Insomnia Patients: A One-Arm Pilot Trial. Int. J. Behav. Med. 2018, 25, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.B.; Tanner, S.M.; Thapa, G.B.; Chang, Y.; Watson, K.L.; Staunton, E.; Harris, N.S. A randomized trial of temazepam versus acetazolamide in high altitude sleep disturbance. High Alt. Med. Biol. 2013, 14, 234–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, G.; O’Connor, R.; Johnston, N. Altitude training for elite endurance athletes: A review for the travel medicine practitioner. Travel. Med. Infect. Dis. 2016, 14, 200–211. [Google Scholar] [CrossRef]
- Luks, A.M. Which medications are safe and effective for improving sleep at high altitude? High Alt. Med. Biol. 2008, 9, 195–198. [Google Scholar] [CrossRef]
- Ellrich, J. Transcutaneous Auricular Vagus Nerve Stimulation. J. Clin. Neurophysiol. 2019, 36, 437–442. [Google Scholar] [CrossRef]
- Yakunina, N.; Kim, S.S.; Nam, E.C. Optimization of Transcutaneous Vagus Nerve Stimulation Using Functional MRI. Neuromodulation 2017, 20, 290–300. [Google Scholar] [CrossRef]
- Dhingra, R.R.; Furuya, W.I.; Bautista, T.G.; Dick, T.E.; Galán, R.F.; Dutschmann, M. Increasing Local Excitability of Brainstem Respiratory Nuclei Reveals a Distributed Network Underlying Respiratory Motor Pattern Formation. Front. Physiol. 2019, 10, 887. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Song, L.; Wang, X.; Li, N.; Zhan, S.; Rong, P.; Liu, A. Transcutaneous Vagus Nerve Stimulation Could Improve the Effective Rate on the Quality of Sleep in the Treatment of Primary Insomnia: A Randomized Control Trial. Brain Sci. 2022, 12, 1296. [Google Scholar] [CrossRef]
- Jackowska, M.; Koenig, J.; Vasendova, V.; Jandackova, V.K. A two-week course of transcutaneous vagal nerve stimulation improves global sleep: Findings from a randomised trial in community-dwelling adults. Auton. Neurosci. 2022, 240, 102972. [Google Scholar] [CrossRef]
- Laborde, S.; Hosang, T.; Mosley, E.; Dosseville, F. Influence of a 30-Day Slow-Paced Breathing Intervention Compared to Social Media Use on Subjective Sleep Quality and Cardiac Vagal Activity. J. Clin. Med. 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broncel, A.; Bocian, R.; Kłos-Wojtczak, P.; Konopacki, J. Medial septal cholinergic mediation of hippocampal theta rhythm induced by vagal nerve stimulation. PLoS ONE 2018, 13, e0206532. [Google Scholar] [CrossRef] [PubMed]
- Manta, S.; El Mansari, M.; Debonnel, G.; Blier, P. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int. J. Neuropsychopharmacol. 2013, 16, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bemmel, A.L.; Kerkhof, G.A. Slaap-waakstoornissen en DSM-5 [Sleep-wake disorders and DSM-5]. Tijdschr. Voor Psychiatr. 2014, 56, 192–195. [Google Scholar]
- Yin, X.; Dong, B.; Liang, T.; Yin, P.; Li, X.; Lin, X.; Xu, S. Efficacy and safety of electroacupuncture on treating depression-related insomnia: A study protocol for a multicentre randomised controlled trial. BMJ Open 2019, 9, e021484. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Reliability and validity of the Pittsburgh sleep quality index. Chin. J. Psychiatry 1996, 29, 103. [Google Scholar]
- Lu, T.; Li, Y.; Xia, P. Analysis on reliability and validity of the Pittsburgh sleep quality index. Chongqing Med. 2014, 43, 260–263. [Google Scholar]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993; p. 238. [Google Scholar]
- Bai, C.; Ji, D.; Chen, L. Reliability and validity of Insomnia Severity Index in clinical insomnia patients. Chin. J. Pract. Nurs. 2018, 34, 2182–2186. [Google Scholar]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: A web-based cross-sectional survey. Psychiatry Res. 2020, 288, 112954. [Google Scholar] [CrossRef]
- Komisaruk, B.R.; Frangos, E. Vagus nerve afferent stimulation: Projection into the brain, reflexivephysiological, perceptual, and behavioral responses, and clinical relevance. Auton. Neurosci. 2022, 237, 102908. [Google Scholar] [CrossRef]
- Miglis, M.G. Autonomic dysfunction in primary sleep disorders. Sleep Med. 2016, 19, 40–49. [Google Scholar] [CrossRef]
- Luo, M.; Qu, X.; Li, S.; Zhao, J.; Zhao, Y.; Jiao, Y.; Rong, P. Transcutaneous vagus nerve stimulation for primary insomnia and affective disorder: A report of 35 cases. Zhongguo Zhen Jiu 2017, 37, 269–273. [Google Scholar]
- Rangon, C.M. Reconsidering Sham in Transcutaneous Vagus Nerve Stimulation studies. Clin. Neurophysiol. 2018, 129, 2501–2502. [Google Scholar] [CrossRef]
- Marrosu, F.; Serra, A.; Maleci, A. Correlation between GABA(A) receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Res. 2003, 55, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Manta, S.; El Mansari, M.; Blier, P. Novel attempts to optimize vagus nerve stimulation parameters on serotonin neuronal firing activity in the rat brain. Brain Stimul. 2012, 5, 422–429. [Google Scholar] [CrossRef]
- Manta, S.; Dong, J.; Debonnel, G. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatry Neurosci. 2009, 34, 272–280. [Google Scholar]
- Valdés-Cruz, A.; Magdaleno-Madrigal, V.M.; Martínez-Vargas, D.; Fernández-Mas, R.; Almazán-Alvarado, S. Long-term changes in sleep and electroencephalographic activity by chronic vagus nerve stimulation in cats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 828–834. [Google Scholar] [CrossRef]
- Rasskazova, E.; Zavalko, I.; Tkhostov, A. High intention to fall asleep causes sleep fragmentation. J. Sleep Res. 2014, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018, 11, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, T.E.; Frick, C.; Zobel, A. Vagus nerve stimulation for depression: Efficacy and safety in a European study. Psychol. Med. 2008, 38, 651–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Rong, P.; Hong, Y. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 2016, 79, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Items | tVNS Group | Control Group | CBTI Group | F/χ2 | p |
|---|---|---|---|---|---|
| Sample size | 33 | 32 | 35 | ||
| Age (years) | 23.12 ± 2.56 | 23.96 ± 2.25 | 24.38 ± 1.95 | 1.797 | 0.185 |
| Years of education | 13.54 ± 2.01 | 14.17 ± 2.51 | 13.63 ± 2.43 | 0.458 | 0.637 |
| Marital status | 1.347 | 0.652 | |||
| Never married | 25 (75.76%) | 22 (68.75%) | 27 (77.14%) | ||
| Married | 5 (15.15%) | 8 (25%) | 6 (17.14%) | ||
| Divorced | 3 (9.09%) | 2 (6.25%) | 2 (5.71%) | ||
| Pre-treatment | |||||
| PSQI | 12.02 ± 2.54 | 11.35 ± 2.95 | 11.00 ± 1.91 | 1.577 | 0.212 |
| ISI | 17.07 ± 2.46 | 17.30 ± 1.99 | 16.57 ± 1.31 | 0.948 | 0.392 |
| GAD-7 | 9.94 ± 5.43 | 9.22 ± 3.50 | 9.20 ± 3.32 | 0.305 | 0.738 |
| TST (min) | 403.90 ± 47.76 | 385.94 ± 82.88 | 406.68 ± 82.86 | 0.568 | 0.569 |
| SOL (min) | 77.99 ± 39.37 | 69.03 ± 24.72 | 71.23 ± 44.23 | 0.063 | 0.173 |
| N3 (min) | 50.08 ± 16.00 | 49.94 ± 24.17 | 52.00 ± 26.24 | 0.229 | 0.919 |
| SE (%) | 81.07 ± 8.59 | 82.97 ± 10.46 | 82.23 ± 11.87 | 0.084 | 0.796 |
| WASO (min) | 42.12 ± 31.61 | 42.40 ± 37.11 | 45.45 ± 53.31 | 0.419 | 0.939 |
| Baseline | 4 Weeks Post-Treatment | 4-Week Follow-Up | 8-Week Follow-Up | F, p | |
|---|---|---|---|---|---|
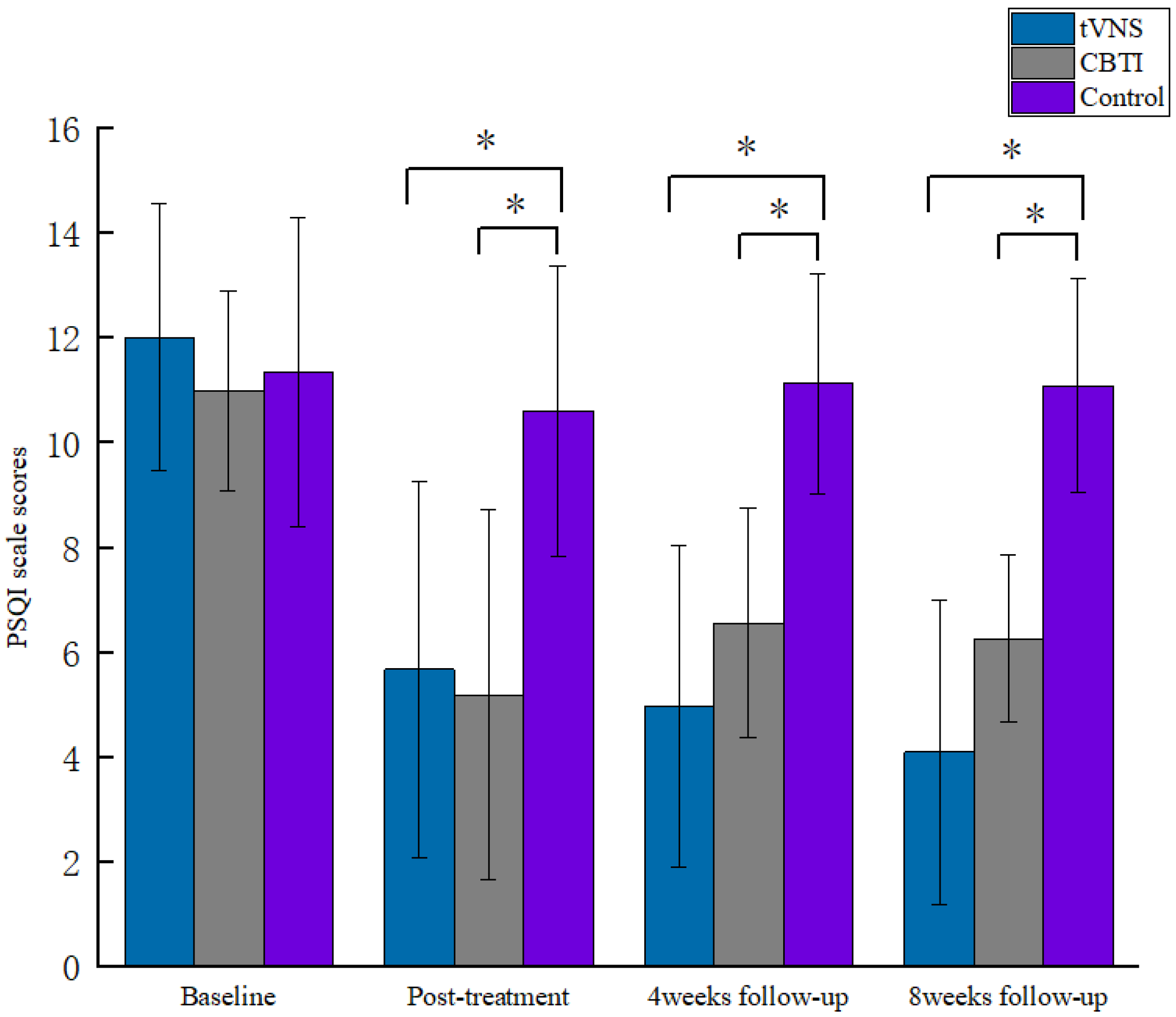
| tVNS | 12.02 ± 2.54 | 5.68 ± 3.58 c | 4.98 ± 3.06 bc | 4.10 ± 2.91 bc | 162.51, 0.001 * |
| PSQI CBTI | 11.00 ± 1.91 | 5.20 ± 3.54 c | 6.57 ± 2.19 ac | 6.27 ± 1.60 ac | 20.79, 0.001 * |
| Control | 11.35 ± 2.95 | 10.61 ± 2.76 ab | 11.13 ± 2.10 ab | 11.09 ± 2.04 ab | 71.43, 0.804 |
| F | 1.58 | 20.12 | 42.16 | 65.03 | |
| p | 0.212 | 0.001 * | 0.001 * | 0.001 * | |
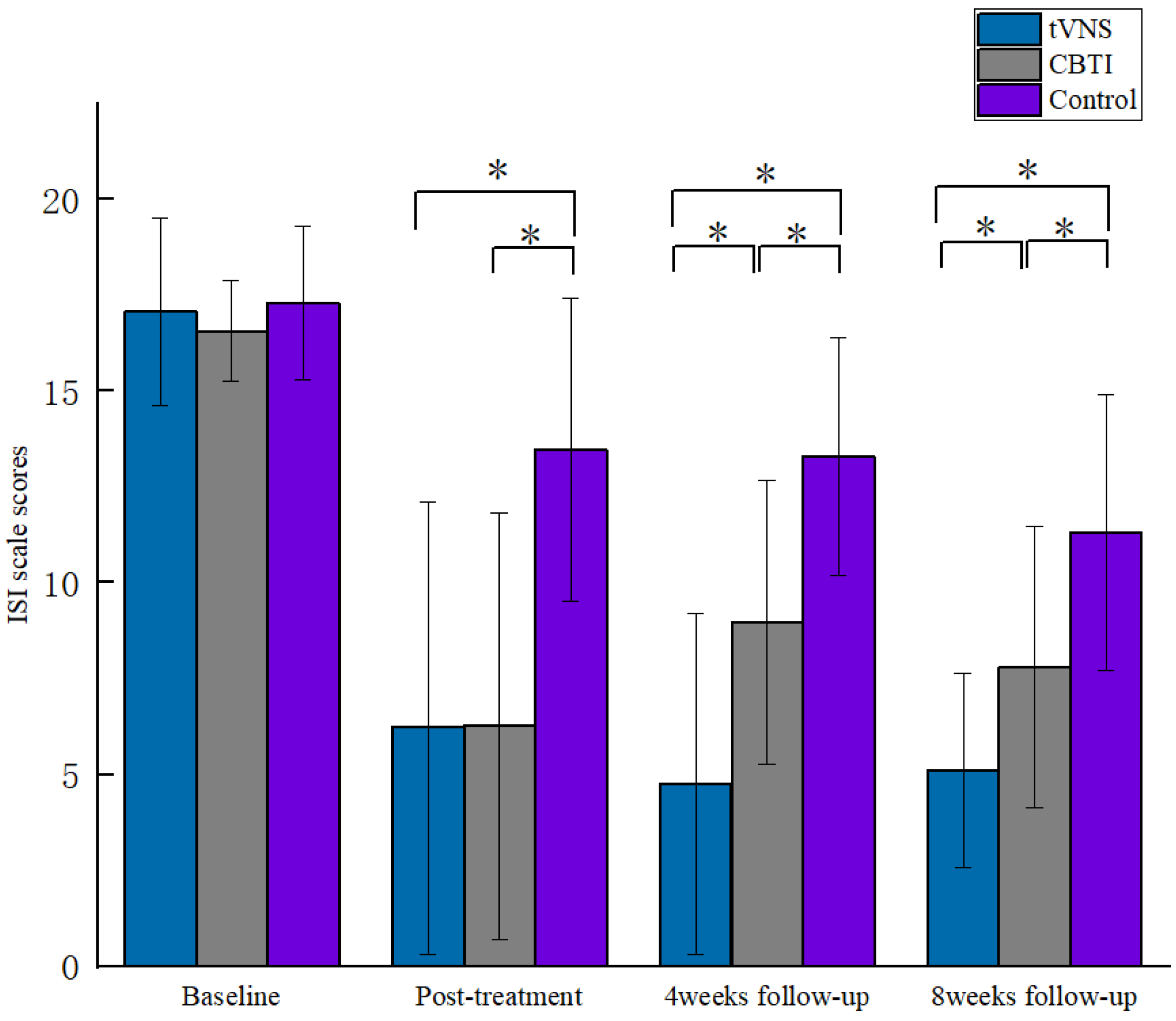
| tVNS | 17.07 ± 2.46 | 6.22 ± 5.90 c | 4.76 ± 4.44 bc | 5.10 ± 2.54 bc | 159.27, 0.001 * |
| ISI CBTI | 16.57 ± 1.31 | 6.27 ± 5.56 c | 8.97 ± 3.71 ac | 7.80 ± 3.67 ac | 69.08, 0.001 * |
| Control | 17.30 ± 1.99 | 13.48 ± 3.95 ab | 13.30 ± 3.11 ab | 11.30 ± 3.59 ab | 20.41, 0.001 * |
| F | 0.95 | 28.51 | 35.87 | 27.96 | |
| p | 0.392 | 0.001 * | 0.001 * | 0.001 * | |
| tVNS | 9.94 ± 5.43 | 3.31 ± 2.54 c | 3.26 ± 3.13 c | 3.31 ± 2.54 c | 30.99, 0.001 * |
| GAD-7 CBTI | 9.20 ± 3.32 | 3.77 ± 3.00 c | 4.13 ± 3.76 c | 3.77 ± 3.00 c | 19.81, 0.001 * |
| Control | 9.22 ± 3.50 | 6.70 ± 3.98 ab | 8.43 ± 4.21 ab | 7.74 ± 3.22 ab | 2.94, 0.096 |
| F | 0.31 | 9.25 | 14.97 | 18.29 | |
| p | 0.74 | 0.001 * | 0.001 * | 0.001 * |
| Baseline | 4 Weeks Post-Treatment | F, p | |
|---|---|---|---|
| tVNS | 403.90 ± 47.76 | 421.34 ± 57.41 | 1.07, 0.303 |
| TST CBTI | 406.66 ± 82.86 | 407.69 ± 69.47 | 0.01, 0.907 |
| Control | 385.94 ± 82.88 | 388.61 ± 82.61 | 0.004, 0.952 |
| F | 0.57 | 1.43 | |
| p | 0.569 | 0.244 | |
| tVNS | 42.11 ± 31.61 | 29.32 ± 24.95 | 1.71, 0.194 |
| WASO CBTI | 45.45 ± 53.31 | 45.56 ± 49.56 | 0.001, 0.991 |
| Control | 42.40 ± 37.11 | 30.17 ± 20.89 | 0.85, 0.359 |
| F | 0.06 | 2.08 | |
| p | 0.939 | 0.131 | |
| tVNS | 81.07 ± 8.59 | 86.69 ± 7.41 | 6.70, 0.01 * |
| SE CBTI | 82.23 ± 11.87 | 83.94 ± 6.40 | 0.64, 0.428 |
| Control | 82.97 ± 10.46 | 81.88 ± 8.61 | 0.14, 0.711 |
| F | 0.23 | 2.90 | |
| p | 0.796 | 0.06 | |
| tVNS | 50.08 ± 16.00 | 72.97 ± 19.88 c | 40.87, 0.001 * |
| N3 CBTI | 52.00 ± 26.24 | 67.24 ± 24.69 | 18.62, 0.001 * |
| Control | 49.95 ± 24.17 | 56.29 ± 24.81 a | 1.70, 0.195 |
| F | 0.08 | ||
| p | 0.919 | ||
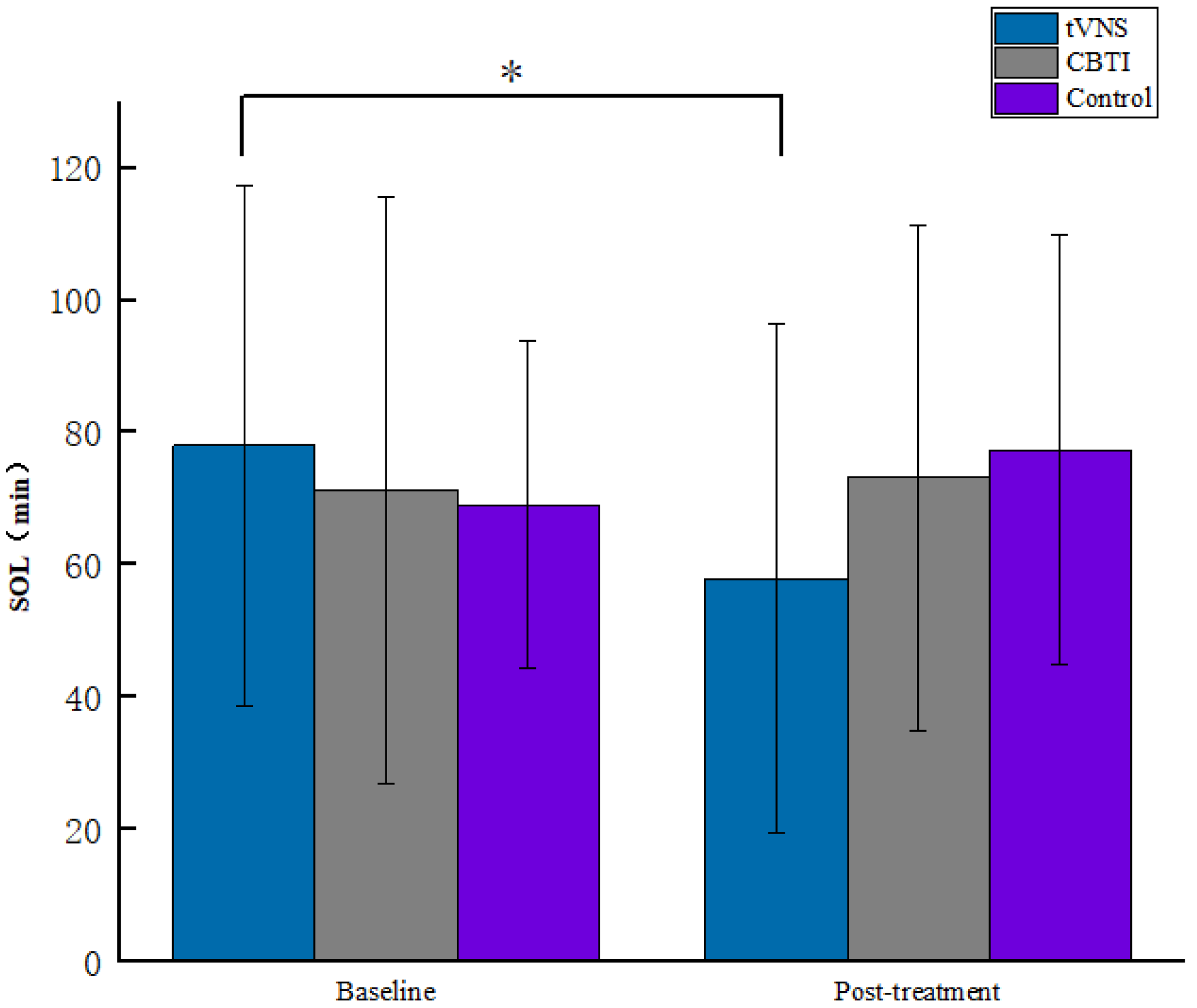
| tVNS | 77.99 ± 39.37 | 57.93 ± 38.35 | 5.23, 0.025 * |
| SOL CBTI | 71.23 ± 44.23 | 73.17 ± 38.19 | 0.05, 0.823 |
| Control | 69.03 ± 24.72 | 77.37 ± 32.33 | 0.491, 0.486 |
| F | 0.42 | 2.24 | |
| p | 0.659 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jin, Y.; Zhang, Q.; Liu, H.; Chen, C.; Song, L.; Li, X.; Ma, Z.; Yang, Q. Transcutaneous Vagus Nerve Stimulation for Insomnia in People Living in Places or Cities with High Altitudes: A Randomized Controlled Trial. Brain Sci. 2023, 13, 985. https://doi.org/10.3390/brainsci13070985
Zhang L, Jin Y, Zhang Q, Liu H, Chen C, Song L, Li X, Ma Z, Yang Q. Transcutaneous Vagus Nerve Stimulation for Insomnia in People Living in Places or Cities with High Altitudes: A Randomized Controlled Trial. Brain Sciences. 2023; 13(7):985. https://doi.org/10.3390/brainsci13070985
Chicago/Turabian StyleZhang, Liang, Yinchuan Jin, Qintao Zhang, Hongyao Liu, Chen Chen, Lei Song, Xiao Li, Zhujing Ma, and Qun Yang. 2023. "Transcutaneous Vagus Nerve Stimulation for Insomnia in People Living in Places or Cities with High Altitudes: A Randomized Controlled Trial" Brain Sciences 13, no. 7: 985. https://doi.org/10.3390/brainsci13070985
APA StyleZhang, L., Jin, Y., Zhang, Q., Liu, H., Chen, C., Song, L., Li, X., Ma, Z., & Yang, Q. (2023). Transcutaneous Vagus Nerve Stimulation for Insomnia in People Living in Places or Cities with High Altitudes: A Randomized Controlled Trial. Brain Sciences, 13(7), 985. https://doi.org/10.3390/brainsci13070985






