Comparison between the Effects of Acute Physical and Psychosocial Stress on Feedback-Based Learning
Abstract
:1. Introduction
Current Study
2. Method
2.1. Subjects
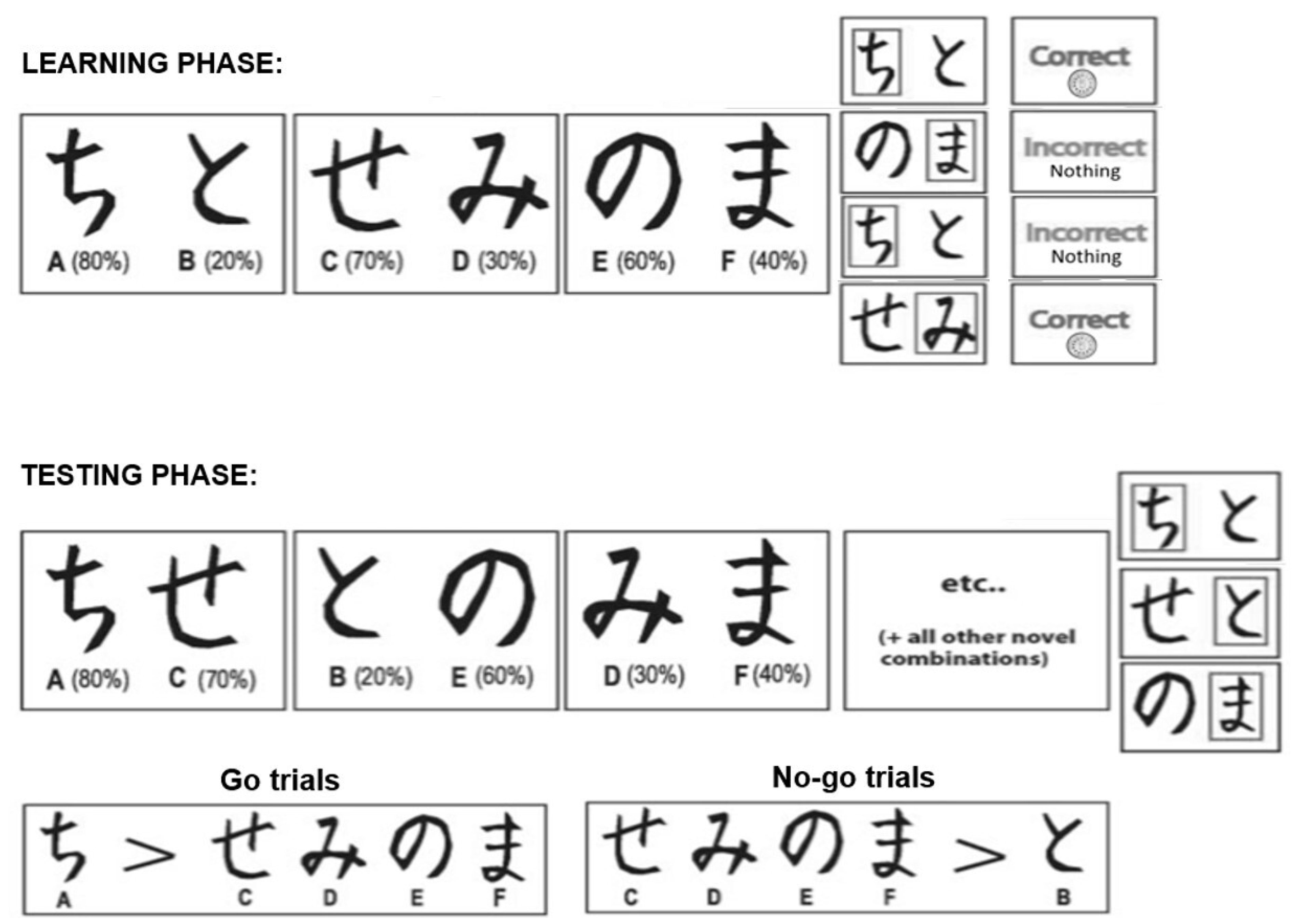
2.2. Feedback-Based Learning Task
2.2.1. Learning Phase
2.2.2. Testing Phase
2.3. Cold Pressor Task
2.4. Mental Arithmetic Task
2.5. Physiological Recording
2.6. Procedure
2.7. Data Reduction
2.8. Analytic Approach
3. Results
3.1. Descriptive Characteristics
3.2. Effects of Stressors on Learning Outcomes
3.3. Effects of Stress Valence and Cardiovascular Reactivity on Learning Outcomes
4. Discussion
4.1. Implications
4.2. Limitations, Future Directions, and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychological Association. Stress in America: The State of Our Nation. 2017. Available online: https://www.apa.org/news/press/releases/stress/2017/state-nation.pdf (accessed on 1 January 2018).
- Jollant, F.; Bellivier, F.; Leboyer, M.; Astruc, B.; Torres, S.; Verdier, R.; Castelnau, D.; Malafosse, A.; Courtet, P. Impaired decision making in suicide attempters. Am. J. Psychiatry 2005, 162, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Salomon, K.; Brady, S.S.; Allen, M.T. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosom. Med. 2003, 65, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.E.; Joormann, J. Stress-induced changes in executive control are associated with depression symptoms: Examining the role of rumination. Clin. Psychol. Sci. 2015, 3, 628–636. [Google Scholar] [CrossRef]
- Jollant, F.; Lawrence, N.L.; Olié, E.; Guillaume, S.; Courtet, P. The suicidal mind and brain: A review of neuropsychological and neuroimaging studies. World J. Biol. Psychiatry 2011, 12, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R. Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. Int. J. Psychophysiol. 2013, 90, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Giannouli, V.; Tsolaki, M. Stressful life events, general cognitive performance, and financial capacity in healthy older adults and Alzheimer’s disease patients. Neuropsychiatrie 2023, 37, 76–79. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, W.; Lin, X.; Que, J.; Huang, Y.; Zheng, H.; Liu, L.; Deng, J.; Lu, L.; Chang, S. The effect of perceived stress on cognition is mediated by personality and the underlying neural mechanism. Transl. Psychiatry 2022, 12, 199. [Google Scholar] [CrossRef]
- Shields, G.S. Stress and cognition: A user’s guide to designing and interpreting studies. Psychoneuroendocrinology 2020, 112, 104475. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; McCullough, A.M.; Yonelinas, A.P. The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol. Bull. 2017, 143, 636–675. [Google Scholar] [CrossRef]
- Frank, M.J.; Seeberger, L.C.; O’Reilly, R.C. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science 2004, 306, 1940–1943. [Google Scholar] [CrossRef] [Green Version]
- de Berker, A.O.; Tirole, M.; Rutledge, R.B.; Cross, G.F.; Dolan, R.J.; Bestmann, S. Acute stress selectively impairs learning to act. Sci. Rep. 2016, 6, 29816. [Google Scholar] [CrossRef] [Green Version]
- Lighthall, N.R.; Gorlick, M.A.; Schoeke, A.; Mather, M. Stress modulates reinforcement learning in younger and older adults. Psychol. Aging 2013, 28, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, A.R.; Raio, C.M.; Chiang, A.; Phelps, E.A.; Daw, N.A. Working-memory capacity protects model-based learning from stress. Proc. Natl. Acad. Sci. USA 2013, 110, 20941–20946. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A.; Plessow, F.; Goschke, T.; Kirschbaum, C. Stress reduces use of negative feedback in a feedback-based learning task. Behav. Neurosci. 2010, 124, 248–255. [Google Scholar] [CrossRef]
- Hartley, C.A.; Phelps, E.A. Anxiety and decision-making. Biol. Psychiatry 2012, 72, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starcke, K.; Brand, M. Decision making under stress: A selective review. Neurosci. Biobehav. Rev. 2012, 36, 1228–1248. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J.; Allen, J.J. Social stress reactivity alters reward and punishment learning. Soc. Cogn. Affect. Neurosci. 2010, 6, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lighthall, N.R.; Pearson, J.M.; Huettel, S.A.; Cabeza, R. Feedback-based learning in aging: Contributions and trajectories of change in striatal and hippocampal systems. J. Neurosci. 2018, 38, 8453–8462. [Google Scholar] [CrossRef] [Green Version]
- Kirschbaum, C.; Pirke, K.M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Preston, S.D.; Buchanan, T.W.; Stansfield, R.B.; Bechara, A. Effects of anticipatory stress on decision making in a gambling task. Behav. Neurosci. 2007, 121, 257–263. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Champagne, F.; Meaney, M.J.; Dagher, A. Dopamine release in response to a psychological stress in human and its relationship to early life maternal care: A positron emission tomography study using 11C raclopride. J. Neurosci. 2004, 24, 2825–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, S.S.; Kemeny, M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004, 130, 355–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovallo, W.R. Stress and Health: Biological and Psychological Interactions, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Storbeck, J.; Clore, G.L. With sadness comes accuracy; with happiness, false memory: Mood and the false memory effect. Psychol. Sci. 2005, 16, 785–791. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Pincomb, G.A.; Wilson, M.F. Predicting response to a reaction time task: Heart rate reactivity compared with Type A behavior. Psychophysiology 1986, 23, 648–656. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Wilson, M.F.; Pincomb, G.A.; Edwards, G.L.; Tompkins, P.; Brackett, D. Activation patterns to aversive stimulation in man: Passive exposure versus effort to control. Psychophysiology 1985, 22, 283–291. [Google Scholar] [CrossRef]
- Al’Absi, M.; Bongard, S.; Buchanan, T.; Pincomb, G.A.; Licinio, J.; Lovallo, W.R. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology 1997, 34, 266–275. [Google Scholar] [CrossRef]
- al’Absi, M.; Buchanan, T.W.; Lovallo, W.R. Cardiovascular and pain responses to cold pressor in persons with positive parental history of hypertension. Psychophysiology 1996, 33, 655–661. [Google Scholar] [CrossRef]
- Spangler, D.P.; Friedman, B.H. Effortful control and resiliency exhibit different patterns of cardiac autonomic control. Int. J. Psychophysiol. 2015, 96, 95–103. [Google Scholar] [CrossRef]
- Veldhuijzen van Zanten JC, S.; Ring, C.; Burns, V.E.; Edwards, K.M.; Drayson, M.; Carroll, D. Mental stress-induced hemoconcentration: Sex differences and mechanisms. Psychophysiology 2004, 41, 541–551. [Google Scholar] [CrossRef]
- Gendolla, G.H.; Wright, R.A.; Richter, M. Effort intensity: Some insights from the cardiovascular system. In The Oxford Handbook of Human Motivation; Ryan, R.M., Ed.; Oxford University Press Inc.: New York, NY, USA, 2012; pp. 420–438. [Google Scholar]
- Grant, S.; Kim, K.; Friedman, B.H. How long is long enough? Controlling for acute caffeine intake in cardiovascular research. Brain Sci. 2023, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.J.; Friedman, B.H. Autonomic response to lateralized cold pressor and facial cooling tasks. Psychophysiology 2015, 52, 416–424. [Google Scholar] [CrossRef]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Linden, W. What do arithmetic stress tests measure? Protocol variations and cardiovascular responses. Psychophysiology 1991, 28, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Kamarck, T.; Stewart, C.; Eddy, M.; Johnson, P. Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology 1992, 29, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D.; Tversky, A. Prospect theory: An analysis of decision under risk. Econometrica 1979, 47, 263–291. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef]
- Schwabe, L. Memory under stress: From single systems to network changes. Eur. J. Neurosci. 2017, 45, 478–489. [Google Scholar] [CrossRef]
- Ginty, A.T. Blunted responses to stress and reward: Reflections on biological disengagement. Int. J. Psychophysiol. 2013, 90, 90–94. [Google Scholar] [CrossRef]
- Philips, A.C.; Ginty, A.T.; Hughes, B.M. The other side of the coin: Blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int. J. Psychophysiol. 2013, 90, 1–7. [Google Scholar] [CrossRef]
- Wright, R.A. Brehm’s theory of motivation as a model of effort and cardiovascular response. In The Psychology of Action: Linking Cognition and Motivation to Behavior; Gollwitzer, P.M., Bargh, J.A., Eds.; Guildford Press: New York, NY, USA, 1996; pp. 424–453. [Google Scholar]
- Pizzagalli, D.A.; Evins, A.E.; Schetter, E.C.; Frank, M.J.; Petra, E.P.; Santesso, D.L.; Culhane, M. Single dose of a dopamine agonist impairs reinforcement learning in humans: Behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology 2008, 196, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, R.; Pizzagalli, D.A. Acute stress reduces reward responsiveness: Implication for depression. Biol. Psychiatry 2006, 60, 1147–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hains, A.B.; Arnsten, A.F. Molecular mechanisms of stress-induced prefrontal cortical impairment: Implication for mental illness. Learn. Mem. 2008, 15, 551–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumming, E.; Henry, W.E. Growing Old: The Process of Disengagement; Basic Books: New York, NY, USA, 1961. [Google Scholar]
- Carstensen, L.L. The influence of a sense of time on human development. Science 2006, 312, 1913–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.; Rottenberg, J. Listening to the blues: An ecological momentary assessment of music choice in depression. Emotion 2021, 21, 1177–1187. [Google Scholar] [CrossRef]
- Westbrook, A.; Yang, X.; Bylsma, L.M.; Daches, S.; George, C.J.; Seidman, A.J.; Jennings, J.R.; Kovacs, M. Economic choice and heart rate fractal scaling indicate that cognitive effort is reduced by depression and boosted by sad mood. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 687–694. [Google Scholar] [CrossRef]
- Light, K.C.; Obrist, P.A. Cardiovascular response to stress: Effects of opportunity to avoid, shock experience, and performance feedback. Psychophysiology 1980, 17, 243–252. [Google Scholar] [CrossRef]
- Kasch, K.L.; Rottenberg, J.; Arrnow, B.A.; Gotlib, H. Behavioral activation and inhibition systems and the severity and course of depression. J. Abnorm. Psychol. 2002, 111, 589–597. [Google Scholar] [CrossRef]
- Brenner, S.L.; Beauchaine, T.P. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology 2011, 48, 1588–1596. [Google Scholar] [CrossRef]
- Harrison, A.; Treasure, J.; Smillie, L.D. Approach and avoidance motivation in eating disorders. Psychiatry Res. 2011, 188, 396–401. [Google Scholar] [CrossRef]
- Atkinson, J.; Sharp, C.; Schmitz, J.; Yaroslavsky, I. Behavioral activation and inhibition, negative affect, and gambling severity in a sample of young adult college students. J. Gambl. Stud. 2012, 28, 437–449. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, S.R.; Roseboom, T.J. Further evidence for an association between self-reported health and cardiovascular as well as cortisol reactions to acute psychological stress. Psychophysiology 2010, 47, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Ginty, A.T.; Phillips, A.C.; Der, G.; Deary, I.J.; Carroll, D. Heart rate reactivity is associated with future cognitive ability and cognitive change in a large community sample. Int. J. Psychophysiol. 2011, 82, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variable | MA Group (n = 48) | CPT Group (n = 48) | F or χ2 Statistics |
|---|---|---|---|
| Female (n) | 26 (45.8%) | 24 (50.0%) | 0.17 |
| Age (years, SD) | 18.92 (1.18) | 19.31 (1.60) | 1.90 |
| BMI (kg/m2, SD) | 22.51 (3.25) | 23.92 (4.25) | 3.32 |
| Stressor Valence | 4.05 (1.93) | 2.63 (1.86) | 13.41 *** |
| HR (bpm, SD) | |||
| Resting | 73.55 (10.46) | 77.40 (11.09) | 3.07 |
| Stressor | 79.59 (10.38) | 81.25 (10.28) | 0.62 |
| SBP (mmHg, SD) | |||
| Resting | 111.21 (14.33) | 111.27 (11.54) | 0.01 |
| Stressor | 121.91 (18.04) | 122.67 (16.41) | 0.05 |
| DBP (mmHg, SD) | |||
| Resting | 67.13 (11.11) | 66.96 (9.64) | 0.01 |
| Stressor | 75.53 (14.74) | 75.61 (12.25) | 0.01 |
| Number of Learning Blocks Needed (SD) | |||
| Pre-stressor | 1.98 (0.95) | 2.21 (1.01) | 1.32 |
| Post-stressor | 1.90 (0.87) | 2.06 (1.00) | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Nackley, B.; Friedman, B.H. Comparison between the Effects of Acute Physical and Psychosocial Stress on Feedback-Based Learning. Brain Sci. 2023, 13, 1127. https://doi.org/10.3390/brainsci13081127
Yang X, Nackley B, Friedman BH. Comparison between the Effects of Acute Physical and Psychosocial Stress on Feedback-Based Learning. Brain Sciences. 2023; 13(8):1127. https://doi.org/10.3390/brainsci13081127
Chicago/Turabian StyleYang, Xiao, Brittany Nackley, and Bruce H. Friedman. 2023. "Comparison between the Effects of Acute Physical and Psychosocial Stress on Feedback-Based Learning" Brain Sciences 13, no. 8: 1127. https://doi.org/10.3390/brainsci13081127
APA StyleYang, X., Nackley, B., & Friedman, B. H. (2023). Comparison between the Effects of Acute Physical and Psychosocial Stress on Feedback-Based Learning. Brain Sciences, 13(8), 1127. https://doi.org/10.3390/brainsci13081127





