Task Cortical Connectivity Reveals Different Network Reorganizations between Mild Stroke Patients with Cortical and Subcortical Lesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
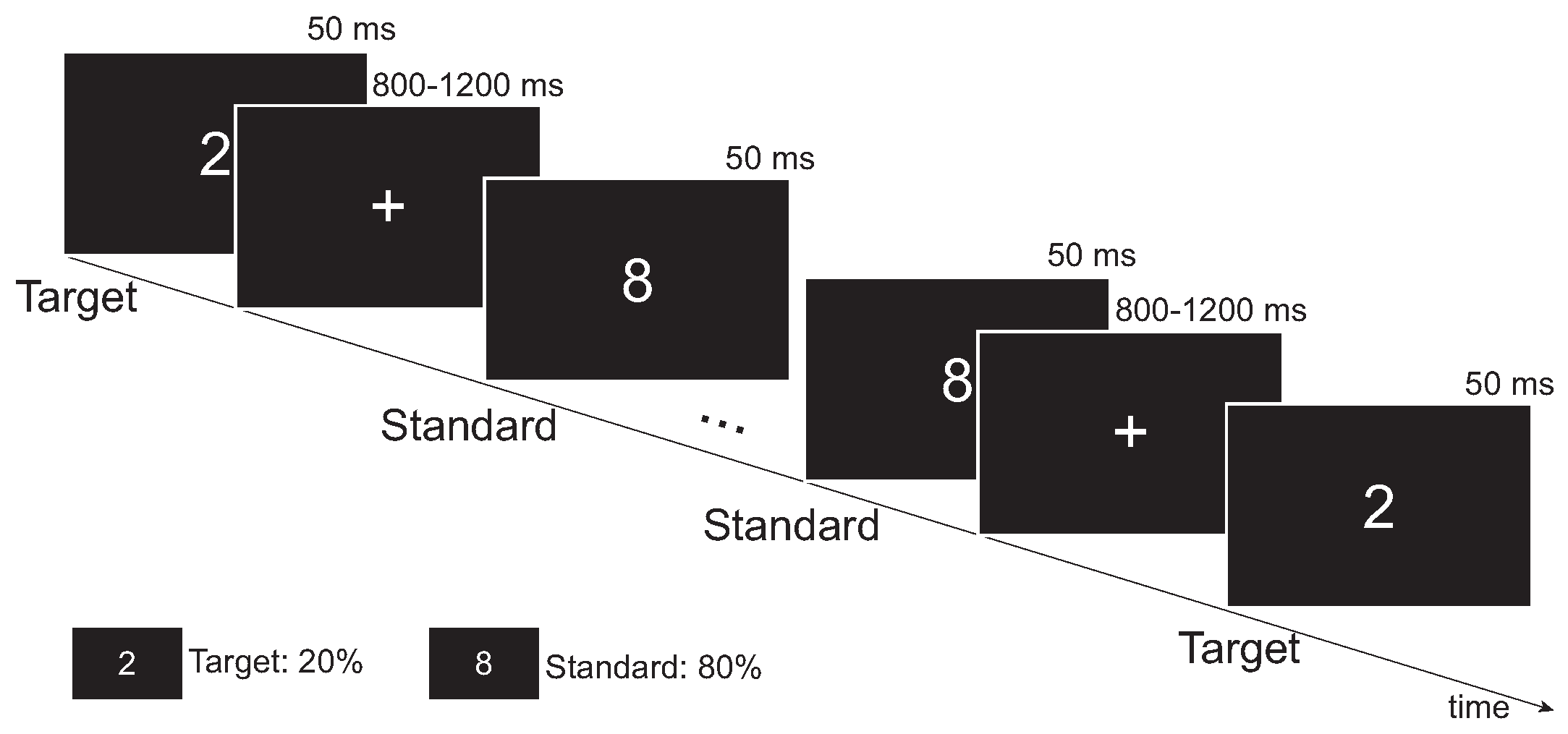
2.2. Experimental Paradigms
2.3. EEG Recordings and Preprocessing
2.4. Source-Space PEC Calculation
2.4.1. EEG Source Reconstruction
2.4.2. EEG Functional Connectivity Measurement
2.5. Network Analysis
2.6. Feature Selection and Classifier
| Algorithm 1: Classification framework. |
Input: PEC features: Subject Index: Labels of health/patient: Output: Classification accuracy: The selected features: Begin: for do = FS for do end for end for Optimal number of features: Optimal feature sets: Rank features by occurrence rate: Get index of K optimal features: End |
2.7. Statistical Analysis
3. Results
3.1. Visual Task Results
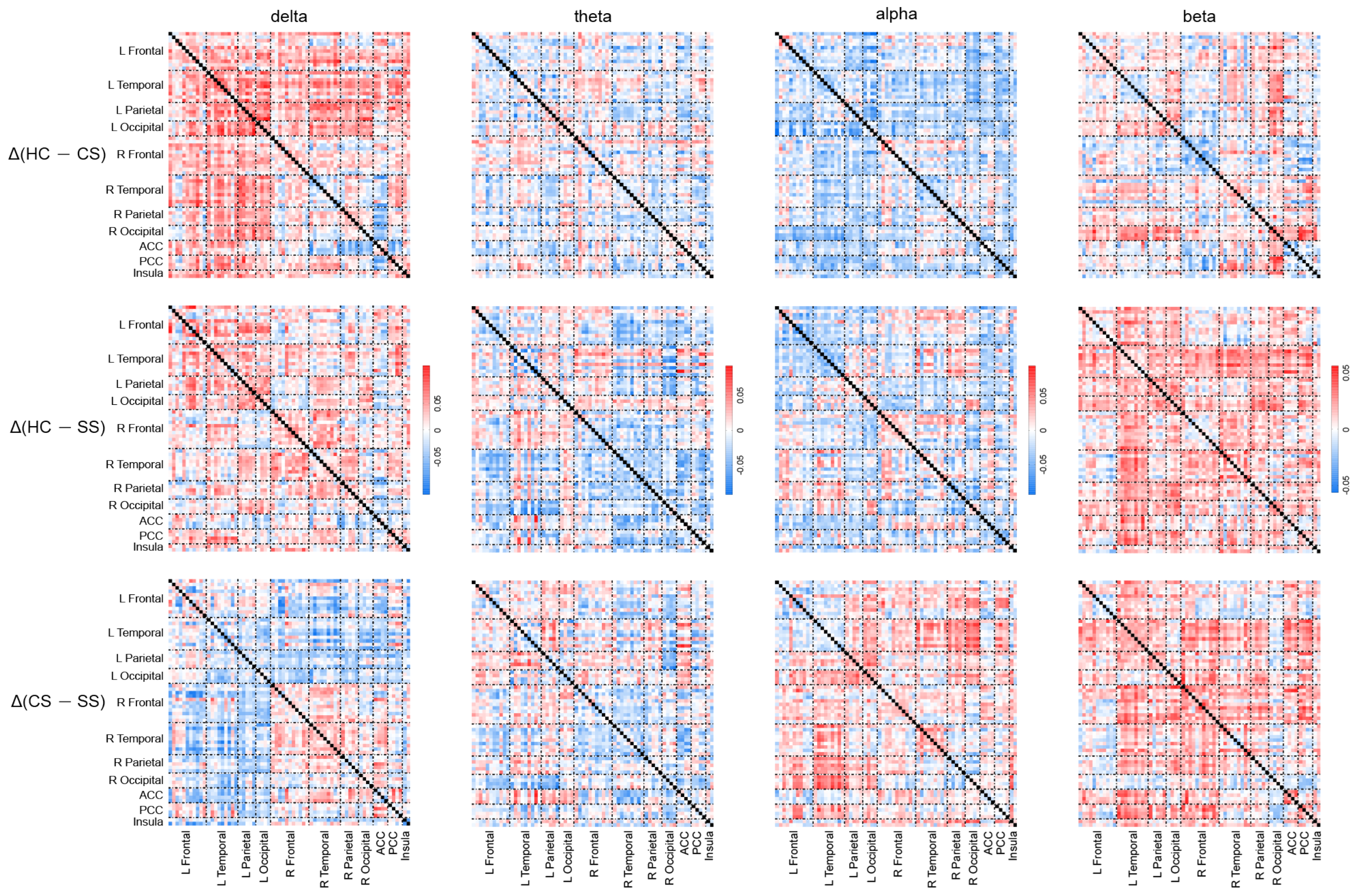
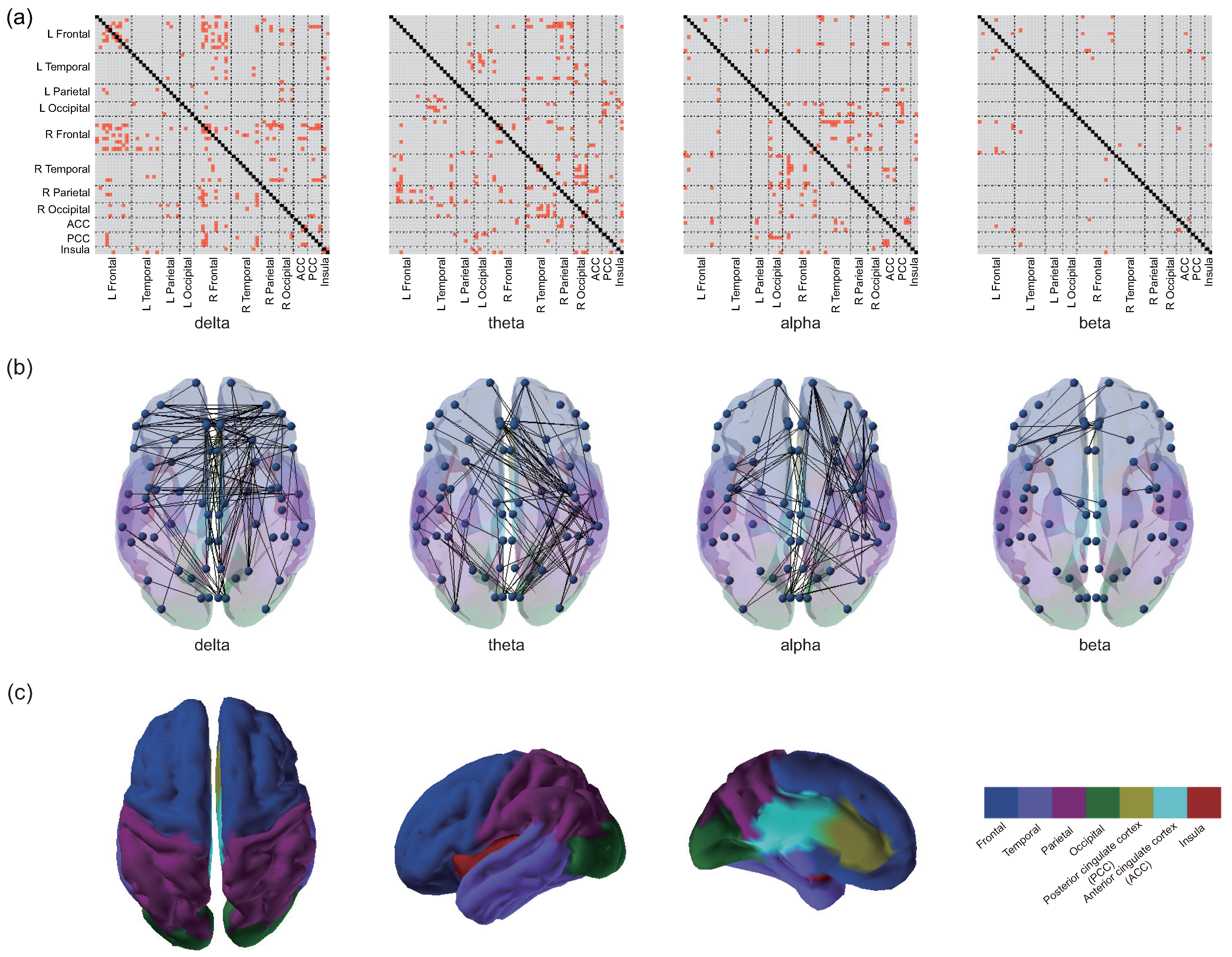
3.2. Differences in EEG Functional Connectivity
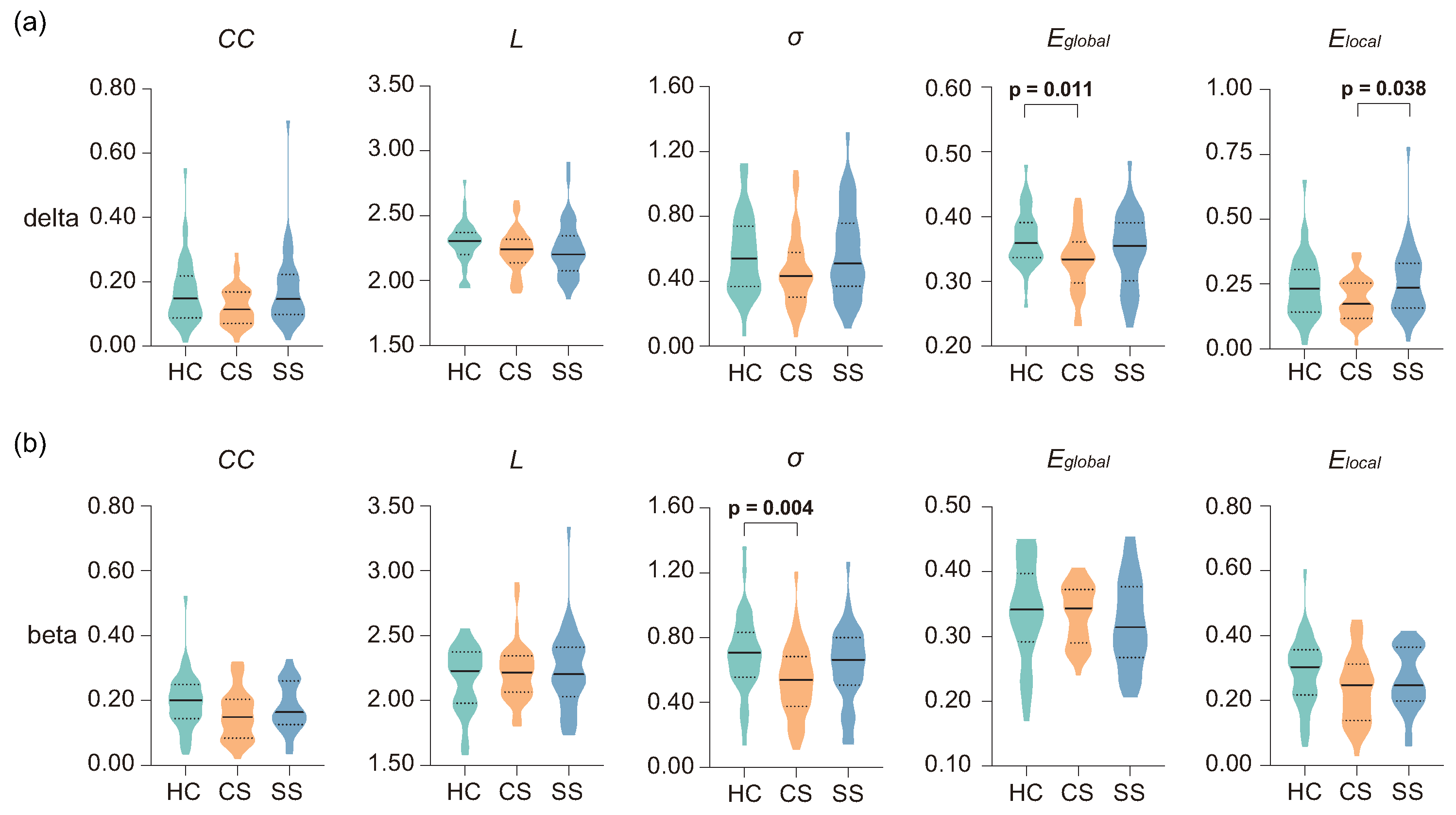
3.3. Analysis of Networks Metrics
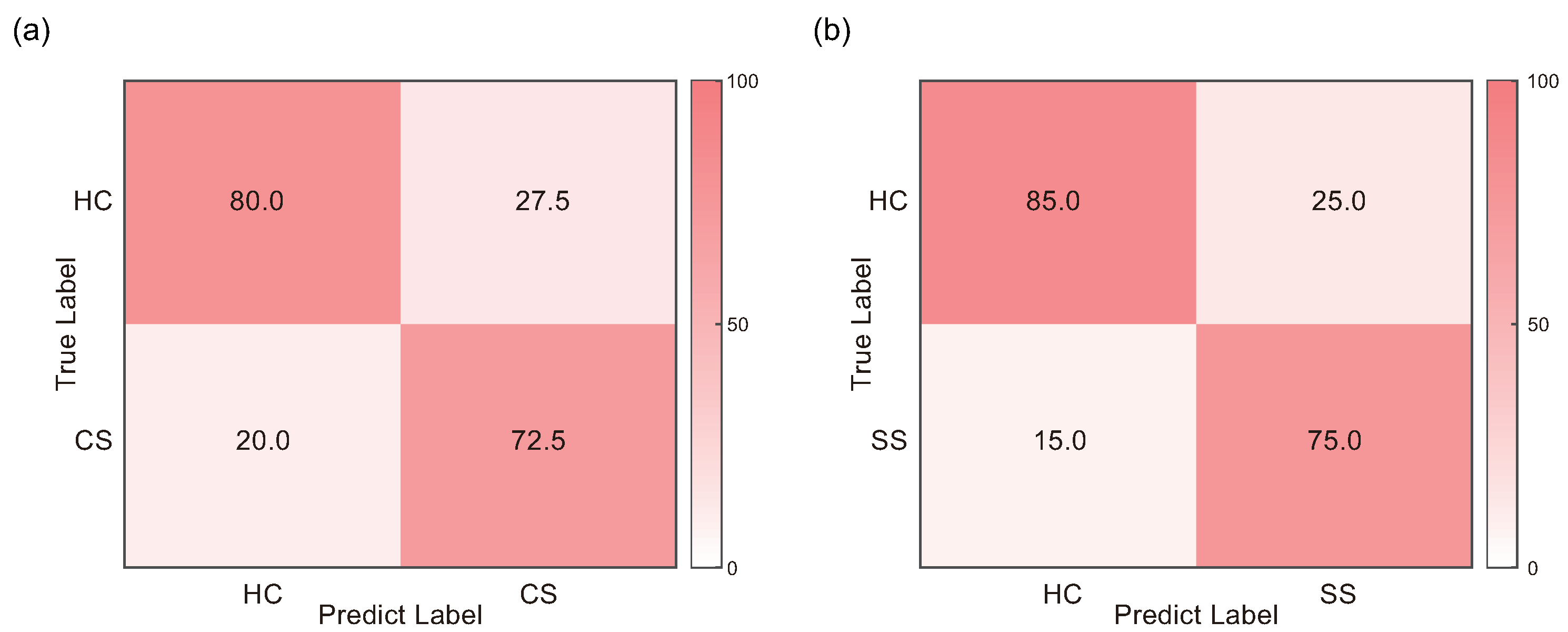
3.4. Classification Performance
4. Discussion
4.1. Worse Task Performance in Patient Groups
4.2. Complex Functional Connectivity Distribution
4.3. Topological Alterations of Brain Network
4.4. Classification Performance
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Supplementary Materials for Patients with Mild Stroke
| Rehabilitation Form | Number of Patients |
|---|---|
| Medication | 60 |
| Medication; Acupuncture treatment | 2 |
| Medication; Exercise therapy | 1 |
| Medication; Acupuncture treatment; Exercise therapy; Occupational therapy | 6 |
| Medication; Cerebral angiography | 2 |
Appendix A.2. Supplementary Classification Results
| Classifiers | HC vs. CS (ACC (Sensitivity/Specificity) (%)) | HC vs. SS (ACC (Sensitivity/Specificity) (%)) | ||||||
|---|---|---|---|---|---|---|---|---|
| Corr | Fisher | Relief | LARS | Corr | Fisher | Relief | LARS | |
| LR | 66.25 (66.67/65.85) | 67.50 (68.42/66.67) | 65.00 (63.64/66.67) | 71.25 (71.79/70.73) | 65.00 (66.67/63.64) | 65.00 (66.67/63.64) | 46.25 (46.34/46.15) | 55.00 (55.26/54.76) |
| Boost | 63.75 (62.79/64.86) | 63.75 (62.79/64.86) | 68.75 (67.44/70.27) | 76.25 (78.38/74.42) | 70.00 (68.18/72.22) | 70.00 (68.18/72.20) | 51.25 (52.17/50.88) | 60.00 (60.00/60.00) |
| Tree | 60.00 (59.52/60.53) | 60.00 (60.00/60.00) | 61.25 (61.54/60.98) | 65.00 (64.29/65.79) | 63.75 (64.10/63.41) | 63.75 (64.10/63.41) | 80.00 (83.33/77.27) | 58.75 (58.54/58.97) |
| RF | 57.50 (57.89/57.14) | 58.75 (58.97/58.54) | 63.75 (64.10/63.41) | 70.00 (68.18/72.22) | 55.00 (55.00/55.00) | 55.00 (55.00/55.00) | 53.75 (54.55/53.19) | 57.50 (60.00/56.00) |

Appendix A.3. Classification Results for CS and SS Groups
| Classifiers | ACC (Sensitivity/Specificity) (%) | |||
|---|---|---|---|---|
| Corr | Fisher | Relief | LARS | |
| LR | 67.50 (67.50/67.50) | 67.50 (67.50/67.50) | 62.50 (62.50/62.50) | 55.00 (55.56/54.55) |
| Boost | 63.75 (64.86/62.79) | 63.75 (64.86/62.79) | 63.75 (64.10/63.41) | 63.75 (64.86/62.79) |
| Tree | 76.25 (73.33/80.00) | 76.25 (73.33/80.00) | 63.75 (62.22/65.71) | 65.00 (64.29/65.79) |
| RF | 67.50 (66.67/68.42) | 67.50 (66.67/68.42) | 58.75 (58.97/58.54) | 57.50 (57.50/57.50) |
Appendix A.4. 3-Class Classification
References
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, S.D.; Leng, X.Y.; Kuo, K.; Wang, Z.T.; Cui, M.; Tan, L.; Wang, K.; Dong, Q.; Yu, J.T. Post-stroke cognitive impairment: Epidemiology, risk factors, and management. J. Alzheimer’s Dis. 2022, 86, 983–999. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Tan, L.; Yu, J.T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [PubMed]
- Yuan, M.; Guo, Y.S.; Han, Y.; Gao, Z.K.; Shen, X.Y.; Bi, X. Effectiveness and mechanisms of enriched environment in post-stroke cognitive impairment. Behav. Brain Res. 2021, 410, 113357. [Google Scholar] [CrossRef] [PubMed]
- Umarova, R.M. Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: Implications for understanding neglect. Cortex 2017, 97, 327–338. [Google Scholar] [CrossRef]
- Gassert, R.; Dietz, V. Rehabilitation robots for the treatment of sensorimotor deficits: A neurophysiological perspective. J. Neuroeng. Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Wolf, T.J.; Barbee, A.R.; White, D. Executive dysfunction immediately after mild stroke. OTJR Occup. Particip. Health 2011, 31, S23–S29. [Google Scholar] [CrossRef]
- Edwards, D.F.; Hahn, M.; Baum, C.; Dromerick, A.W. The impact of mild stroke on meaningful activity and life satisfaction. J. Stroke Cerebrovasc. Dis. 2006, 15, 151–157. [Google Scholar] [CrossRef]
- Rochette, A.; Desrosiers, J.; Bravo, G.; St-Cyr-Tribble, D.; Bourget, A. Changes in participation after a mild stroke: Quantitative and qualitative perspectives. Top. Stroke Rehabil. 2007, 14, 59–68. [Google Scholar] [CrossRef]
- Saa, J.P.; Tse, T.; Baum, C.; Cumming, T.; Josman, N.; Rose, M.; Carey, L. Longitudinal evaluation of cognition after stroke—A systematic scoping review. PLoS ONE 2019, 14, e0221735. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.C. Lesion location and repeatable battery for the assessment of neuropsychological status performance in acute ischemic stroke. Clin. Neuropsychol. 2010, 24, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.T.; Cushman, L.A. Intellectual and memory functions after cortical and subcortical stroke. Neurorehabilitation 1992, 2, 45–52. [Google Scholar] [CrossRef]
- Su, C.Y.; Chen, H.M.; Kwan, A.L.; Lin, Y.H.; Guo, N.W. Neuropsychological impairment after hemorrhagic stroke in basal ganglia. Arch. Clin. Neuropsychol. 2007, 22, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Planton, M.; Peiffer, S.; Albucher, J.F.; Barbeau, E.; Tardy, J.; Pastor, J.; Januel, A.; Bezy, C.; Lemesle, B.; Puel, M.; et al. Neuropsychological outcome after a first symptomatic ischaemic stroke with ‘good recovery’. Eur. J. Neurol. 2012, 19, 212–219. [Google Scholar] [CrossRef]
- Vecchio, F.; Pappalettera, C.; Miraglia, F.; Deinite, G.; Manenti, R.; Judica, E.; Caliandro, P.; Rossini, P.M. Prognostic role of hemispherical functional connectivity in stroke: A study via graph theory versus coherence of electroencephalography rhythms. Stroke 2023, 54, 499–508. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Huster, R.J.; Herrmann, C.S. EEG-neurofeedback as a tool to modulate cognition and behavior: A review tutorial. Front. Hum. Neurosci. 2017, 11, 51. [Google Scholar] [CrossRef]
- Caliandro, P.; Vecchio, F.; Miraglia, F.; Reale, G.; Della Marca, G.; La Torre, G.; Lacidogna, G.; Iacovelli, C.; Padua, L.; Bramanti, P.; et al. Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabil. Neural Repair. 2017, 31, 81–94. [Google Scholar] [CrossRef]
- Fanciullacci, C.; Panarese, A.; Spina, V.; Lassi, M.; Mazzoni, A.; Artoni, F.; Micera, S.; Chisari, C. Connectivity measures differentiate cortical and subcortical sub-acute ischemic stroke patients. Front. Hum. Neurosci. 2021, 15, 669915. [Google Scholar] [CrossRef]
- Dubovik, S.; Ptak, R.; Aboulafia, T.; Magnin, C.; Gillabert, N.; Allet, L.; Pignat, J.M.; Schnider, A.; Guggisberg, A.G. EEG alpha band synchrony predicts cognitive and motor performance in patients with ischemic stroke. Behav. Neurol. 2013, 26, 187–189. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J.M. Discovering oscillatory interaction networks with M/EEG: Challenges and breakthroughs. Trends Cogn. Sci. 2012, 16, 219–230. [Google Scholar] [CrossRef]
- Hipp, J.F.; Hawellek, D.J.; Corbetta, M.; Siegel, M.; Engel, A.K. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat. Neurosci. 2012, 15, 884–890. [Google Scholar] [CrossRef]
- Toll, R.T.; Wu, W.; Naparstek, S.; Zhang, Y.; Narayan, M.; Patenaude, B.; De Los Angeles, C.; Sarhadi, K.; Anicetti, N.; Longwell, P.; et al. An electroencephalography connectomic profile of posttraumatic stress disorder. Am. J. Psychiatry 2020, 177, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, W.; Toll, R.T.; Naparstek, S.; Maron-Katz, A.; Watts, M.; Gordon, J.; Jeong, J.; Astolfi, L.; Shpigel, E.; et al. Identification of psychiatric disorder subtypes from functional connectivity patterns in resting-state electroencephalography. Nat. Biomed. Eng. 2021, 5, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Liao, X.; Vasilakos, A.V.; He, Y. Small-world human brain networks: Perspectives and challenges. Neurosci. Biobehav. Rev. 2017, 77, 286–300. [Google Scholar] [CrossRef]
- Yan, J.; Sun, J.; Guo, X.; Jin, Z.; Li, Y.; Li, Z.; Tong, S. Motor imagery cognitive network after left ischemic stroke: Study of the patients during mental rotation task. PLoS ONE 2013, 8, e77325. [Google Scholar] [CrossRef]
- Fallani, F.D.V.; Pichiorri, F.; Morone, G.; Molinari, M.; Babiloni, F.; Cincotti, F.; Mattia, D. Multiscale topological properties of functional brain networks during motor imagery after stroke. Neuroimage 2013, 83, 438–449. [Google Scholar] [CrossRef]
- Vecchio, F.; Tomino, C.; Miraglia, F.; Iodice, F.; Erra, C.; Di Iorio, R.; Judica, E.; Alù, F.; Fini, M.; Rossini, P.M. Cortical connectivity from EEG data in acute stroke: A study via graph theory as a potential biomarker for functional recovery. Int. J. Psychophysiol. 2019, 146, 133–138. [Google Scholar] [CrossRef]
- Leśniak, M.; Bak, T.; Czepiel, W.; Seniów, J.; Członkowska, A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement. Geriatr. Cogn. Disord. 2008, 26, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, Y.; Kanno, A.; Kambara, T.; Nozawa, T.; Sugiura, M.; Okumura, E.; Kawashima, R. Spatiotemporal dynamics of high-gamma activities during a 3-stimulus visual oddball task. PLoS ONE 2013, 8, e59969. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.G.; Balsters, J.H.; Kilcullen, S.M.; Campbell, W.; Bokde, A.W.; Lai, R.; Upton, N.; Robertson, I.H. A simultaneous ERP/fMRI investigation of the P300 aging effect. Neurobiol. Aging 2012, 33, 2448–2461. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-stroke cognitive impairment and dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Venkataraman, A.; Whitford, T.J.; Westin, C.F.; Golland, P.; Kubicki, M. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr. Res. 2012, 139, 7–12. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Wang, L.; Li, W. Functional brain network classification with compact representation of SICE matrices. IEEE. Trans. Biomed. Eng. 2015, 62, 1623–1634. [Google Scholar] [CrossRef]
- Rahim, M.; Thirion, B.; Comtat, C.; Varoquaux, G. Transmodal learning of functional networks for Alzheimer’s disease prediction. IEEE J. Sel. Top. Signal Process. 2016, 10, 1204–1213. [Google Scholar] [CrossRef]
- Xu, M.; Feng, Z.; Wang, S.; Gao, H.; Cai, J.; Wu, B.; Cai, H.; Sun, Y.; Guan, C. Machine learning technique reveals intrinsic EEG connectivity characteristics of patients with mild stroke during cognitive task performing. IEEE Trans. Cogn. Develop. Syst. 2023. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Hauk, O. Keep it simple: A case for using classical minimum norm estimation in the analysis of EEG and MEG data. Neuroimage 2004, 21, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.S.; Evans, A.C.; McKinstry, R.C.; Almli, C.R.; Collins, D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Mosher, J.C.; Leahy, R.M.; Lewis, P.S. EEG and MEG: Forward solutions for inverse methods. IEEE. Trans. Biomed. Eng. 1999, 46, 245–259. [Google Scholar] [CrossRef]
- Gramfort, A.; Papadopoulo, T.; Olivi, E.; Clerc, M. OpenMEEG: Opensource software for quasistatic bioelectromagnetics. Biomed. Eng. Online 2010, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Hassan, M.; Wendling, F. Electroencephalography source connectivity: Aiming for high resolution of brain networks in time and space. IEEE Signal Process. Mag. 2018, 35, 81–96. [Google Scholar] [CrossRef]
- Bensmann, W.; Zink, N.; Mückschel, M.; Beste, C.; Stock, A.K. Neuronal networks underlying the conjoint modulation of response selection by subliminal and consciously induced cognitive conflicts. Brain Struct. Funct. 2019, 224, 1697–1709. [Google Scholar] [CrossRef]
- Takacs, A.; Zink, N.; Wolff, N.; Münchau, A.; Mückschel, M.; Beste, C. Connecting EEG signal decomposition and response selection processes using the theory of event coding framework. Hum. Brain Mapp. 2020, 41, 2862–2877. [Google Scholar] [CrossRef]
- Bassett, D.S.; Bullmore, E. Small-world brain networks. Neuroscientist 2006, 12, 512–523. [Google Scholar] [CrossRef]
- Pernet, C.R.; Latinus, M.; Nichols, T.; Rousselet, G. Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: A simulation study. J. Neurosci. Methods 2015, 250, 85–93. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Liu, H.; Ye, J. On similarity preserving feature selection. IEEE Trans. Knowl. Data Eng. 2011, 25, 619–632. [Google Scholar] [CrossRef]
- Shinde, P.P.; Shah, S. A review of machine learning and deep learning applications. In Proceedings of the 2018 Fourth International Conference on Computing Communication Control and Automation (ICCUBEA), Pune, India, 16–18 August 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–6. [Google Scholar]
- Kakkos, I.; Dimitrakopoulos, G.N.; Sun, Y.; Yuan, J.; Matsopoulos, G.K.; Bezerianos, A.; Sun, Y. EEG fingerprints of task-independent mental workload discrimination. IEEE J. Biomed. Health Inform. 2021, 25, 3824–3833. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Tourville, J. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci. 2012, 6, 171. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Lou, W.; Zhao, S. Dynamic information flow analysis in vascular dementia patients during the performance of a visual oddball task. Neurosci. Lett. 2014, 580, 108–113. [Google Scholar] [CrossRef]
- Xu, J.; Lou, W.; Zhao, S.; Wang, C. Altered directed connectivity in patients with early vascular dementia during a visual oddball task. Brain Topogr. 2015, 28, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Lo, S.F.; Lin, C.Y.; Chen, F.F.; Lo, Y.C.; Chou, L.W.; Kuo, C.L.; Tien, Y.M. Impact of putamen stroke on task context updating: Evidence from P300 brain waves. J. Clin. Neurosci. 2018, 55, 45–51. [Google Scholar] [CrossRef]
- Carter, A.R.; Astafiev, S.V.; Lang, C.E.; Connor, L.T.; Rengachary, J.; Strube, M.J.; Pope, D.L.W.; Shulman, G.L.; Corbetta, M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 2010, 67, 365–375. [Google Scholar] [PubMed]
- Carrera, E.; Tononi, G. Diaschisis: Past, present, future. Brain 2014, 137, 2408–2422. [Google Scholar] [CrossRef]
- Digiacomo, M.R.; Marco-Pallarés, J.; Flores, A.B.; Gómez, C.M. Wavelet analysis of the EEG during the neurocognitive evaluation of invalidly cued targets. Brain Res. 2008, 1234, 94–103. [Google Scholar] [CrossRef]
- Nácher, V.; Ledberg, A.; Deco, G.; Romo, R. Coherent delta-band oscillations between cortical areas correlate with decision making. Proc. Natl. Acad. Sci. USA 2013, 110, 15085–15090. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, R.F.; Huang, M.; Wilson, G.; Knight, R.T. Prefrontal cortex modulates posterior alpha oscillations during top-down guided visual perception. Proc. Natl. Acad. Sci. USA 2017, 114, 9457–9462. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; File, B.; Boha, R.; Kardos, Z.; Hidasi, Z.; Gaál, Z.A.; Csibri, É.; Salacz, P.; Stam, C.J.; Molnár, M. EEG network connectivity changes in mild cognitive impairment—Preliminary results. Int. J. Psychophysiol. 2014, 92, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Grady, C.L.; Ng, C.; Hasher, L. Age differences in the frontoparietal cognitive control network: Implications for distractibility. Neuropsychologia 2012, 50, 2212–2223. [Google Scholar] [CrossRef]
- Jung, R.E.; Haier, R.J. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav. Brain Sci. 2007, 30, 135–154. [Google Scholar] [CrossRef]
- Vakhtin, A.A.; Ryman, S.G.; Flores, R.A.; Jung, R.E. Functional brain networks contributing to the Parieto-Frontal Integration Theory of Intelligence. Neuroimage 2014, 103, 349–354. [Google Scholar] [CrossRef]
- Meppelink, A.M.; de Jong, B.M.; Renken, R.; Leenders, K.L.; Cornelissen, F.W.; van Laar, T. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain 2009, 132, 2980–2993. [Google Scholar] [CrossRef]
- Evans, J.R.; Budzynski, T.H.; Budzynski, H.K.; Abarbanel, A. Introduction to Quantitative EEG and Neurofeedback: Advanced Theory and Applications; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Meeuwissen, E.B.; Takashima, A.; Fernández, G.; Jensen, O. Increase in posterior alpha activity during rehearsal predicts successful long-term memory formation of word sequences. Hum. Brain Mapp. 2011, 32, 2045–2053. [Google Scholar] [CrossRef]
- Dubovik, S.; Pignat, J.M.; Ptak, R.; Aboulafia, T.; Allet, L.; Gillabert, N.; Magnin, C.; Albert, F.; Momjian-Mayor, I.; Nahum, L.; et al. The behavioral significance of coherent resting-state oscillations after stroke. Neuroimage 2012, 61, 249–257. [Google Scholar] [CrossRef]
- Eggenberger, P.; Wolf, M.; Schumann, M.; De Bruin, E.D. Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front. Aging Neurosci. 2016, 8, 66. [Google Scholar] [CrossRef]
- Stoll, F.M.; Wilson, C.R.; Faraut, M.C.; Vezoli, J.; Knoblauch, K.; Procyk, E. The effects of cognitive control and time on frontal beta oscillations. Cereb. Cortex 2016, 26, 1715–1732. [Google Scholar] [CrossRef] [PubMed]
- Noudoost, B.; Chang, M.H.; Steinmetz, N.A.; Moore, T. Top-down control of visual attention. Curr. Opin. Neurobiol. 2010, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Laney, J.; Adalı, T.; Waller, S.M.; Westlake, K.P. Quantifying motor recovery after stroke using independent vector analysis and graph-theoretical analysis. NeuroImage Clin. 2015, 8, 298–304. [Google Scholar] [CrossRef]
- Bassett, D.S.; Meyer-Lindenberg, A.; Achard, S.; Duke, T.; Bullmore, E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. USA 2006, 103, 19518–19523. [Google Scholar] [CrossRef] [PubMed]
- Reijmer, Y.D.; Freeze, W.M.; Leemans, A.; Biessels, G.J. The effect of lacunar infarcts on white matter tract integrity. Stroke 2013, 44, 2019–2021. [Google Scholar] [CrossRef]
- Philips, G.R.; Daly, J.J.; Príncipe, J.C. Topographical measures of functional connectivity as biomarkers for post-stroke motor recovery. J. Neuroeng. Rehabil. 2017, 14, 67. [Google Scholar] [CrossRef]
- Xin, X.; Duan, F.; Kranz, G.S.; Shu, D.; Fan, R.; Gao, Y.; Yan, Z.; Chang, J. Functional network characteristics based on EEG of patients in acute ischemic stroke: A pilot study. NeuroRehabilitation 2022, 51, 455–465. [Google Scholar] [CrossRef]
- Tóth, B.; Boha, R.; Pósfai, M.; Gaál, Z.A.; Kónya, A.; Stam, C.J.; Molnár, M. EEG synchronization characteristics of functional connectivity and complex network properties of memory maintenance in the delta and theta frequency bands. Int. J. Psychophysiol. 2012, 83, 399–402. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Xu, J.; Schoenfeld, M.A.; Rossini, P.M.; Tatlisumak, T.; Nürnberger, A.; Antal, A.; He, H.; Gao, Y.; Sabel, B.A. Adaptive and maladaptive brain functional network reorganization after stroke in hemianopia patients: An electroencephalogram-tracking study. Brain Connect. 2022, 12, 725–739. [Google Scholar] [CrossRef]
- Rahma, O.N.; Wijaya, S.K.; Prawito; Badri, C. Electroencephalogram analysis with extreme learning machine as a supporting tool for classifying acute ischemic stroke severity. In Proceedings of the 2017 International Seminar on Sensors, Instrumentation, Measurement and Metrology (ISSIMM), Surabaya, Indonesia, 25–26 August 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 180–186. [Google Scholar]
- Wilkinson, C.M.; Burrell, J.I.; Kuziek, J.W.; Thirunavukkarasu, S.; Buck, B.H.; Mathewson, K.E. Predicting stroke severity with a 3-min recording from the Muse portable EEG system for rapid diagnosis of stroke. Sci. Rep. 2020, 10, 18465. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. Quantitative evaluation of task-induced neurological outcome after stroke. Brain Sci. 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, S.; Wong, A.; Read, S. Defining abnormal slow EEG activity in acute ischaemic stroke: Delta/alpha ratio as an optimal QEEG index. Clin. Neurophysiol. 2016, 127, 1452–1459. [Google Scholar] [CrossRef]
- Klados, M.A.; Kanatsouli, K.; Antoniou, I.; Babiloni, F.; Tsirka, V.; Bamidis, P.D.; Micheloyannis, S. A graph theoretical approach to study the organization of the cortical networks during different mathematical tasks. PLoS ONE 2013, 8, e71800. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, G.N.; Kakkos, I.; Dai, Z.; Wang, H.; Sgarbas, K.; Thakor, N.; Bezerianos, A.; Sun, Y. Functional connectivity analysis of mental fatigue reveals different network topological alterations between driving and vigilance tasks. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 740–749. [Google Scholar] [CrossRef]
- Pego-Pérez, E.R.; Fernández-Rodríguez, I.; Pumar-Cebreiro, J.M. National Institutes of Health Stroke Scale, modified Rankin Scale, and modified Thrombolysis in Cerebral Infarction as autonomy predictive tools for stroke patients. Rev. Neurosci. 2019, 30, 701–708. [Google Scholar] [CrossRef]
- Green, T.L.; King, K.M. Functional and psychosocial outcomes 1 year after mild stroke. J. Stroke Cerebrovasc. Dis. 2010, 19, 10–16. [Google Scholar] [CrossRef]
- Abzhandadze, T.; Reinholdsson, M.; Stibrant Sunnerhagen, K. NIHSS is not enough for cognitive screening in acute stroke: A cross-sectional, retrospective study. Sci. Rep. 2020, 10, 534. [Google Scholar] [CrossRef]
- Barrett, A.B.; Barnett, L. Granger causality is designed to measure effect, not mechanism. Front. Neuroinform. 2013, 7, 6. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Lu, J.; Tian, T.; Zhang, G.; Zhou, Y.; Wu, D.; Zhang, S.; Zhu, W. Structural and functional alterations within the Papez circuit in subacute stroke patients. Brain Imaging Behav. 2022, 16, 2681–2689. [Google Scholar] [CrossRef]
- Adhikari, M.H.; Griffis, J.; Siegel, J.S.; Thiebaut de Schotten, M.; Deco, G.; Instabato, A.; Gilson, M.; Corbetta, M. Effective connectivity extracts clinically relevant prognostic information from resting state activity in stroke. Brain Commun. 2021, 3, fcab233. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Safont, G.; Salazar, A.; Vergara, L. Vector score alpha integration for classifier late fusion. Pattern Recognit. Lett. 2020, 136, 48–55. [Google Scholar] [CrossRef]
- Efron, B.; Hastie, T.; Johnstone, I.; Tibshirani, R. Least angle regression. Ann. Stat. 2004, 32, 407–499. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, D. Feature selection and analysis on correlated gas sensor data with recursive feature elimination. Sens. Actuators B Chem. 2015, 212, 353–363. [Google Scholar] [CrossRef]

| HC (N = 40) | CS (N = 40) | SS (N = 40) | p-Value | |
|---|---|---|---|---|
| Gender (M/F) | 15/25 | 22/18 | 22/18 | 0.195 a |
| Age (years) | 62.58 ± 0.92 | 64.93 ± 1.73 | 62.20 ± 1.68 | 0.376 b |
| NIHSS | - | 1.74 ± 0.36 c | 1.67 ± 0.27 d | 0.377 e |
| Educational attainment (years) | 6.83 ± 0.65 | 7.50 ± 0.69 | 6.83 ± 0.67 | 0.647 e |
| Time after stroke (days) | - | 72.05 ± 27.53 | 64.80 ± 39.14 | 0.379 f |
| HC | CS | SS | H-Value | p-Value | Multiple Comparison Test (p-Value a) | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | HC/CS | HC/SS | CS/SS | |||
| RT | 316.754 ± 37.880 | 366.390 ± 82.805 | 388.662 ± 76.989 | 28.342 | <0.001 | 0.006 | <0.001 | 0.087 |
| RA | 0.993 ± 0.009 | 0.976 ± 0.037 | 0.974 ± 0.034 | 16.081 | <0.001 | 0.010 | <0.001 | 1.000 |
| Delta | Theta | Alpha | Beta | |||||
|---|---|---|---|---|---|---|---|---|
| H-Value | p-Value | H-Value | p-Value | H-Value | p-Value | H-Value | p-Value | |
| 6.839 | 0.033 | 0.235 | 0.889 | 3.019 | 0.221 | 5.222 | 0.073 | |
| L | 3.862 | 0.145 | 0.726 | 0.696 | 0.199 | 0.905 | 0.487 | 0.784 |
| 4.950 | 0.084 | 0.505 | 0.777 | 0.824 | 0.662 | 10.313 | 0.006 | |
| 8.481 | 0.014 | 1.639 | 0.441 | 4.544 | 0.103 | 1.952 | 0.377 | |
| 6.802 | 0.033 | 0.565 | 0.754 | 3.344 | 0.188 | 4.987 | 0.083 | |
| Classifiers | HC vs. CS (ACC ± SEM (%)) | HC vs. SS (ACC ± SEM (%)) | ||||||
|---|---|---|---|---|---|---|---|---|
| Corr | Fisher | Relief | LARS | Corr | Fisher | Relief | LARS | |
| LR | 66.25 ± 5.32 | 67.50 ± 5.27 | 65.00 ± 5.37 | 71.25 ± 5.09 | 65.00 ± 5.37 | 65.00 ± 5.37 | 46.25 ± 5.61 | 55.00 ± 5.60 |
| Boost | 63.75 ± 5.41 | 63.75 ± 5.41 | 68.75 ± 5.21 | 76.25 ± 4.79 | 70.00 ± 5.16 | 70.00 ± 5.16 | 51.25 ± 5.62 | 60.00 ± 5.51 |
| Tree | 60.00 ± 5.51 | 60.00 ± 5.51 | 61.25 ± 5.21 | 65.00 ± 4.79 | 63.75 ± 5.41 | 63.75 ± 5.41 | 80.00 ± 4.50 | 58.75 ± 5.54 |
| RF | 57.50 ± 5.56 | 58.75 ± 5.54 | 63.75 ± 5.41 | 70.00 ± 5.16 | 55.00 ± 5.60 | 55.00 ± 5.60 | 53.75 ± 5.61 | 57.50 ± 5.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Xu, M.; Cai, H.; Jiang, Y.; Zheng, X.; Sun, H.; Sun, Y.; Sun, Y. Task Cortical Connectivity Reveals Different Network Reorganizations between Mild Stroke Patients with Cortical and Subcortical Lesions. Brain Sci. 2023, 13, 1143. https://doi.org/10.3390/brainsci13081143
Cai J, Xu M, Cai H, Jiang Y, Zheng X, Sun H, Sun Y, Sun Y. Task Cortical Connectivity Reveals Different Network Reorganizations between Mild Stroke Patients with Cortical and Subcortical Lesions. Brain Sciences. 2023; 13(8):1143. https://doi.org/10.3390/brainsci13081143
Chicago/Turabian StyleCai, Jiaye, Mengru Xu, Huaying Cai, Yun Jiang, Xu Zheng, Hongru Sun, Yu Sun, and Yi Sun. 2023. "Task Cortical Connectivity Reveals Different Network Reorganizations between Mild Stroke Patients with Cortical and Subcortical Lesions" Brain Sciences 13, no. 8: 1143. https://doi.org/10.3390/brainsci13081143
APA StyleCai, J., Xu, M., Cai, H., Jiang, Y., Zheng, X., Sun, H., Sun, Y., & Sun, Y. (2023). Task Cortical Connectivity Reveals Different Network Reorganizations between Mild Stroke Patients with Cortical and Subcortical Lesions. Brain Sciences, 13(8), 1143. https://doi.org/10.3390/brainsci13081143





