A Study of Brain Function Characteristics of Service Members at High Risk for Accidents in the Military
Abstract
:1. Introduction
1.1. Background and Purpose of the Study
1.2. Brain Function and Autonomic Nervous System Characteristics
1.3. Stress, Depression, and Their Relationship to Brain Function and the Autonomic Nervous System
2. Previous Research
2.1. Characteristics of Military Organizations
2.2. Relationship between Stress, Depression, and Traumatic Events
3. Research Methods
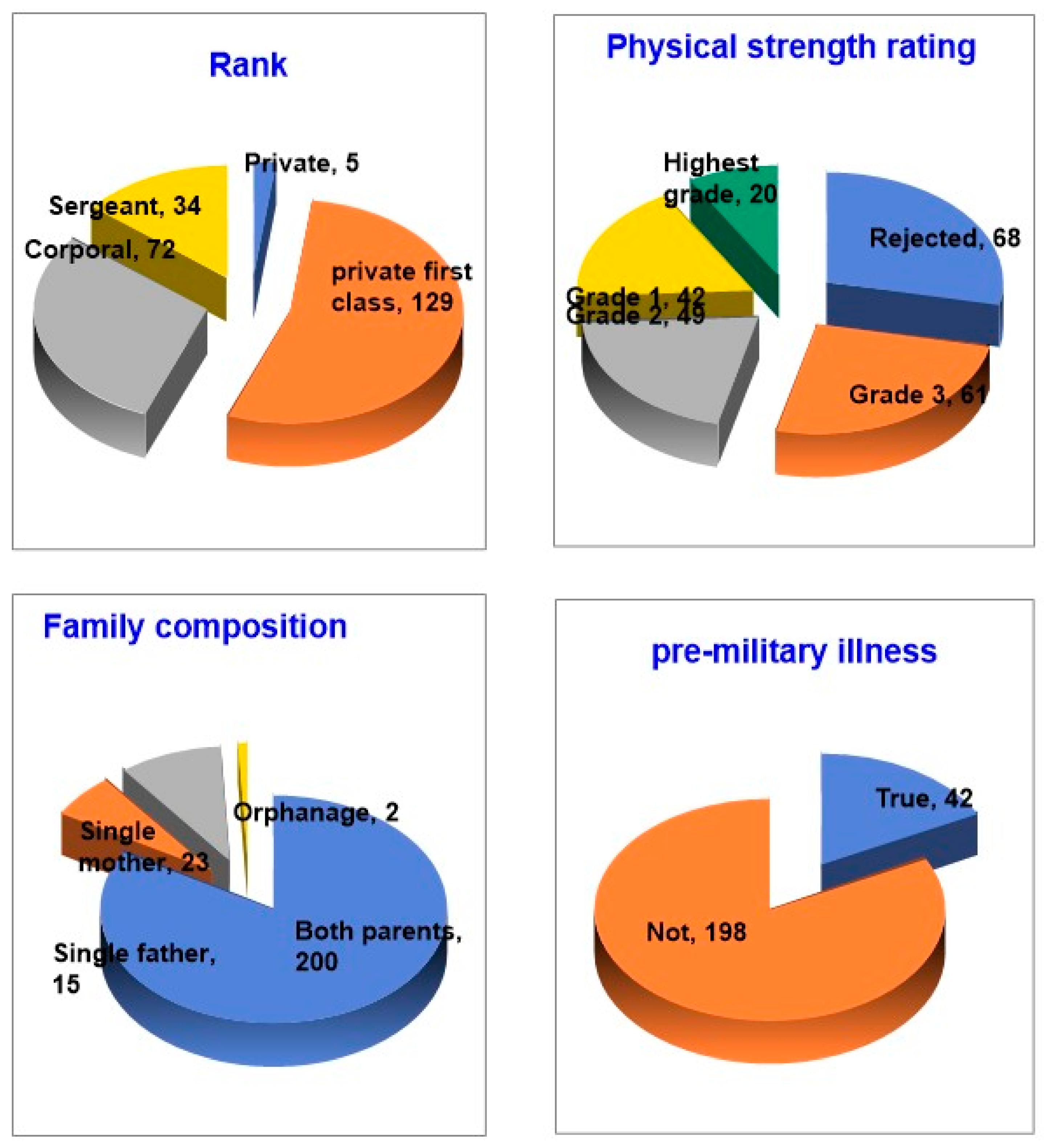
3.1. Choosing Who to Study
3.2. Scope and Procedure of the Study
3.3. Measurement Equipment
3.4. Data Collection and Analysis
3.4.1. Brain Waves
- All spectral power values in the 4–13 Hz frequency domain were summed and divided by 2.
- The frequency where the cumulative power in the 4–13 Hz frequency domain first exceeded the value calculated in step 1 was selected.
3.4.2. Pulse Wave
4. Group Design and Participants
5. Data Collection and Analysis Approaches
6. EEG, Pulse Waves, and Survey Results
6.1. Analyze the EEG Index of Four Populations
6.1.1. Compare between-Group Means by EEG Index
6.1.2. Compare Groups by EEG Index
6.1.3. Brain Wave Post-Test
6.2. Analyze Pulse Wave Indices for Four Populations
6.2.1. Compare Group Means by Pulse Wave Index
6.2.2. Compare Groups by Pulse Wave Index
6.2.3. Pulse Wave Post-Test
6.3. Correlate EEG, Pulse Waves, and Survey Results
6.3.1. Analyzing the Correlation between EEG and Pulse Wave Indices
6.3.2. Correlating Brainwave Indices with Survey Results
6.3.3. Correlate Pulse Wave Indices with Survey Results
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, M.J. The study of soldier’s human right and the program for preventing suicide incidentin Korean Military. Democr. Hum. Rights 2008, 8, 79–109. [Google Scholar]
- Yang, M.; Song, M.; Shin, H. The Military Peer Counselling Training Program. Youth Couns. Res. 2009, 17, 19–31. [Google Scholar]
- Lee, J.-H.; Cho, J.-Y. Anxiety, Depression and Impulsiveness according to Military Service Duration in Army-Enlisted Males. J. Korean Neuropsychiatr. Assoc. 1999, 966–972. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-49526 (accessed on 1 March 2022).
- Military Culture Innovation Team, Ministry of National Defense Personnel Welfare Center. Status of Military Deaths; e-Country Indicators; Military Culture Innovation Team, Ministry of National Defense Personnel Welfare Center: Seoul, Republic of Korea, 2022. [Google Scholar]
- Sampson, L.; Gradus, J.L.; Cabral, H.J.; Rosellini, A.J.; Fink, D.S.; Cohen, G.H.; Liberzon, I.; Galea, S. Stressful life events and incident depression among U.S. military personnel. Soc. Psychiatry Psychiatr. Epidemiol. 2023, 58, 1009–1018. [Google Scholar] [CrossRef]
- Yoon, M.J. The Sociological Analysis of Military Culture and Suicide Accidents in Korea. Korean Soc. Soc. Hist. 2008, 201, 165–193. [Google Scholar]
- Lewandowski-Romps, L.; Peterson, C.; Berglund, P.A.; Collins, S.; Cox, K.; Hauret, K.; Jones, B.; Kessler, R.C.; Mitchell, C.; Park, N.; et al. Risk Factors for Accident Death in the U.S. Army, 2004−2009. Am. J. Prev. Med. 2014, 47, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Greden, J.F.; Valenstein, M.; Spinner, J.; Blow, A.; Gorman, L.A.; Dalack, G.W.; Marcus, S.; Kees, M. Buddy-to-Buddy, a citizen soldier peer support program to counteract stigma, PTSD, depression, and suicide. Ann. N. Y. Acad. Sci. 2010, 1208, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scofield, D.E.; Proctor, S.P.; Kardouni, J.R.; Hill, O.T.; McKinnon, C.J. Risk Factors for Mild Traumatic Brain Injury and Subsequent Post-Traumatic Stress Disorder and Mental Health Disorders among United States Army Soldiers. J. Neurotrauma 2017, 34, 3249–3255. [Google Scholar] [CrossRef]
- Ge, Q.-Q.; Zhou, X.-N.; Liu, Y.-Z.; Su, W.-J.; Wang, W. Role of neuroticism in depressive symptoms among officers and soldiers: Mediating effects of negative automatic thoughts and response psychological stress. Acad. J. Nav. Med. Univ. 2022, 43, 821–826. [Google Scholar]
- Bandoli, G.; Campbell-Sills, L.; Kessler, R.C.; Heeringa, S.G.; Nock, M.K.; Rosellini, A.J.; Sampson, N.A.; Schoenbaum, M.; Ursano, R.J.; Stein, M.B. Childhood adversity, adult stress, and the risk of major depression or generalized anxiety disorder in US soldiers: A test of the stress sensitization hypothesis. Psychol. Med. 2017, 47, 2379–2392. [Google Scholar] [CrossRef]
- Vanhollebeke, G.; De Smet, S.; De Raedt, R.; Baeken, C.; van Mierlo, P.; Vanderhasselt, M.-A. The neural correlates of psychosocial stress: A systematic review and meta-analysis of spectral analysis EEG studies. Neurobiol. Stress 2022, 18, 100452. [Google Scholar] [CrossRef] [PubMed]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological links between stress and anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef]
- Mazure, C.M. Life stressors as risk factors in depression. Clin. Psychol. Sci. Pract. 1998, 5, 291–313. [Google Scholar] [CrossRef]
- Lazarus, R.S. Stress, Appraisal, and Coping; Springer Publishing Company: Berlin/Heidelberg, Germany, 1984; ISBN 0-8261-4191-9. [Google Scholar]
- Folkman, S. Stress: Appraisal and Coping. In Encyclopedia of Behavioral Medicine; Springer International Publishing: Cham, Switzerland, 2020; pp. 2177–2179. [Google Scholar]
- Demichelis, O.P.; Grainger, S.A.; Hubbard, R.E.; Henry, J.D. Emotion regulation mediates the relationship between social frailty and stress, anxiety, and depression. Sci. Rep. 2023, 13, 6430. [Google Scholar] [CrossRef] [PubMed]
- Steffy, B.D.; Jones, J.W.; Murphy, L.R.; Kunz, L. A demonstration of the impact of stress abatement program on reducing employee’s accidents and their costs. Am. J. Health Promot. 1986, 1, 25–32. [Google Scholar] [CrossRef]
- Sutherland, V.J.; Cooper, C.L. Stress and Accidents in the Offshore Oil and Gas Industry; Gulf Publishing Company: Houston, TX, USA, 1991; p. 227. [Google Scholar]
- Bakhtiari, A.; Basirat, Z.; Nasiri-Amiri, F. Sexual dysfunction in women undergoing fertility treatment in Iran: Prevalence and associated risk factors. J. Reprod. Infertil. 2016, 17, 26–33. [Google Scholar]
- Ramya, S.; Poornima, P.; Jananisri, A.; Geofferina, I.P.; Bavyataa, V.; Divya, M.; Priyanga, P.; Vadivukarasi, J.; Sujitha, S.; Elamathi, S.; et al. Role of Hormones and the Potential Impact of Multiple Stresses on Infertility. J. Stress. 2023, 3, 454–474. [Google Scholar] [CrossRef]
- Ghahvehchi-Hosseini, F.; Manshadi, E.; Mohammadi, A.; Jahromi, G.P.; Hatef, B. Evaluation of the persistence effect acute social stress test on the alpha band power. J. Mil. Med. 2018, 20, 509–518. [Google Scholar]
- Mane, S.A.M.; Shinde, A. StressNet: Hybrid model of LSTM and CNN for stress detection from electroencephalogram signal (EEG). Results Control Optim. 2023, 11, 100231. [Google Scholar] [CrossRef]
- Morris, A.T.; Temereanca, S.; Zandvakili, A.; Thorpe, R.; Sliva, D.D.; Greenberg, B.D.; Carpenter, L.L.; Philip, N.S.; Jones, S.R. Fronto-central resting-state 15–29 Hz transient beta events change with therapeutic transcranial magnetic stimulation for posttraumatic stress disorder and major depressive disorder. Sci. Rep. 2023, 13, 6366. [Google Scholar] [CrossRef]
- Anijärv, T.E.; Can, A.T.; Gallay, C.C.; Forsyth, G.A.; Dutton, M.; Mitchell, J.S.; Hermens, D.F.; Lagopoulos, J. Spectral Changes of EEG Following a 6-Week Low-Dose Oral Ketamine Treatment in Adults With Major Depressive Disorder and Chronic Suicidality. Int. J. Neuropsychopharmacol. 2023, 26, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lytaev, S. Psychological and Neurophysiological Screening Investigation of the Collective and Personal Stress Resilience. Behav. Sci. 2023, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Loganovsky, K.N.; Zdanevich, N.A.; Gresko, M.V.; Marazziti, D.; Loganovskaja, T.K. Neuropsychiatric characteristics of antiterrorist operation combatants in the Donbass (Ukraine). CNS Spectr. 2018, 23, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedford, C.E.; Nakamura, Y.; Marchand, W.R.; Garland, E.L. Heightened autonomic reactivity to negative affective stimuli among activeduty soldiers with PTSD and opioid-treated chronic pain. Psychiatry Res. 2022, 309, 114394. [Google Scholar] [CrossRef] [PubMed]
- Stojar, R.; Fučík, J.; Frank, L. Wars without Soldiers and Casualties or Victories in Hearts and Minds? In Proceedings of the Modelling and Simulation for Autonomous Systems: 6th International Conference, MESAS 2019, Palermo, Italy, 29–31 October 2019; Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 372–378. [Google Scholar]
- Britton, J.W.; Frey, L.C.; Hopp, J.L.; Korb, P.; Koubeissi, M.Z.; Lievens, W.E.; Pestana-Knight, E.M.; Louis, E.K.S. Electroencephalography (EEG): An Introductory Text and Atlas of Normal and Abnormal Findings in Adults, Children, and Infants; Louis, E.K.S., Frey, L.C., Eds.; American Epilepsy Society: Chicago, IL, USA, 2016. [Google Scholar]
- Soininen, H.; Reinikainen, K.; Partanen, J.; Mervaala, E.; Paljarvi, L.; Helkala, E. Slowing of the dominant occipital rhythm in electroencephalogram is associated with low concentration of noradrenaline in the thalamus in patients with Alzheimer’s disease. Neurosci. Lett. 1992, 137, 5–8. [Google Scholar] [CrossRef]
- Lee, G.; Shin, J. Effects of the barefoot walking experience program on EEG and brain utilization ability of elementary school students. J. Korean Assoc. Learn.-Centered Curric. Instr. 2019, 19, 219–238. [Google Scholar] [CrossRef]
- Park, Y.-J.; Lim, U.-N.; Park, S.; Shin, J.-H. Effect of Brain and Pulse Waves on Safety Consciousness and Safety Commitment of Workers at Construction Sites. Sensors 2021, 21, 2753. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Xu, L.; Song, T.; Peng, Z.; Zhang, Z.; An, X.; Chen, S.; Zhong, X.; Shao, Y. Reduced Resting-State EEG Power Spectra and Functional Connectivity after 24 and 36 Hours of Sleep Deprivation. Brain Sci. 2023, 13, 949. [Google Scholar] [CrossRef]
- Koudelková, Z.; Strmiska, M.; Jašek, R. Analysis of brain waves according to their frequency. Int. J. Biol. Biomed. Eng. 2018, 12, 202–207. [Google Scholar]
- Mody, C.; McIntyre, H.; Miller, B.; Altman, K.; Read, S. Computerized EEG frequency analysis and topographic brain mapping in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1991, 620, 45–56. [Google Scholar] [CrossRef]
- Du, J.; Chen, X.; Xi, L.; Jiang, B.; Ma, J.; Yuan, G.; Hassan, A.; Fu, E.; Huang, Y. Electroencephalography-Based Neuroemotional Responses in Cognitively Normal and Cognitively Impaired Elderly by Watching the Ardisia mamillata Hance with Fruits and without Fruits. Int. J. Environ. Res. Public Health 2022, 19, 10020. [Google Scholar] [CrossRef] [PubMed]
- Jelic, V.; Kowalski, J. Evidence-based evaluation of diagnostic accuracy of resting EEG in dementia and mild cognitive impairment. Clin. EEG Neurosci. 2009, 40, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yin, J.; Chen, X.; Hassan, A.; Fu, E.; Li, X. Electroencephalography (EEG)-Based Neural Emotional Response to Flower Arrangements (FAs) on Normal Elderly (NE) and Cognitively Impaired Elderly (CIE). Int. J. Environ. Res. Public Health 2022, 19, 3971. [Google Scholar] [CrossRef] [PubMed]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Nagendra, B.; Abhishek, M.L.; Balakrishnan, A.; Javali, M.; Rao, N.P.; et al. Stimulus-induced gamma rhythms are weaker in human elderly with mild cognitive impairment and Alzheimer’s disease. eLife 2021, 10, e61666. [Google Scholar] [CrossRef]
- Kelly, R.; Hayward, C.; Avolio, A.; O’Rourke, M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 1989, 80, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Takazawa, K.; Tanaka, N.; Takeda, K.; Kurosu, F.; Ibukiyama, C. Underestimation of vasodilator effects of nitroglycerin by upper limb blood pressure. Hypertension 1995, 26, 520–523. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology; The North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Casolo, G.C.; Stroder, P.; Signorini, C.; Calzolari, F.; Zucchini, M.; Balli, E.; Sulla, A.; Lazzerini, S. Heart rate variability during the acute phase of myocardial infarction. Circulation 1992, 85, 2073–2079. [Google Scholar] [CrossRef] [Green Version]
- Stein, K.M.; Borer, J.S.; Hochreiter, C.; Okin, P.M.; Herrold, E.M.; Devereux, R.B.; Kligfield, P. Prognostic value and physiological correlates of heart rate variability in chronic severe mitral regurgitation. Circulation 1993, 88, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Smeets, T.; Cornelisse, S.; Quaedflieg, C.; Meyer, T.; Jelicic, M.; Merckelbach, H. Introducing the maastricht acute stress test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 2012, 37, 1998–2008. [Google Scholar] [CrossRef]
- Duman, R.S. Depression: A case of neuronal life and death? Biol. Psychiatry 2004, 56, 140–145. [Google Scholar] [CrossRef]
- Duman, R.S.; Malberg, J.; Nakagawa, S. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 2001, 299, 401–407. [Google Scholar]
- Posener, J.A.; Wang, L.; Price, J.L.; Gado, M.H.; Province, M.A.; Miller, M.I.; Babb, C.M.; Csernansky, J.G. High-dimensional mapping of the hippocampus in depression. Am. J. Psychiatry 2003, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Chickens, eggs and hippocampal atrophy. Nat. Neurosci. 2002, 5, 1111–1113. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur. J. Neurosci. 2002, 16, 129–136. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999, 22, 105–122. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef] [Green Version]
- Bessa, J.M.; Ferreira, D.; Melo, I.; Marques, F.; Cerqueira, J.J.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry 2009, 14, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated Stress Causes Cognitive Impairment by Suppressing Glutamate Receptor Expression and Function in Prefrontal Cortex. Neuron 2012, 73, 962–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Pillai, A.G.; Chattarji, S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004, 128, 667–673. [Google Scholar] [CrossRef]
- Bessa, J.M.; Morais, M.; Marques, F.; Pinto, L.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl. Psychiatry 2013, 3, e266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevets, W.C. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 2001, 11, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Czéh, B.; Michaelis, T.; Watanabe, T.; Frahm, J.; De Biurrun, G.; Van Kampen, M.; Bartolomucci, A.; Fuchs, E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc. Natl. Acad. Sci. USA 2001, 98, 12796–12801. [Google Scholar] [CrossRef]
- Manji, H.K.; Drevets, W.C.; Charney, D.S. The cellular neurobiology of depression. Nat. Med. 2001, 7, 541–547. [Google Scholar] [CrossRef]
- Bremner, J.D.; Narayan, M.; Anderson, E.R.; Staib, L.H.; Miller, H.L.; Charney, D.S. Hippocampal volume reduction in major depression. Am. J. Psychiatry 2000, 157, 115–117. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Simpson, J.R., Jr.; Todd, R.D.; Reich, T.; Vannier, M.; Raichle, M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997, 386, 824–827. [Google Scholar] [CrossRef]
- Nasca, C.; Bigio, B.; Zelli, D.; Nicoletti, F.; McEwen, B.S. Mind the gap: Glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol. Psychiatry 2015, 20, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.J.; Ebmeier, K.P.; Glabus, M.F.; Goodwin, G.M. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression: Controlled magnetic resonance imaging study. Br. J. Psychiatry 1998, 172, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffens, D.C.; Byrum, C.E.; McQuoid, D.R.; Greenberg, D.L.; Payne, M.E.; Blitchington, T.F.; MacFall, J.R.; Krishnan, K.R.R. Hippocampal volume in geriatric depression. Biol. Psychiatry 2000, 48, 301–309. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.M.; Campbell, S.; McEwen, B.S.; Macdonald, K.; Amano, S.; Joffe, R.T.; Nahmias, C.; Trevor Young, L. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA 2003, 100, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Kan, D.P.X.; Lee, P.F. Decrease alpha waves in depression: An electroencephalogram(EEG) study. In Proceedings of the International Conference on BioSignal Analysis, Processing and Systems, Kuala Lumpur, Malaysia, 26–28 May 2015; ICBAPS 7292237. pp. 156–161. [Google Scholar]
- Galderisi, S.; Mucci, A.; Bucci, P.; Romano, G.; Maj, M. Quantitative EEG test dose procedure in the prediction of response to treatment with antipsychotic drugs. Psychiatry Res. Neuroimaging 1997, 68, 162–163. [Google Scholar] [CrossRef]
- Hughes, J.R.; John, E.R. Conventional and quantitative elecroencephalography in psychiatry. J. Neuropsychiatry Clin. Neurosci. 1999, 11, 190–208. [Google Scholar] [CrossRef] [Green Version]
- Allen, B.; Urry, H.L.; Hitt, S.K.; Coan, J.A. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 2004, 41, 269–280. [Google Scholar] [CrossRef]
- Debener, S.; Beauducel, A.; Nessler, D.; Brocke, B.; Heilemann, H.; Kayser, J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology 2000, 41, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, P.; Yang, H.; Roy, P.P.; van Vugt, M. Comparing resting state and task-based EEG using machine learning to predict vulnerability to depression in a non-clinical population. Sci. Rep. 2023, 13, 7467. [Google Scholar] [CrossRef]
- Chaudhury, D.; Liu, H.; Han, M.-H. Neuronal correlates of depression. Cell. Mol. Life Sci. 2015, 72, 4825–4848. [Google Scholar] [CrossRef] [Green Version]
- Hammond, D.C. Neurofeedback treatment of depression and anxiety. J. Adult Dev. 2005, 12, 131–137. [Google Scholar] [CrossRef]
- Hosseinifard, B.; Hassan, M.; Rostami, R. Classifying depression patients and normal subjects using machine learning techniques and nonlinear features from EEG signal. Comput. Methods Program. Biomed. 2012, 109, 339–345. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Rosenfeld, C.J.P. Frontal EEG Alpha Asymmetry Depression and Cognitive Functioning. Cogn. Emot. 1998, 12, 449–478. [Google Scholar] [CrossRef]
- Mohammadzadeh, B.; Sattari, K.; Lotfizadeh, M. Determining the relationship between depression and brain waves in depressed subjects using Pearson correlation and regression. Int. J. Epidemiol. Res. 2016, 3, 375–384. [Google Scholar]
- Smit, D.J.; Posthuma, D.; Boomsma, D.I.; De Geus, E.J. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biol. Psychol. 2007, 74, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Canli, T.; Qiu, M.; Omura, K.; Lesch, K.P. Neural correlates of epigenesis. Biol. Sci. 2006, 103, 16033–16038. [Google Scholar] [CrossRef]
- Allen, J.J.B.; Coan, J.A.; Nazarian, M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 2004, 67, 183–218. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Andrzej, W. Beta activity: A carrier for visual attention. Acta Neurobiol. Exp. 2000, 60, 247–260. [Google Scholar]
- Lin, T.-W.; Kuo, Y.-M. Exercise Benefits Brain Function: The Monoamine Connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciolino, N.R.; Holmes, P.V. Exercise offers anxiolytic potential: A role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci. Biobehav. Rev. 2012, 36, 1965–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, H.; Duman, H.; Puşuroğlu, M.; Yılmaz, A.S. Anxiety disorders and depression are associated with resistant hypertension. Adv. Clin. Exp. Med. 2023; ahead of print. [Google Scholar] [CrossRef]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Johnson, H.M. Anxiety and Hypertension: Is There a Link? A Literature Review of the Comorbidity Relationship between Anxiety and Hypertension. Curr. Hypertens. Rep. 2019, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, O.Y.; Tashchuk, V.K.; Barchuk, N.I.; Amelina, T.M.; Hrechko, S.I.; Trefanenko, I.V. Anxiety and Depressive Disorders in Patients with Arterial Hypertension. Wiad. Lek. 2021, 74, 455–459. [Google Scholar] [CrossRef]
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2,3-Dioxygenase 1 Influenced the Age Onset of Parkinson’s Disease. Front. Biosci.—Landmark 2022, 27, 265. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Medica Indones. 2017, 49, 158–165. [Google Scholar]
- Sfera, A.; Hazan, S.; Kozlakidis, Z.; Klein, C. Microbiota-derived psychedelics: Lessons from COVID-19. Adv. Clin. Exp. Med. 2023, 32, 395–399. [Google Scholar] [CrossRef]
- Asano, M.; Kajiwara, Y.; Shimakawa, H. Estimating Working Stressor Based on Pulse Wave. In Proceedings of the 9th International Conference on Machine Learning and Computing, Singapore, 24–26 February 2017; pp. 23–27. [Google Scholar]
- Sriramprakash, S.; Prasanna, V.D.; Murthy, O.V.R. Stress Detection in Working People. Procedia Comput. Sci. 2017, 115, 359–366. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Liu, C.; Du, G.; Zhao, L.; Dong, H.; Zhao, S.; Liu, Y. Feasibility study for detection of mental stress and depression using pulse rate variability metrics via various durations. Biomed. Signal Process. Control 2023, 79, 104145. [Google Scholar] [CrossRef]
- Watkins, L.L.; Grossman, P.; Krishnan, R.; Blumenthal, J.A. Anxiety reduces baroreflex cardiac control in older adults with major depression. Psychosom. Med. 1999, 61, 334–340. [Google Scholar] [CrossRef]
- Seldenrijk, A.; van Hout, H.P.J.; van Marwijk, H.W.J. Depression, Anxiety, and Arterial Stiffness. Biol. Psychiatry 2011, 69, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Yeragani, V.K.; Tancer, M.; Seema, K.P.; Josyula, K.; Desai, N. Increased pulse-wave velocity in patients with anxiety: Implications for autonomic dysfunction. J. Psychosom. Res. 2006, 61, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hernando, A.; Lazaro, J.; Gil, E.; Arza, A.; Garzon, J.M.; Lopez-Anton, R.; de la Camara, C.; Laguna, P.; Aguilo, J.; Bailon, R. Inclusion of respiratory frequency information in heart rate variability analysis for stress assessment. IEEE J. Biomed. Health Inform. 2016, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Zhao, Y.; Ye, P.F.; Zhang, J.; Zou, J.Z. Detecting driving stress in physiological signals based on multimodal feature analysis and kernel classifiers. Expert Syst. Appl. 2017, 85, 279–291. [Google Scholar] [CrossRef]
- Byun, S.; Kim, A.Y.; Jang, E.H.; Kim, S.; Choi, K.W.; Yu, H.Y.; Jeon, H.J. Detection of major depressive disorder from linear and nonlinear heart rate variability features during mental task protocol. Comput. Biol. Med. 2019, 112, 103381. [Google Scholar] [CrossRef]
- Healey, J.A.; Picard, R.W. Detecting stress during real-world driving tasks using physiological sensors. IEEE Trans. Intell. Transp. Syst. 2005, 6, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Betti, S.; Lova, R.M.; Rovini, E.; Acerbi, G.; Santarelli, L.; Cabiati, M.; Del Ry, S.; Cavallo, F. Evaluation of an integrated system of wearable physiological sensors for stress monitoring in working environments by using biological markers. IEEE Trans. Biomed. Eng. 2018, 65, 1748–1758. [Google Scholar]
- Kang, J.S.; Jang, G.J.; Lee, M.H. Stress status classification based on EEG signals. J. Inst. Internet Broadcast. Commun. 2016, 16, 103–108. [Google Scholar] [CrossRef]
- Kwon, I. A Study on the Consciousness Tendency and the Military Stress and Adjustment of New-Generation Soldiers; Hannam University: Daejeon, Republic of Korea, 2004. [Google Scholar]
- Stansfeld, S.; Candy, B. Psychosocial work environment and mental health-a meta-analytic review. Scand. J. Work Environ. Health 2006, 32, 443–462. [Google Scholar] [CrossRef]
- Reason, J. Managing the Risks of Organizational Accidents; Routledge: London, UK, 2016; pp. 1–252. [Google Scholar]
- Mazure, C.M.; Bruce, M.L.; Maciejewski, P.K.; Jacobs, S.C. Adverse life events and cognitive-personality characteristics in the prediction of major depression and antidepressant response. Am. J. Psychiatry 2000, 157, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C.L.; Mayol, A. Depression and cognitive characteristics of stressful life-event types. J. Abnorm. Psychol. 1982, 91, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Beckes, L.; Coan, J.A. Social Baseline Teory and the Social Regulation of Emotion. Sci. Couple 2013, 12, 93–106. [Google Scholar]
- Beckes, L.; Sbarra, D.A. Social baseline theory: State of the science and new directions. Curr. Opin. Psychol. 2022, 43, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Poudel, L.; Bhandari, N.; Adhikari, N.; Shrestha, B.; Poudel, B.; Bishwokarma, A.; Kuikel, B.S.; Timalsena, D.; Paneru, B.; et al. Prevalence and factors associated with depression, anxiety and stress symptoms among construction workers in Nepal. PLoS ONE 2023, 18, e0284696. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, H.; Serbaya, S.H.; Rizwan, A.; Saleem, M.; Maleh, Y.; Alamgir, Z. Impact of emotional intelligence on the stress and safety of construction workers’ in Saudi Arabia. Eng. Constr. Archit. Manag. 2023, 30, 1365–1378. [Google Scholar] [CrossRef]
- Alonso, F.; Esteban, C.; Gonzalez-Marin, A.; Alfaro, E.; Useche, S.A. Job stress and emotional exhaustion at work in Spanish workers: Does unhealthy work affect the decision to drive? PLoS ONE 2020, 15, e0227328. [Google Scholar] [CrossRef] [Green Version]
- Monllor, P.; Cervera-Ferri, A.; Lloret, M.-A.; Esteve, D.; Lopez, B.; Leon, J.-L.; Lloret, A. Electroencephalography as a non-invasive biomarker of Alzheimer’s disease: A forgotten candidate to substitute CSF molecules? Int. J. Mol. Sci. 2021, 22, 10889. [Google Scholar] [CrossRef]
- Müller-Putz, G.R. Chapter 18—Electroencephalography. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 168, pp. 249–262. [Google Scholar]
- Malver, L.P.; Brokjær, A.; Staahl, C.; Graversen, C.; Andresen, T. Electroencephalography and analgesics. Br. J. Clin. Pharmacol. 2013, 77, 72–95. [Google Scholar] [CrossRef] [Green Version]
- Tsolaki, A.; Kazis, D.; Kompatsiaris, I.; Kosmidou, V.; Tsolaki, M. Electroencephalogram and Alzheimer’s disease: Clinical and research approaches. Int. J. Alzheimers Dis. 2014, 2014, 349249. [Google Scholar] [CrossRef] [Green Version]
- de Aguiar Neto, F.S.; Rosa, J.L.G. Depression biomarkers using non-invasive EEG: A review. Neurosci. Biobehav. Rev. 2019, 105, 83–93. [Google Scholar] [CrossRef]
- Mantri, S.; Patil, D.; Agrawal, P.; Wadhai, V. Noninvasive EEG signal processing framework for real time depression analysis. In Proceedings of the 2015 SAI Intelligent Systems Conference (IntelliSys), London, UK, 10–11 November 2015. [Google Scholar] [CrossRef]
- Adamis, D.; Sahu, S.; Treloar, A. The utility of EEG in dementia: A clinical perspective. Int. J. Geriatr. Psychiatry 2005, 20, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Segerstrom, S.C.; Nes, L.S. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol. Sci. 2007, 18, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Tran, Y.; Wijesuriya, N.; Tarvainen, M.; Karjalainen, P.; Craig, A. The relationship between spectral changes in heart rate variability and fatigue. J. Psychophysiol. 2009, 23, 143–151. [Google Scholar] [CrossRef]
- Malik, M.; Xia, R.; Odemuyiwa, O.; Staunton, A.; Poloniecki, J.; Camm, A. Influence of the recognition artefact in automatic analysis of longterm electrocardiograms on time-domain measurement of heart rate variability. Med. Biol. Eng. Comput. 1993, 31, 539–544. [Google Scholar] [CrossRef]
- Mongin, D.; Chabert, C.; Extremera, M.G.; Hue, O.; Courvoisier, D.S.; Carpena, P.; Bernaola Galvan, P.A. Decrease of heart rate variability during exercise: An index of cardiorespiratory fitness. PLoS ONE 2022, 17, e0273981. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-term heart rate variability—Influence of gender and age in healthy subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef] [Green Version]
- Inoue, N.; Kawakami, H.; Yamamoto, H.; Ito, C.; Fujiwara, S.; Sasaki, H.; Kihara, Y. Second derivative of the finger photoplethysmogram and cardiovascular mortality in middle-aged and elderly Japanese women. Hypertens. Res. 2017, 40, 207–211. [Google Scholar] [CrossRef]
- Asyrafi, H.; Sunarno, F.; Waruwu, M.M.; Wijaya, R. Photoplethysmography (PPG) Signal-Based Chronological Age Estimation Algorithm. In Proceedings of the 2022 International Conference of Science and Information Technology in Smart Administration, Denpasar, Indonesia, 10–12 November 2022; ICSINTESA 2022. pp. 128–132. [Google Scholar]
- Tsai, P.-Y.; Huang, C.-H.; Guo, J.-W.; Li, Y.-C.; Wu, A.-Y.A.; Lin, H.-J.; Wang, T.-D. Coherence between decomposed components of wrist and finger ppg signals by imputing missing features and resolving ambiguous features. Sensors 2021, 21, 4315. [Google Scholar] [CrossRef]
- Rinkevĭcius, M.; Kontaxis, S.; Gil, E.; Bailón, R.; Lázaro, J.; Laguna, P.; Marozas, V. Photoplethysmogram Signal Morphology-Based Stress Assessment. In Proceedings of the 2019 Computing in Cardiology (CinC), Singapore, 8–11 September 2019. [Google Scholar]
- Santos, S.A.; Venema, B.; Leonhardt, S. Accelerometer-assisted PPG measurement during physical exercise using the LAVIMO sensor system. Acta Polytech. 2012, 52, 80–85. [Google Scholar] [CrossRef]
- Georgieva-Tsaneva, G.; Gospodinova, E.; Gospodinov, M.; Cheshmedzhiev, K. Portable sensor system for registration, processing and mathematical analysis of PPG signals. Appl. Sci. 2020, 10, 1051. [Google Scholar] [CrossRef] [Green Version]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Chanraud, S.; Pitel, A.-L.; Shankaranarayanan, A.; Alsop, D.C.; Rohlfing, T.; Sullivan, E.V. Volumetric cerebral perfusion imaging in healthy adults: Regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL). Psychiatry Res.—Neuroimaging 2010, 182, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Tian, X.; Wong, C.-W.; Wu, M. Learning Your Heart Actions from Pulse: ECG Waveform Reconstruction from PPG. IEEE Internet Things J. 2021, 8, 16734–16748. [Google Scholar] [CrossRef]
- Huotari, M.; Maatta, K.; Kostamovaara, J. A pulse waveform data decomposition based on multi component curve fit compared with second derivative photoplethysmography and phase plane plot. In Proceedings of the BIOSIGNALS 2011—International Conference on Bio-Inspired Systems and Signal Processing, Rome, Italy, 26–29 January 2011; pp. 477–480. [Google Scholar]
- Reddy, G.N.K.; Manikandan, M.S.; Murty, N.V.L.N. Evaluation of Objective Distortion Measures for Automatic Quality Assessment of Processed PPG Signals for Real-Time Health Monitoring Devices. IEEE Access 2022, 10, 15707–15745. [Google Scholar] [CrossRef]
- Ryu, M.-O.; Lee, S.; Baek, K.-K. A study on the Brain function specialty based on the Maladaptive Soldier by Brain waves analysis. J. Korean Soc. Ind. Eng. Technol. 2014, 15, 1916–1922. [Google Scholar]
- Moghaddam, B. Stress activation of glutamate neurotransmission in the prefrontal cortex: Implications for dopamine-associated psychiatric disorders. Biol. Psychiatry 2002, 51, 775–787. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012, 13, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Swanson, C.J.; Bures, M.; Johnson, M.P.; Linden, A.-M.; Monn, J.A.; Schoepp, D.D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005, 4, 131–144. [Google Scholar] [CrossRef]
- Hennebelle, M.; Champeil-Potokar, G.; Lavialle, M.; Vancassel, S.; Denis, I. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr. Rev. 2014, 72, 99–112. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, M.A.; May, R.W.; Koutnik, A.P.; Kabbaj, M.; Fincham, F.D. Sympathetic vasomotor tone is associated with depressive symptoms in young females: A potential link between depression and cardiovascular disease. Am. J. Hypertens. 2013, 26, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubzansky, L.D.; Koenen, K.C.; Spiro, A., III; Vokonas, P.S.; Sparrow, D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the normative aging study. Arch. Gen. Psychiatry 2007, 64, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherrer, J.F.; Chrusciel, T.; Zeringue, A.; Garfield, L.D.; Hauptman, P.J.; Lustman, P.J.; Freedland, K.E.; Carney, R.M.; Bucholz, K.K.; Owen, R.; et al. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed Veterans Administration patients. Am. Heart J. 2010, 159, 772–779. [Google Scholar] [CrossRef]
- Boscarino, J.A. A prospective study of PTSD and early-age heart disease mortality among vietnam veterans: Implications for surveillance and prevention. Psychosom. Med. 2008, 70, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into structural and functional organization of the brain: Evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the role of vmPFC in the acquisition of pavlovian threat conditioning in humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer’s disease with depression as the first symptom: A case report with literature review. Front. Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]

| N | Average | Standard Deviation | Standard Error | 95% for the Mean Confidence Interval | Minimum | Maximum | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||||
| Concentration Level | General soldiers | 136 | 6.555 | 2.0031 | 0.1718 | 6.215 | 6.895 | 2.0 | 10.0 |
| Accident risk level 1 soldier | 42 | 6.517 | 2.0204 | 0.3118 | 5.887 | 7.146 | 3.2 | 9.9 | |
| Accident risk level 2 soldier | 47 | 5.649 | 1.5998 | 0.2334 | 5.179 | 6.119 | 2.6 | 9.8 | |
| Accident risk level 3 soldier | 26 | 5.169 | 0.9768 | 0.1916 | 4.775 | 5.564 | 3.5 | 7.2 | |
| Subtotal | 251 | 6.235 | 1.9116 | 0.1207 | 5.998 | 6.473 | 2.0 | 10.0 | |
| Brain activity | General soldiers | 136 | 77.43 | 7.883 | 0.676 | 76.10 | 78.77 | 43 | 93 |
| Accident risk level 1 soldier | 42 | 78.00 | 8.175 | 1.261 | 75.45 | 80.55 | 61 | 95 | |
| Accident risk level 2 soldier | 47 | 73.68 | 6.763 | 0.987 | 71.70 | 75.67 | 52 | 87 | |
| Accident risk level 3 soldier | 26 | 72.69 | 6.638 | 1.302 | 70.01 | 75.37 | 57 | 81 | |
| Subtotal | 251 | 76.33 | 7.821 | 0.494 | 75.36 | 77.31 | 43 | 95 | |
| Psychological stress | General soldiers | 136 | 6.096 | 1.2835 | 0.1101 | 5.878 | 6.313 | 3.7 | 10.0 |
| Accident risk level 1 soldier | 42 | 5.707 | 1.0086 | 0.1556 | 5.393 | 6.021 | 4.2 | 8.2 | |
| Accident risk level 2 soldier | 47 | 5.870 | 1.2360 | 0.1803 | 5.507 | 6.233 | 3.8 | 8.9 | |
| Accident risk level 3 soldier | 26 | 5.688 | 1.0203 | 0.2001 | 5.276 | 6.101 | 4.0 | 8.5 | |
| Subtotal | 251 | 5.946 | 1.2130 | 0.0766 | 5.795 | 6.097 | 3.7 | 10.0 | |
| Imbalance of left and right brain activity | General soldiers | 136 | 5.007 | 0.6024 | 0.0517 | 4.905 | 5.110 | 3.0 | 7.0 |
| Accident risk level 1 soldier | 42 | 5.167 | 0.4897 | 0.0756 | 5.014 | 5.319 | 5.0 | 7.0 | |
| Accident risk level 2 soldier | 47 | 5.085 | 0.4582 | 0.0668 | 4.951 | 5.220 | 4.0 | 7.0 | |
| Accident risk level 3 soldier | 26 | 5.038 | 0.8237 | 0.1615 | 4.706 | 5.371 | 3.0 | 7.0 | |
| Subtotal | 251 | 5.052 | 0.5876 | 0.0371 | 4.979 | 5.125 | 3.0 | 7.0 | |
| Levene Statistics | df1 | df2 | CTT Significance | |
|---|---|---|---|---|
| Concentration Level | 6.734 | 3 | 247 | 0.000 |
| Brain activity | 0.756 | 3 | 247 | 0.520 |
| Psychological stress | 1.577 | 3 | 247 | 0.195 |
| Imbalance of left and right brain activity | 0.453 | 3 | 247 | 0.715 |
| The Sum of Squares | The Degree of Freedom | Mean Square | F | Significance Probability | ||
|---|---|---|---|---|---|---|
| Concentration Level | Between groups | 62.947 | 3 | 20.982 | 6.093 | 0.001 |
| Within the group | 850.628 | 247 | 3.444 | |||
| The entire | 913.574 | 250 | ||||
| Brain activity | Between groups | 956.733 | 3 | 318.911 | 5.495 | 0.001 |
| Within the group | 14,335.156 | 247 | 58.037 | |||
| The entire | 15,291.888 | 250 | ||||
| Psychological stress | Between groups | 7.434 | 3 | 2.478 | 1.698 | 0.168 |
| Within the group | 360.410 | 247 | 1.459 | |||
| The entire | 367.844 | 250 | ||||
| Imbalance of left and right brain activity | Between groups | 0.880 | 3 | 0.293 | 0.848 | 0.469 |
| Within the group | 85.447 | 247 | 0.346 | |||
| The entire | 86.327 | 250 | ||||
| Dependent Variable | Group (I) | Group (J) | Average Difference (I–J) | Standardization Error | Probability of Significance | 95% Confidence Interval for the Average | ||
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| Dunnett T3 | Concentration level | General soldiers | Accident risk level 2 soldier | 0.9062 * | 0.2898 | 0.014 | 0.129 | 1.683 |
| Accident risk level 3 soldier | 1.3859 * | 0.2573 | 0.000 | 0.691 | 2.081 | |||
| Accident risk level 1 soldier | Accident risk level 3 soldier | 1.3474 * | 0.3659 | 0.003 | 0.355 | 2.340 | ||
| Scheffe | Brain activity | General soldiers General soldiers | Accident risk level 1 soldier | −0.566 | 1.345 | 0.981 | −4.35 | 3.22 |
| Accident risk level 2 soldier | 3.753 * | 1.289 | 0.039 | 0.12 | 7.38 | |||
| Accident risk level 3 soldier | 4.742 * | 1.631 | 0.040 | 0.15 | 9.33 | |||
| N | Average | Standard Deviation | Standard Error | 95% for the Mean Confidence Interval | Minimum | Maximum | |||
|---|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||||
| Heart health | General soldiers | 136 | 13.7765 | 3.57091 | 0.30620 | 13.1709 | 14.3820 | 2.00 | 24.50 |
| Accident risk level 1 soldier | 42 | 13.7271 | 3.73957 | 0.57703 | 12.5618 | 14.8925 | 6.30 | 23.50 | |
| Accident risk level 2 soldier | 47 | 12.3670 | 3.25648 | 0.47501 | 11.4109 | 13.3232 | 6.68 | 25.00 | |
| Accident risk level 3 soldier | 26 | 13.3885 | 4.11029 | 0.80609 | 11.7283 | 15.0486 | 5.85 | 24.00 | |
| Subtotal | 251 | 13.4641 | 3.62098 | 0.22855 | 13.0140 | 13.9142 | 2.00 | 25.00 | |
| Body stress | General soldiers | 136 | 43.684 | 4.9166 | 0.4216 | 42.850 | 44.518 | 30.0 | 62.0 |
| Accident risk level 1 soldier | 42 | 44.214 | 5.6935 | 0.8785 | 42.440 | 45.989 | 33.0 | 66.0 | |
| Accident risk level 2 soldier | 47 | 46.362 | 4.9142 | 0.7168 | 44.919 | 47.805 | 34.0 | 58.0 | |
| Accident risk level 3 soldier | 26 | 46.885 | 6.1535 | 1.2068 | 44.399 | 49.370 | 35.0 | 69.0 | |
| Subtotal | 251 | 44.606 | 5.3111 | 0.3352 | 43.945 | 45.266 | 30.0 | 69.0 | |
| Cumulative fatigue | General soldiers | 136 | 4.809 | 0.4475 | 0.0384 | 4.733 | 4.885 | 2.0 | 5.0 |
| Accident risk level 1 soldier | 42 | 4.857 | 0.3542 | 0.0546 | 4.747 | 4.968 | 4.0 | 5.0 | |
| Accident risk level 2 soldier | 47 | 4.681 | 0.4712 | 0.0687 | 4.543 | 4.819 | 4.0 | 5.0 | |
| Accident risk level 3 soldier | 26 | 4.731 | 0.5335 | 0.1046 | 4.515 | 4.946 | 3.0 | 5.0 | |
| Subtotal | 251 | 4.785 | 0.4489 | 0.0283 | 4.729 | 4.841 | 2.0 | 5.0 | |
| Physical Vitality | General soldiers | 136 | 4.934 | 0.3031 | 0.0260 | 4.882 | 4.985 | 3.0 | 5.0 |
| Accident risk level 1 soldier | 42 | 4.833 | 0.5372 | 0.0829 | 4.666 | 5.001 | 3.0 | 5.0 | |
| Accident risk level 2 soldier | 47 | 4.851 | 0.4159 | 0.0607 | 4.729 | 4.973 | 3.0 | 5.0 | |
| Accident risk level 3 soldier | 26 | 4.654 | 0.7452 | 0.1462 | 4.353 | 4.955 | 3.0 | 5.0 | |
| Subtotal | 251 | 4.873 | 0.4378 | 0.0276 | 4.818 | 4.927 | 3.0 | 5.0 | |
| Autonomic nervous system health | General soldiers | 136 | 7.6746 | 0.75925 | 0.06510 | 7.5458 | 7.8033 | 5.68 | 9.17 |
| Accident risk level 1 soldier | 42 | 7.6667 | 0.81587 | 0.12589 | 7.4124 | 7.9209 | 5.46 | 9.78 | |
| Accident risk level 2 soldier | 47 | 7.3209 | 0.83791 | 0.12222 | 7.0748 | 7.5669 | 5.63 | 8.79 | |
| Accident risk level 3 soldier | 26 | 7.5112 | 0.76082 | 0.14921 | 7.2039 | 7.8185 | 5.54 | 8.73 | |
| Subtotal | 251 | 7.5901 | 0.79162 | 0.04997 | 7.4917 | 7.6885 | 5.46 | 9.78 | |
| Levene Statistics | df1 | df2 | CTT Significance | |
|---|---|---|---|---|
| Heart health | 0.924 | 3 | 247 | 0.430 |
| Body Stress | 0.100 | 3 | 247 | 0.960 |
| Cumulative fatigue | 4.186 | 3 | 247 | 0.006 |
| Physical Vitality | 13.008 | 3 | 247 | 0.000 |
| Autonomic nervous system health | 0.205 | 3 | 247 | 0.893 |
| The Sum of Squares | The Degree of Freedom | Mean Square | F | Significance Probability | ||
|---|---|---|---|---|---|---|
| heart health | Between groups | 72.893 | 3 | 24.298 | 1.873 | 0.135 |
| Within the group | 3204.976 | 247 | 12.976 | |||
| The entire | 3277.869 | 250 | ||||
| Body Stress | Between groups | 401.971 | 3 | 133.990 | 4.977 | 0.002 |
| Within the group | 6649.981 | 247 | 26.923 | |||
| The entire | 7051.952 | 250 | ||||
| Cumulative fatigue | Between groups | 0.882 | 3 | 0.294 | 1.467 | 0.224 |
| Within the group | 49.500 | 247 | 0.200 | |||
| The entire | 50.382 | 250 | ||||
| Physical vitality | Between groups | 1.841 | 3 | 0.614 | 3.289 | 0.021 |
| Within the group | 46.080 | 247 | 0.187 | |||
| The entire | 47.921 | 250 | ||||
| Autonomic nervous system health | Between groups | 4.786 | 3 | 1.595 | 2.594 | 0.053 |
| Within the group | 151.880 | 247 | 0.615 | |||
| The entire | 156.666 | 250 | ||||
| Dependent Variable | Group (I) | Group (J) | Average Difference (I–J) | Standardization Error | Probability of Significance | 95% Confidence Interval for the Average | ||
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| Scheffe | Body stress | General soldiers | Accident risk level 1 soldier | −0.5305 | 0.9160 | 0.953 | −3.109 | 2.048 |
| Accident risk level 2 soldier | −2.6779 * | 0.8779 | 0.027 | −5.149 | −0.207 | |||
| Accident risk level 3 soldier | −3.2008 * | 1.1106 | 0.042 | −6.327 | −0.075 | |||
| Dunnett t | Physical vitality | General soldiers | Accident risk level 3 soldier | 0.2008 * | 0.0952 | 0.007 | −0.066 | 0.494 |
| Accident risk level 1 soldier | Accident risk level 3 soldier | 0.1795 | 0.1078 | 0.200 | −0.070 | 0.429 | ||
| Accident risk level 2 soldier | Accident risk level 3 soldier | 0.1970 * | 0.1056 | 0.135 | 0.047 | 0.441 | ||
| Average | Standard Deviation | N | |
|---|---|---|---|
| Concentration Level | 6.235 | 1.9116 | 251 |
| Brain activity | 76.33 | 7.821 | 251 |
| Psychological stress | 5.946 | 1.2130 | 251 |
| Imbalance of left and right brain activity | 5.052 | 0.5876 | 251 |
| Body Stress | 44.606 | 5.3111 | 251 |
| Cumulative fatigue | 4.785 | 0.4489 | 251 |
| heart health | 13.4641 | 3.62098 | 251 |
| Physical vitality | 4.873 | 0.4378 | 251 |
| Autonomic nervous system health | 7.5901 | 0.79162 | 251 |
| Maladjustment | 2.2083 | 1.11388 | 240 |
| Suicidal concerns | 1.9269 | 1.13635 | 240 |
| Body Stress | Cumulated Fatigue | Heart Health | Physical Vitality | Autonomic Neuronal System Activity | ||
|---|---|---|---|---|---|---|
| Concentration Level | Pearson correlation | −0.102 | 0.051 | 0.062 | 0.066 | 0.134 * |
| Probability of significance (both sides) | 0.109 | 0.418 | 0.327 | 0.300 | 0.034 | |
| N | 251 | 251 | 251 | 251 | 251 | |
| Brain activity | Pearson correlation | −0.036 | −0.039 | 0.038 | −0.095 | 0.034 |
| Probability of significance (both sides) | 0.569 | 0.542 | 0.545 | 0.134 | 0.588 | |
| N | 251 | 251 | 251 | 251 | 251 | |
| Psychological stress | Pearson correlation | −0.077 | 0.062 | 0.004 | 0.109 | 0.050 |
| Probability of significance (both sides) | 0.221 | 0.331 | 0.951 | 0.085 | 0.432 | |
| N | 251 | 251 | 251 | 251 | 251 | |
| Imbalance of left and right brain activity | Pearson correlation | −0.042 | 0.073 | −0.048 | −0.021 | 0.034 |
| Probability of significance (both sides) | 0.506 | 0.251 | 0.449 | 0.742 | 0.596 | |
| N | 251 | 251 | 251 | 251 | 251 | |
| Concentration Level | Brain Activity | Psychological Stress | Imbalance of Left and Right Brain Activity | ||
|---|---|---|---|---|---|
| Maladjustment | Pearson correlation | −0.203 ** | −0.198 ** | −0.101 | −0.004 |
| Probability of significance (both sides) | 0.002 | 0.002 | 0.117 | 0.951 | |
| N | 240 | 240 | 240 | 240 | |
| Suicidal concerns | Pearson correlation | −0.216 ** | −0.229 ** | −0.073 | −0.004 |
| Probability of significance (both sides) | 0.001 | 0.000 | 0.259 | 0.946 | |
| N | 240 | 240 | 240 | 240 | |
| Body Stress | Cumulative Fatigue | Heart Health | Physical Vitality | Autonomic Nervous System Health | ||
|---|---|---|---|---|---|---|
| Maladjustment | Pearson correlation | 0.201 ** | −0.088 | −0.068 | −0.182 ** | −0.114 |
| Probability of significance (both sides) | 0.002 | 0.172 | 0.296 | 0.005 | 0.078 | |
| N | 240 | 240 | 240 | 240 | 240 | |
| Suicidal concerns | Pearson correlation | 0.231** | −0.108 | −0.086 | −0.131 * | −0.123 |
| Probability of significance (both sides) | 0.000 | 0.095 | 0.184 | 0.043 | 0.057 | |
| N | 240 | 240 | 240 | 240 | 240 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-O.; Choi, J.-G.; Yun, J.-Y. A Study of Brain Function Characteristics of Service Members at High Risk for Accidents in the Military. Brain Sci. 2023, 13, 1157. https://doi.org/10.3390/brainsci13081157
Choi S-O, Choi J-G, Yun J-Y. A Study of Brain Function Characteristics of Service Members at High Risk for Accidents in the Military. Brain Sciences. 2023; 13(8):1157. https://doi.org/10.3390/brainsci13081157
Chicago/Turabian StyleChoi, Sung-Oh, Jong-Geun Choi, and Jong-Yong Yun. 2023. "A Study of Brain Function Characteristics of Service Members at High Risk for Accidents in the Military" Brain Sciences 13, no. 8: 1157. https://doi.org/10.3390/brainsci13081157
APA StyleChoi, S.-O., Choi, J.-G., & Yun, J.-Y. (2023). A Study of Brain Function Characteristics of Service Members at High Risk for Accidents in the Military. Brain Sciences, 13(8), 1157. https://doi.org/10.3390/brainsci13081157





