Revealing the Acute Effects of Dietary Components on Mood and Cognition: The Role of Autonomic Nervous System Responses
Abstract
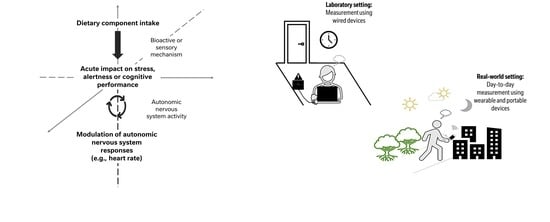
:1. Introduction
2. Dietary Components to Relieve Stress Acutely
3. Dietary Components That Acutely Enhance Alertness
4. Dietary Components to Improve Cognitive Performance Acutely
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langner, R. Cognitive-Energetic Mechanisms and Neural Basis of Alertness Regulation. Doctoral Dissertation, RWTH Aachen University, Aachen, Germany, 2010. [Google Scholar]
- Selye, H. Stress and disease. Science 1955, 122, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.G.; Haupt, I.A. The Emotions; Williams & Wilkins Co.: Philadelphia, PA, USA, 1922. [Google Scholar]
- Cannon, W.B. The James-Lange theory of emotions: A critical examination and an alternative theory. Am. J. Psychol. 1927, 39, 106–124. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Fisher, C.; Scott, T.R. Food Flavours: Biology and Chemistry; Royal Society of Chemistry: London, UK, 1997. [Google Scholar]
- Hammersley, R.; Reid, M.; Atkin, S.L. How to measure mood in nutrition research. Nutr. Res. Rev. 2014, 27, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Polak, M.A.; Richardson, A.C.; Flett, J.A.; Brookie, K.L.; Conner, T.S. Measuring mood: Considerations and innovations for nutrition science. Nutr. Brain Health Cogn. Perform. 2015, 3, 95–122. [Google Scholar]
- Blundell, J.E.; de Vries, J.; Edwards, D.; Kallus, W.; Milon, H.; Pannemans, D.; Tuijtelaars, S.; Tuorila, H. PASSCLAIM–Mental state and performance. Eur. J. Nutr. 2004, 43, 2. [Google Scholar]
- Reece, J.B.; Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Campbell Biology; Pearson Boston: Boston, MA, USA, 2014. [Google Scholar]
- Seyle, H. Thymus and adrenals in response of the organism to injuries and intoxication. Br. J. Exp. Pathol. 1936, 17, 234–248. [Google Scholar]
- Russell, J.A. A circumplex model of affect. J. Personal. Soc. Psychol. 1980, 39, 1161. [Google Scholar] [CrossRef]
- Pressman, S.D.; Cohen, S. Does positive affect influence health? Psychol. Bull. 2005, 131, 925. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.O.; Little, W.; Scholey, A.B. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon Balm). Psychosom. Med. 2004, 66, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Bonnlander, B.; Lang, S.C.; Pischel, I.; Forster, J.; Khan, J.; Jackson, P.A.; Wightman, E.L. Acute and Chronic Effects of Green Oat (Avena sativa) Extract on Cognitive Function and Mood during a Laboratory Stressor in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled Study in Healthy Humans. Nutrients 2020, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.L.; Brockfeld, A.; Haupt, S.; Schierbauer, J.R.; Zimmer, R.T.; Wachsmuth, N.B.; Zunner, B.E.M.; Zimmermann, P.; Erlmann, M.; Obermayer-Pietsch, B. Acute Changes in Heart Rate Variability to Glucose and Fructose Supplementation in Healthy Individuals: A Double-Blind Randomized Crossover Placebo-Controlled Trial. Biology 2022, 11, 338. [Google Scholar] [CrossRef]
- Moss, M.; Cook, J.; Wesnes, K.; Duckett, P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int. J. Neurosci. 2003, 113, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Redondo, B.; Vera, J.; Carreño--Rodríguez, C.; Molina-Romero, R.; Jiménez, R. Acute effects of caffeine on dynamic accommodative response and pupil size: A placebo-controlled, double-blind, balanced crossover study. Curr. Eye Res. 2020, 45, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; Owusu-Mensah, J.; Osei, K. Caffeine intake is associated with pupil dilation and enhanced accommodation. Eye 2017, 31, 615–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanini, J.; Galduróz, J.C.F.; Pompéia, S. Acute personalized habitual caffeine doses improve attention and have selective effects when considering the fractionation of executive functions. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 29–43. [Google Scholar] [CrossRef]
- Mikalsen, A.; Bertelsen, B.; Flaten, M. Effects of caffeine, caffeine-associated stimuli, and caffeine-related information on physiological and psychological arousal. Psychopharmacology 2001, 157, 373–380. [Google Scholar] [CrossRef]
- Michael, N.; Johns, M.; Owen, C.; Patterson, J. Effects of caffeine on alertness as measured by infrared reflectance oculography. Psychopharmacology 2008, 200, 255–260. [Google Scholar] [CrossRef]
- Bruce, S.E.; Werner, K.B.; Preston, B.F.; Baker, L.M. Improvements in concentration, working memory and sustained attention following consumption of a natural citicoline–caffeine beverage. Int. J. Food Sci. Nutr. 2014, 65, 1003–1007. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, H.R. The effects of ginseng, ephedrine, and caffeine on cognitive performance, mood and energy. Nutr. Rev. 2001, 59, 91–102. [Google Scholar] [CrossRef]
- Quinlan, P.T.; Lane, J.; Moore, K.L.; Aspen, J.; Rycroft, J.A.; O’Brien, D.C. The acute physiological and mood effects of tea and coffee: The role of caffeine level. Pharmacol. Biochem. Behav. 2000, 66, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, P.; Lane, J.; Aspinall, L. Effects of hot tea, coffee and water ingestion on physiological responses and mood: The role of caffeine, water and beverage type. Psychopharmacology 1997, 134, 164–173. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B. A glucose-caffeine ‘energy drink’ameliorates subjective and performance deficits during prolonged cognitive demand. Appetite 2004, 42, 331–333. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macefield, V.G.; Wallin, B.G. The discharge behaviour of single sympathetic neurones supplying human sweat glands. J. Auton. Nerv. Syst. 1996, 61, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, J.; Baydogan, M.; Chavez-Echeagaray, M.E.; Atkinson, R.K.; Burleson, W. Affect measurement: A roadmap through approaches, technologies, and data analysis. In Emotions and Affect in Human Factors and Human-Computer Interaction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 255–288. [Google Scholar]
- Wright, K.P., Jr.; Hull, J.T.; Czeisler, C.A. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 283, R1370–R1377. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R. Natural Odor Inhalers (AromaStick®) Outperform Red Bull® for Enhancing Cognitive Vigilance: Results from a Four-Armed, Randomized Controlled Study. Percept. Mot. Ski. 2021, 128, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.J.; Lu, K.; Gray, M.; Oliver, C. The neuropharmacology of L-theanine (N-ethyl-L-glutamine) a possible neuroprotective and cognitive enhancing agent. J. Herb. Pharmacother. 2006, 6, 21–30. [Google Scholar]
- Zahar, S.; Schneider, N.; Makwana, A.; Chapman, S.; Corthesy, J.; Amico, M.; Hudry, J. Dietary tryptophan-rich protein hydrolysate can acutely impact physiological and psychological measures of mood and stress in healthy adults. Nutr. Neurosci. 2022, 26, 303–312. [Google Scholar] [CrossRef]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-J.; Cha, J.-Y.; Kim, S.-E.; Ko, I.-G.; Jee, Y.-S. Effects of Ylang-Ylang aroma on blood pressure and heart rate in healthy men. J. Exerc. Rehabil. 2013, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Boyle, N.B.; Billington, J.; Lawton, C.; Quadt, F.; Dye, L. A combination of green tea, rhodiola, magnesium and B vitamins modulates brain activity and protects against the effects of induced social stress in healthy volunteers. Nutr. Neurosci. 2021, 25, 1845–1859. [Google Scholar] [CrossRef]
- Abascal, K.; Yarnell, E. Nervine herbs for treating anxiety. Altern. Complement. Ther. 2004, 10, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell. Mol. Life Sci. CMLS 2004, 61, 857–872. [Google Scholar] [CrossRef]
- Steinhauer, S.R.; Siegle, G.J.; Condray, R.; Pless, M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int. J. Psychophysiol. 2004, 52, 77–86. [Google Scholar] [CrossRef]
- De Longis, E.; Lerond, C.; Costello, S.E.; Hudry, J. The Matrix Matters: Beverage Carbonation Impacts the Timing of Caffeine Effects on Sustained Attention. Nutrients 2022, 14, 2305. [Google Scholar] [CrossRef]
- Illy, E. The complexity of coffee. Sci. Am. 2002, 286, 86–91. [Google Scholar] [CrossRef]
- Bichler, A.; Swenson, A.; Harris, M. A combination of caffeine and taurine has no effect on short term memory but induces changes in heart rate and mean arterial blood pressure. Amino Acids 2006, 31, 471–476. [Google Scholar] [CrossRef]
- Scholey, A.B.; Kennedy, D.O. Cognitive and physiological effects of an “energy drink”: An evaluation of the whole drink and of glucose, caffeine and herbal flavouring fractions. Psychopharmacology 2004, 176, 320–330. [Google Scholar] [CrossRef]
- Smit, H.J.; Rogers, P.J. Effects of ‘energy’drinks on mood and mental performance: Critical methodology. Food Qual. Prefer. 2002, 13, 317–326. [Google Scholar] [CrossRef]
- Hindmarch, I.; Rigney, U.; Stanley, N.; Quinlan, P.; Rycroft, J.; Lane, J. A naturalistic investigation of the effects of day-long consumption of tea, coffee and water on alertness, sleep onset and sleep quality. Psychopharmacology 2000, 149, 203–216. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Kuhn, W.; Morsch, K.; Schäfer, A.; Hillecke, T.K.; Thayer, J.F. Impact of Caffeine on Heart Rate Variability: A Systematic Review. J. Caffeine Res. 2013, 3, 22–37. [Google Scholar] [CrossRef]
- Hirano, Y.; Obata, T.; Kashikura, K.; Nonaka, H.; Tachibana, A.; Ikehira, H.; Onozuka, M. Effects of chewing in working memory processing. Neurosci. Lett. 2008, 436, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Smith, A.P. Chewing gum: Cognitive performance, mood, well-being, and associated physiology. BioMed Res. Int. 2015, 2015, 654806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.P.; Jacob, T.J.; Smith, A.P. Effects and after-effects of chewing gum on vigilance, heart rate, EEG and mood. Physiol. Behav. 2014, 133, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Pomportes, L.; Davranche, K.; Brisswalter, I.; Hays, A.; Brisswalter, J. Heart rate variability and cognitive function following a multi-vitamin and mineral supplementation with added guarana (Paullinia cupana). Nutrients 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [Green Version]
- Burke, T.M.; Markwald, R.R.; McHill, A.W.; Chinoy, E.D.; Snider, J.A.; Bessman, S.C.; Jung, C.M.; O’Neill, J.S.; Wright, K.P. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci. Transl. Med. 2015, 7, 305ra146. [Google Scholar] [CrossRef] [Green Version]
- Elliman, N.A.; Ash, J.; Green, M.W. Pre-existent expectancy effects in the relationship between caffeine and performance. Appetite 2010, 55, 355–358. [Google Scholar] [CrossRef]
- Broadhurst, P.L. Emotionality and the Yerkes-Dodson law. J. Exp. Psychol. 1957, 54, 345. [Google Scholar] [CrossRef] [PubMed]
- Lunenburg, F.C. Goal-setting theory of motivation. Int. J. Manag. Bus. Adm. 2011, 15, 1–6. [Google Scholar]
- Dye, L.; Blundell, J. Functional foods: Psychological and behavioural functions. Br. J. Nutr. 2002, 88, S187–S211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, D.; Tellegen, A. Issues in dimensional structure of affect—Effects of descriptors, measurement error, and response formats: Comment on Russell and Carroll (1999). Psychol. Bull. 1999, 125, 601–610. [Google Scholar] [CrossRef]
- Favieri, F.; Forte, G.; Pazzaglia, M.; Chen, E.Y.; Casagrande, M. High-level executive functions: A possible role of sex and weight condition in planning and decision-making performances. Brain Sci. 2022, 12, 149. [Google Scholar] [CrossRef]
- Dittmar, O.; Krehl, R.; Lautenbacher, S. Interrelation of self-report, behavioural and electrophysiological measures assessing pain-related information processing. Pain Res. Manag. 2011, 16, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Orne, M.T. On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. In Sociological Methods; Routledge: London, UK, 2017; pp. 279–299. [Google Scholar]
- McCambridge, J.; Kypri, K.; Elbourne, D. Research participation effects: A skeleton in the methodological cupboard. J. Clin. Epidemiol. 2014, 67, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Pasman, W.J.; Boessen, R.; Donner, Y.; Clabbers, N.; Boorsma, A. Effect of caffeine on attention and alertness measured in a home-setting, using web-based cognition tests. JMIR Res. Protoc. 2017, 6, e6727. [Google Scholar] [CrossRef] [Green Version]
- Sempionatto, J.R.; Montiel, V.R.-V.; Vargas, E.; Teymourian, H.; Wang, J. Wearable and mobile sensors for personalized nutrition. ACS Sens. 2021, 6, 1745–1760. [Google Scholar] [CrossRef]
- Adams, S.H.; Anthony, J.C.; Carvajal, R.; Chae, L.; Khoo, C.S.H.; Latulippe, M.E.; Matusheski, N.V.; McClung, H.L.; Rozga, M.; Schmid, C.H. Perspective: Guiding principles for the implementation of personalized nutrition approaches that benefit health and function. Adv. Nutr. 2020, 11, 25–34. [Google Scholar]
- Jinnette, R.; Narita, A.; Manning, B.; McNaughton, S.A.; Mathers, J.C.; Livingstone, K.M. Does personalized nutrition advice improve dietary intake in healthy adults? A systematic review of randomized controlled trials. Adv. Nutr. 2021, 12, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.M.; Saleh, A.A.; Jospe, M.R.; Liao, Y.; Schembre, S.M. Using Biological Feedback to Promote Health Behavior Change in Adults: Protocol for a Scoping Review. JMIR Res. Protoc. 2022, 11, e32579. [Google Scholar] [CrossRef] [PubMed]
| Reference | Design | Interventions | Population | Key Endpoints | Key Results |
|---|---|---|---|---|---|
| Zahar S, Schneider N, Makwana A, Chapman S, Corthesy J, Amico M and Hudry J [35] | Randomized, double-blind, 3-arm parallel, placebo-controlled | 0.5 or 1 g tryptophan in an egg protein hydrolysate | 45 healthy subjects (mean age 33.3 ± 6.15 SD yo) |
| Tryptophan interventions versus placebo: Higher HRV during a multi-task stressor |
| Kimura K, Ozeki M, Juneja LR and Ohira H [36] | Randomized, double-blind, 2-arm cross-over, placebo-controlled | 200 mg L-theanine | 12 male undergraduate students (mean age 21.50 ± 1.38 SD yo) |
| L-theanine intervention versus placebo: Lower HR during a task stressor |
| Kennedy DO, Bonnlander B, Lang SC, Pischel I, Forster J, Khan J, Jackson PA and Wightman EL [16] | Randomized, double-blind, 4-arm parallel, placebo-controlled | 430 mg, 860 mg, or 1290 mg green oat extract | 132 healthy subjects (age range 35–65 yo) |
| Green oat intervention (860 mg) versus placebo: Higher HR during an observed multi-task stressor |
| Boyle NB, Billington J, Lawton C, Quadt F and Dye L [38] | Randomized, double-blind, 4-arm parallel, placebo-controlled | Mg + B vitamins + green tea + rhodiola extract, Mg + B vitamins + rhodiola extract or Mg + B vitamins + green tea -Mg: 150 mg elemental -Green tea: 125 mg containing 40% L-Theanine -Rhodiola extract: 222 mg -B vitamins: 0.7, 0.1, and 0.00125 mg of vitamins , and , respectively | 100 healthy subjects (age range 18–50 yo) |
| Intervention of Mg + B vitamins + green tea + rhodiola extract versus a placebo: Lower SACL stress score & HRV during a social stress test |
| Eckstein ML, Brockfeld A, Haupt S, Schierbauer JR, Zimmer RT, Wachsmuth NB, Zunner BEM, Zimmermann P, Erlmann M and Obermayer-Pietsch B [17] | Randomized, double-blind, 4-arm cross-over, placebo-controlled | 1 g/kg BM Glucose, 1 g/kg BM Fructose, or 0.5 g/kg BM of Glucose + Fructose | 15 healthy subjects (age range 18–65 yo) |
| Carbohydrate interventions versus a placebo: Lower HRV |
| Jung D-J, Cha J-Y, Kim S-E, Ko I-G and Jee Y-S [37] | 2-arm parallel, placebo-controlled | 20-min inhalation of Ylang-Ylang essential oil (Cananga odorata) | 15 healthy males in the Ylang-Ylang essential oil group (mean age 21.07 ± 0.43 SEM yo) 14 males in placebo group (mean age 22.00 ± 0.58 SEM yo). |
| Ylang-Ylang essential oil intervention versus a placebo: Lower HR |
| Reference | Design | Interventions | Population | Key Endpoints | Key Results |
|---|---|---|---|---|---|
| Redondo B, Vera J, Carreño--Rodríguez C, Molina-Romero R and Jiménez R [19] | Double-blind, 2-arm cross-over, placebo-controlled | 4 mg of caffeine per 1 kg of body weight | 22 university students (21.68 ± 3.67 SD yo) |
| Caffeine intervention versus a placebo: Higher pupil dilation & alertness score |
| Quinlan PT, Lane J, Moore KL, Aspen J, Rycroft JA and O’Brien DC [26]—Study 1 | Randomized, 5-arm cross-over, placebo-controlled | Hot water, tea (37.5 mg caffeine), tea (75 mg caffeine), or coffee (75 or 150 mg) | 17 healthy subjects (age range 21–51 yo) |
| Caffeine interventions versus hot water: Higher LARS alertness scores (with a dose-related increase) Caffeine and hot water interventions versus a placebo: Higher HR, GSR & skin temperature |
| Quinlan PT, Lane J, Moore KL, Aspen J, Rycroft JA and O’Brien DC [26]—Study 2 | Randomized, double-blind, 5-arm cross-over, placebo-controlled | Hot water, decaffeinated (~5 mg caffeine), or decaffeinated tea with caffeine (30, 55, 105, or 205 mg caffeine) | 17 healthy subjects (age range 22–52 yo) |
| Caffeine interventions versus hot water: Modulation of arousal (with a dose-related U-shaped pattern), lower HR & skin temperature (with a dose-related decrease) and higher GSR Hot water versus placebo: Higher skin temperature, GSR & HR |
| Schneider R [33] | Randomized, double-blind, 4-arm cross-over, placebo-controlled | 100% natural essential oils from peppermint, rosemary, and grapefruit (ratio: 50-30-20), 100% natural peppermint, rosemary, and cinnamon oil (ratio: 86-11-3), or 250 mL of commercialized energy drink | 50 health subjects (mean age 34.2 ± 6.9 SD yo) |
| Essential oil versus an energy drink intervention or a placebo: Higher HRV & RT |
| Reference | Design | Interventions | Population | Key Endpoints | Key Results |
|---|---|---|---|---|---|
| Bichler A, Swenson A and Harris M [44] | Randomized, double-blind, 2-arm cross-over, placebo-controlled | 100 mg caffeine + 1000 mg taurine | 14 undergraduate students (mean age 20.5 ± 1.5 SD yo) |
| Caffeine + taurine versus placebo: Lower HR |
| Scholey AB and Kennedy DO [45] | Randomized, double-blind, 5-arm cross-over, placebo-controlled | 250 mL water with 75 mg caffeine, 37.5 g glucose, herbal flavoring fractions, or 75 mg caffeine, 37.5 g glucose, and herbal flavoring fractions. | 20 undergraduate volunteers (age range 18–32 yo) |
| Glucose intervention versus placebo: Higher HR Caffeine intervention versus placebo: Lower HR Glucose + flavoring fractions intervention versus placebo: Higher cognitive performance |
| Allen AP and Smith AP [50]—Study 4 | 2-arm, cross-over, placebo-controlled | 1 full packet of chewing gum | 30 full-time university staff (mean age 30.4 ± 6.9 SD yo) |
| Chewing gum intervention versus placebo: Respectively higher and lower scores associated with the work done and the cognitive problems during a workday |
| Allen AP, Jacob TJ and Smith AP [51] | Randomized, parallel, 2-arm, placebo-controlled | Gum (gum base, glycerine, lecithin, sorbitol, emulsifier and sweeteners including aspartame and acesulfame K) | Gum condition: 18 participants (mean age 22.33 ± 2.5 SD yo) Control: 24 participants (mean age 24.4 ± 3 SD yo) |
| Chewing gum intervention versus placebo: Higher Hits and, lower RT &HR |
| Pomportes et al. [52] | Randomized, double-blind, 3-arm, cross-over, placebo-controlled | A supplement with vitamin, mineral and, 300 mg guarana supplement or a 100 mg caffeine supplement | 56 participants (males age range 19–45 yo; females age range 18–42 yo) |
| Vitamin + mineral + guarana intervention versus a placebo: Lower RT Caffeine intervention and placebo: Decrease of HRV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahar, S.; De Longis, E.; Hudry, J. Revealing the Acute Effects of Dietary Components on Mood and Cognition: The Role of Autonomic Nervous System Responses. Brain Sci. 2023, 13, 1177. https://doi.org/10.3390/brainsci13081177
Zahar S, De Longis E, Hudry J. Revealing the Acute Effects of Dietary Components on Mood and Cognition: The Role of Autonomic Nervous System Responses. Brain Sciences. 2023; 13(8):1177. https://doi.org/10.3390/brainsci13081177
Chicago/Turabian StyleZahar, Sélima, Evelina De Longis, and Julie Hudry. 2023. "Revealing the Acute Effects of Dietary Components on Mood and Cognition: The Role of Autonomic Nervous System Responses" Brain Sciences 13, no. 8: 1177. https://doi.org/10.3390/brainsci13081177