Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion
Abstract
:1. Introduction
2. Methods
2.1. Subjects
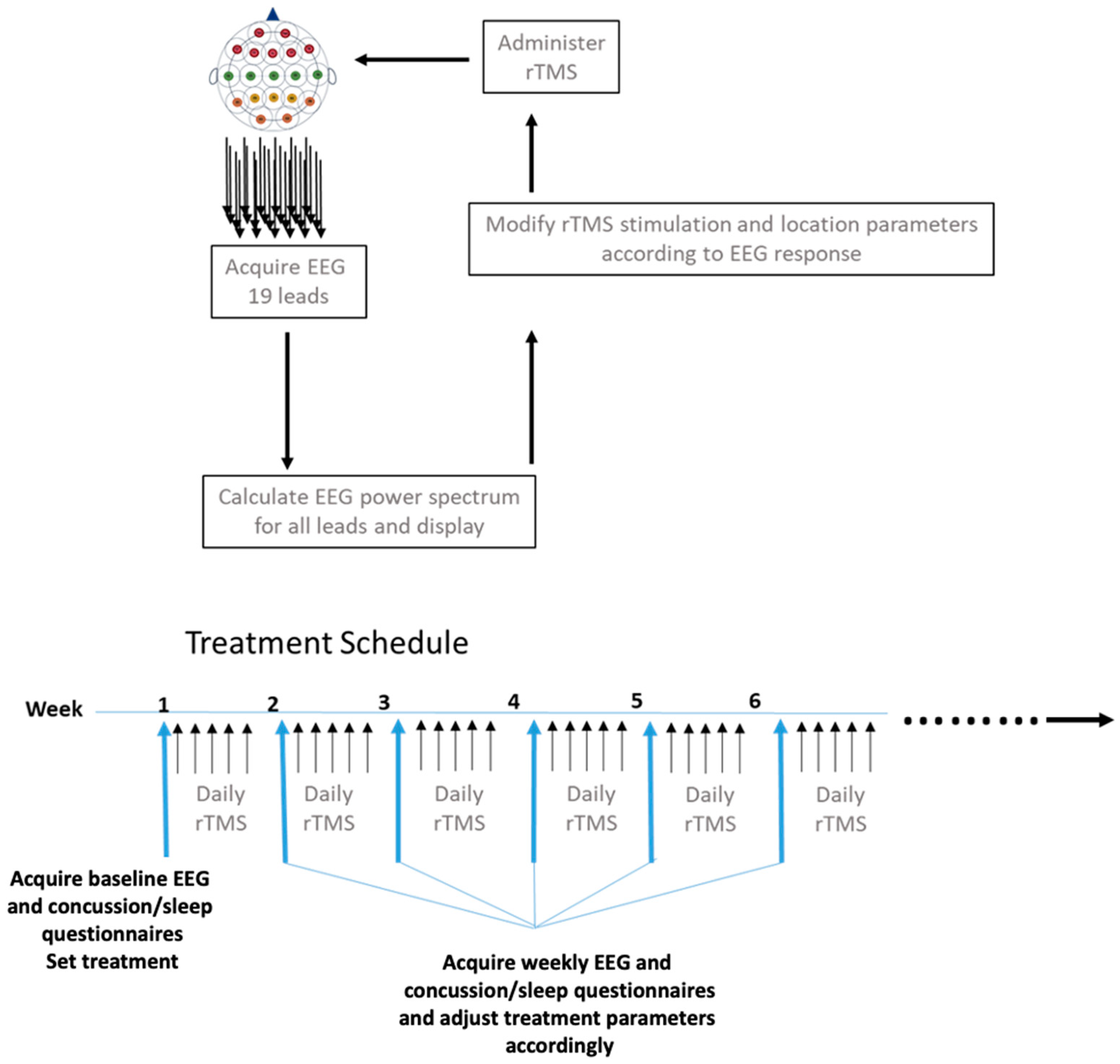
2.2. Treatment Schedule
2.3. EEG Data Acquisition
2.4. Personalized Repetitive Transcranial Magnetic Stimulation (PrTMS)
2.5. Data Analysis and Statistical Methods
2.5.1. Rivermead and Sleep Quality Scores
2.5.2. EEG Spectral Analyses
3. Results
3.1. Concussion Symptom Inventory (CSI)
3.2. Rivermead Concussion Questionnaire (RPQ) Scores
3.3. Sleep Quality and Insomnia Scores
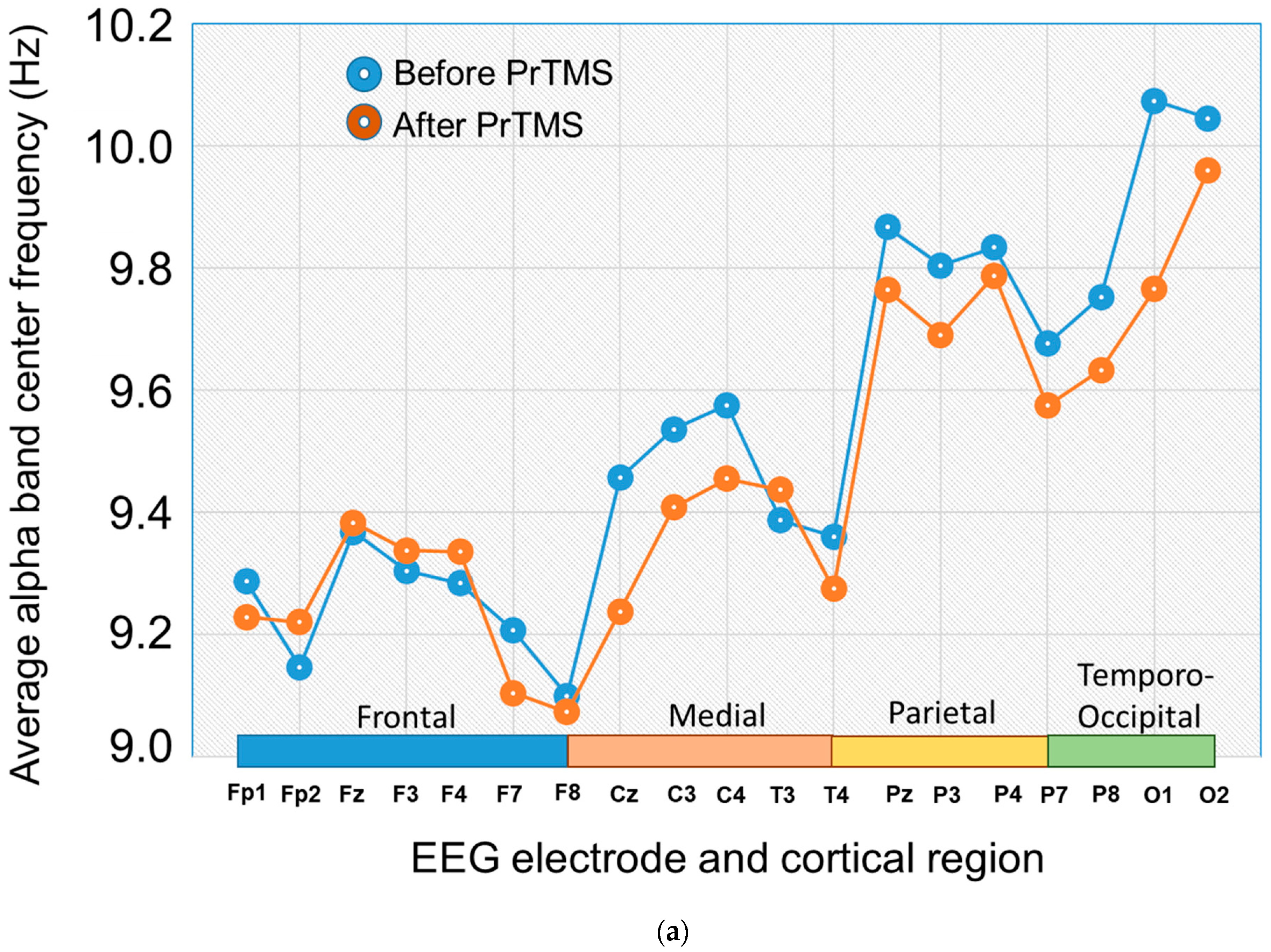
3.4. EEG Alpha Bband Center Frequency and 1/fa Spectral Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karton, C.; Blaine Hoshizaki, T. Concussive and subconcussive brain trauma: The complexity of impact biomechanics and injury risk in contact sport. Handb. Clin. Neurol. 2018, 158, 39–49. [Google Scholar] [CrossRef]
- Mollica, A.; Safavifar, F.; Fralick, M.; Giacobbe, P.; Lipsman, N.; Burke, M.J. Transcranial Magnetic Stimulation for the Treatment of Concussion: A Systematic Review. Neuromodulation 2021, 24, 803–812. [Google Scholar] [CrossRef]
- Suhr, J.A.; Gunstad, J. “Diagnosis Threat”: The effect of negative expectations on cognitive performance in head injury. J. Clin. Exp. Neuropsychol. 2002, 24, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H. Concussions and their consequences: Current diagnosis, management and prevention. Can. Med. Assoc. J. 2013, 185, 975–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stulemeijer, M.; Van der Werf, S.; Borm, G.F.; Vos, P.E. Early prediction of favourable recovery 6 months after mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2008, 79, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.J. Pathophysiology of Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Correction: Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2019, 14, e0218423. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Trapp, N.T.; Shahim, P.; Hacker, C.D.; Laumann, T.O.; Kandala, S.; Carter, A.R.; Brody, D.L. Individualized Connectome-Targeted Transcranial Magnetic Stimulation for Neuropsychiatric Sequelae of Repetitive Traumatic Brain Injury in a Retired NFL Player. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Plassman, B.L.; Havlik, R.J.; Steffens, D.C.; Helms, M.J.; Newman, T.N.; Drosdick, D.; Phillips, C.; Gau, B.A.; Welsh-Bohmer, K.A.; Burke, J.R.; et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000, 55, 1158–1166. [Google Scholar] [CrossRef]
- Gavett, B.E.; Stern, R.A.; McKee, A.C. Chronic traumatic encephalopathy: A potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 2011, 30, 179–188, xi. [Google Scholar] [CrossRef] [Green Version]
- Danna-Dos-Santos, A.; Mohapatra, S.; Santos, M.; Degani, A.M. Long-term effects of mild traumatic brain injuries to oculomotor tracking performances and reaction times to simple environmental stimuli. Sci. Rep. 2018, 8, 4583. [Google Scholar] [CrossRef] [Green Version]
- Sussman, D.; da Costa, L.; Chakravarty, M.M.; Pang, E.W.; Taylor, M.J.; Dunkley, B.T. Concussion induces focal and widespread neuromorphological changes. Neurosci. Lett. 2017, 650, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, P.; Hodge, C.; Rose, K.; Fraser, C.L. Persistent visual disturbances after concussion. Aust. J. Gen. Pract. 2019, 48, 531–536. [Google Scholar] [CrossRef]
- Luo, J.; Feng, Y.; Li, M.; Yin, M.; Qin, F.; Hu, X. Repetitive Transcranial Magnetic Stimulation Improves Neurological Function and Promotes the Anti-inflammatory Polarization of Microglia in Ischemic Rats. Front. Cell Neurosci. 2022, 16, 878345. [Google Scholar] [CrossRef]
- Peng, R.Y.; Gao, Y.B.; Wang, D.W.; Xiao, X.Y.; Chen, H.Y.; Wu, X.H.; Liu, J.; Xu, L.H.; Hu, W.H. Expression and significance of enkephalin and dopamine in experimental rat cerebral concussion tissue. Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi 2003, 19, 232–234. [Google Scholar]
- Sasso, V.; Bisicchia, E.; Latini, L.; Ghiglieri, V.; Cacace, F.; Carola, V.; Molinari, M.; Viscomi, M.T. Repetitive transcranial magnetic stimulation reduces remote apoptotic cell death and inflammation after focal brain injury. J. Neuroinflamm. 2016, 13, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Su, Y.; Guo, F.; Zhang, H.; Zhao, Y.; Huang, Q.; Xu, H. Deep rTMS Mitigates Behavioral and Neuropathologic Anomalies in Cuprizone-Exposed Mice through Reducing Microglial Proinflammatory Cytokines. Front. Integr. Neurosci. 2020, 14, 556839. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Kolivakis, T.; Yu, C.; Chen, J.K.; Delaney, S.; Ptito, A. Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J. Neurotrauma 2015, 32, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Meek, B.P.; Hill, S.; Modirrousta, M. Accelerated repetitive transcranial magnetic stimulation in the treatment of post-concussion symptoms due to mild traumatic brain injury: A pilot study. Brain Inj. 2021, 35, 48–58. [Google Scholar] [CrossRef]
- Moussavi, Z.; Suleiman, A.; Rutherford, G.; Ranjbar Pouya, O.; Dastgheib, Z.; Zhang, W.; Salter, J.; Wang, X.; Mansouri, B.; Lithgow, B. A Pilot Randomised Double-Blind Study of the Tolerability and efficacy of repetitive Transcranial Magnetic Stimulation on Persistent Post-Concussion Syndrome. Sci. Rep. 2019, 9, 5498. [Google Scholar] [CrossRef] [Green Version]
- Oberman, L.M.; Exley, S.; Philip, N.S.; Siddiqi, S.H.; Adamson, M.M.; Brody, D.L. Use of Repetitive Transcranial Magnetic Stimulation in the Treatment of Neuropsychiatric and Neurocognitive Symptoms Associated with Concussion in Military Populations. J. Head. Trauma. Rehabil. 2020, 35, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.; Terstege, D.J.; Poh, E.Z.; Epp, J.R.; Rodger, J. Low intensity repetitive transcranial magnetic stimulation modulates brain-wide functional connectivity to promote anti-correlated c-Fos expression. Sci. Rep. 2022, 12, 20571. [Google Scholar] [CrossRef] [PubMed]
- Zmeykina, E.; Mittner, M.; Paulus, W.; Turi, Z. Weak rTMS-induced electric fields produce neural entrainment in humans. Sci. Rep. 2020, 10, 11994. [Google Scholar] [CrossRef] [PubMed]
- Leuchter, A.F.; Cook, I.A.; Feifel, D.; Goethe, J.W.; Husain, M.; Carpenter, L.L.; Thase, M.E.; Krystal, A.D.; Philip, N.S.; Bhati, M.T.; et al. Efficacy and Safety of Low-field Synchronized Transcranial Magnetic Stimulation (sTMS) for Treatment of Major Depression. Brain Stimul. 2015, 8, 787–794. [Google Scholar] [CrossRef]
- Klooster, D.C.W.; Ferguson, M.A.; Boon, P.; Baeken, C. Personalizing Repetitive Transcranial Magnetic Stimulation Parameters for Depression Treatment Using Multimodal Neuroimaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Gogulski, J.; Ross, J.M.; Talbot, A.; Cline, C.C.; Donati, F.L.; Munot, S.; Kim, N.; Gibbs, C.; Bastin, N.; Yang, J.; et al. Personalized Repetitive Transcranial Magnetic Stimulation for Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 351–360. [Google Scholar] [CrossRef]
- Leuchter, A.F.; Wilson, A.C.; Vince-Cruz, N.; Corlier, J. Novel method for identification of individualized resonant frequencies for treatment of Major Depressive Disorder (MDD) using repetitive Transcranial Magnetic Stimulation (rTMS): A proof-of-concept study. Brain Stimul. 2021, 14, 1373–1383. [Google Scholar] [CrossRef]
- Figueira, J.S.B.; David, I.P.A.; Lobo, I.; Pacheco, L.B.; Pereira, M.G.; de Oliveira, L.; Keil, A. Effects of load and emotional state on EEG alpha-band power and inter-site synchrony during a visual working memory task. Cogn. Affect. Behav. Neurosci. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Roelofs, C.L.; Krepel, N.; Corlier, J.; Carpenter, L.L.; Fitzgerald, P.B.; Daskalakis, Z.J.; Tendolkar, I.; Wilson, A.; Downar, J.; Bailey, N.W.; et al. Individual alpha frequency proximity associated with repetitive transcranial magnetic stimulation outcome: An independent replication study from the ICON-DB consortium. Clin. Neurophysiol. 2021, 132, 643–649. [Google Scholar] [CrossRef]
- Jin, Y.; Potkin, S.G.; Kemp, A.S.; Huerta, S.T.; Alva, G.; Thai, T.M.; Carreon, D.; Bunney, W.E., Jr. Therapeutic effects of individualized alpha frequency transcranial magnetic stimulation (alphaTMS) on the negative symptoms of schizophrenia. Schizophr. Bull. 2006, 32, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Garnaat, S.L.; Fukuda, A.M.; Yuan, S.; Carpenter, L.L. Identification of Clinical Features and Biomarkers that may inform a Personalized Approach to rTMS for Depression. Pers. Med. Psychiatry 2019, 17–18, 4–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munia, T.T.K.; Haider, A.; Schneider, C.; Romanick, M.; Fazel-Rezai, R. A Novel EEG Based Spectral Analysis of Persistent Brain Function Alteration in Athletes with Concussion History. Sci. Rep. 2017, 7, 17221. [Google Scholar] [CrossRef] [Green Version]
- Voytek, B.; Kramer, M.A.; Case, J.; Lepage, K.Q.; Tempesta, Z.R.; Knight, R.T.; Gazzaley, A. Age-Related Changes in 1/f Neural Electrophysiological Noise. J. Neurosci. 2015, 35, 13257–13265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef]
- Ostlund, B.D.; Alperin, B.R.; Drew, T.; Karalunas, S.L. Behavioral and cognitive correlates of the aperiodic (1/f-like) exponent of the EEG power spectrum in adolescents with and without ADHD. Dev. Cogn. Neurosci. 2021, 48, 100931. [Google Scholar] [CrossRef]
- Dave, S.; Brothers, T.A.; Swaab, T.Y. 1/f neural noise and electrophysiological indices of contextual prediction in aging. Brain Res. 2018, 1691, 34–43. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety of TMSCG: Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.F.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 35–48. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmoller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Espie, C.A.; Kyle, S.D.; Hames, P.; Gardani, M.; Fleming, L.; Cape, J. The Sleep Condition Indicator: A clinical screening tool to evaluate insomnia disorder. BMJ Open 2014, 4, e004183. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.; Schreiber, M.; Miller, M.W.; Euler, M.J.; Lohse, K.R. Exploring the reliability and sensitivity of the EEG power spectrum as a biomarker. Int. J. Psychophysiol. 2021, 160, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Yasen, A.L.; Lim, M.M.; Weymann, K.B.; Christie, A.D. Excitability, Inhibition, and Neurotransmitter Levels in the Motor Cortex of Symptomatic and Asymptomatic Individuals Following Mild Traumatic Brain Injury. Front. Neurol. 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Waschke, L.; Wostmann, M.; Obleser, J. States and traits of neural irregularity in the age-varying human brain. Sci. Rep. 2017, 7, 17381. [Google Scholar] [CrossRef] [Green Version]
- Marguet, S.L.; Harris, K.D. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J. Neurosci. 2011, 31, 6414–6420. [Google Scholar] [CrossRef] [Green Version]
- Pachitariu, M.; Lyamzin, D.R.; Sahani, M.; Lesica, N.A. State-dependent population coding in primary auditory cortex. J. Neurosci. 2015, 35, 2058–2073. [Google Scholar] [CrossRef] [Green Version]
- Rudroff, T.; Workman, C.D. Transcranial Direct Current Stimulation as a Treatment Tool for Mild Traumatic Brain Injury. Brain Sci. 2021, 11, 806. [Google Scholar] [CrossRef]
- Peitz, G.W.; Wilde, E.A.; Grandhi, R. Magnetoencephalography in the Detection and Characterization of Brain Abnormalities Associated with Traumatic Brain Injury: A Comprehensive Review. Med. Sci. 2021, 9, 7. [Google Scholar] [CrossRef]
- Kundu, B.; Brock, A.A.; Englot, D.J.; Butson, C.R.; Rolston, J.D. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: A review. Neurosurg. Focus. 2018, 45, E14. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.J.; Gurkoff, G.G.; Izadi, A.; Berman, R.F.; Ekstrom, A.D.; Muizelaar, J.P.; Lyeth, B.G.; Shahlaie, K. Medial septal nucleus theta frequency deep brain stimulation improves spatial working memory after traumatic brain injury. J. Neurotrauma 2013, 30, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.J.; Gurkoff, G.G.; Izadi, A.; Seidl, S.E.; Echeverri, A.; Melnik, M.; Berman, R.F.; Ekstrom, A.D.; Muizelaar, J.P.; Lyeth, B.G.; et al. Septohippocampal Neuromodulation Improves Cognition after Traumatic Brain Injury. J. Neurotrauma 2015, 32, 1822–1832. [Google Scholar] [CrossRef] [Green Version]
- Ghaffarpasand, F.; Razmkon, A.; Khalili, H. Deep Brain Stimulation in Patients with Traumatic Brain Injury; Facts and Figures. Bull. Emerg. Trauma 2014, 2, 101–102. [Google Scholar]
- Cunningham, M.G.; Yadollahikhales, G.; Vitaliano, G.; van Horne, C. Administration of electroconvulsive therapy for depression associated with deep brain stimulation in a patient with post-traumatic Parkinson’s Disease: A case study. BMC Psychiatry 2016, 16, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, P.; Liu, A.; Wu, X.; Yan, Z.; Dai, S.; Wei, J.; Zhang, Z.; Li, X.; Luo, P.; et al. Pulsed Electromagnetic Field Protects against Brain Injury after Intracerebral Hemorrhage: Involvement of Anti-Inflammatory Processes and Hematoma Clearance via CD36. J. Mol. Neurosci. 2022, 72, 2150–2161. [Google Scholar] [CrossRef]
- Yoon, K.J.; Lee, Y.T.; Chung, P.W.; Lee, Y.K.; Kim, D.Y.; Chun, M.H. Effects of Repetitive Transcranial Magnetic Stimulation on Behavioral Recovery during Early Stage of Traumatic Brain Injury in Rats. J. Korean Med. Sci. 2015, 30, 1496–1502. [Google Scholar] [CrossRef] [Green Version]
- Sekar, S.; Zhang, Y.; Miranzadeh Mahabadi, H.; Buettner, B.; Taghibiglou, C. Low-Field Magnetic Stimulation Alleviates MPTP-Induced Alterations in Motor Function and Dopaminergic Neurons in Male Mice. Int. J. Mol. Sci. 2023, 24, 10328. [Google Scholar] [CrossRef]
- Lu, H.; Kobilo, T.; Robertson, C.; Tong, S.; Celnik, P.; Pelled, G. Transcranial magnetic stimulation facilitates neurorehabilitation after pediatric traumatic brain injury. Sci. Rep. 2015, 5, 14769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdugo-Diaz, L.; Estrada-Rojo, F.; Garcia-Espinoza, A.; Hernandez-Lopez, E.; Hernandez-Chavez, A.; Guzman-Uribe, C.; Martinez-Vargas, M.; Perez-Arredondo, A.; Calvario, T.; Elias-Vinas, D.; et al. Effect of Intermediate-Frequency Repetitive Transcranial Magnetic Stimulation on Recovery following Traumatic Brain Injury in Rats. Biomed. Res. Int. 2017, 2017, 4540291. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bao, X.; Li, J.; Zhang, G.; Guan, J.; Gao, Y.; Wu, P.; Zhu, Z.; Huo, X.; Wang, R. High-frequency repetitive transcranial magnetic stimulation for treating moderate traumatic brain injury in rats: A pilot study. Exp. Ther. Med. 2017, 13, 2247–2254. [Google Scholar] [CrossRef] [Green Version]
- Grohn, H.; Gillick, B.T.; Tkac, I.; Bednarik, P.; Mascali, D.; Deelchand, D.K.; Michaeli, S.; Meekins, G.D.; Leffler-McCabe, M.J.; MacKinnon, C.D.; et al. Influence of Repetitive Transcranial Magnetic Stimulation on Human Neurochemistry and Functional Connectivity: A Pilot MRI/MRS Study at 7 T. Front. Neurosci. 2019, 13, 1260. [Google Scholar] [CrossRef]
- Levitt, J.G.; Kalender, G.; O’Neill, J.; Diaz, J.P.; Cook, I.A.; Ginder, N.; Krantz, D.; Minzenberg, M.J.; Vince-Cruz, N.; Nguyen, L.D.; et al. Dorsolateral prefrontal gamma-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy. J. Psychiatry Neurosci. 2019, 44, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.P.; Noda, Y.; Nabeshima, T. Involvement of activation of dopaminergic neuronal system in learning and memory deficits associated with experimental mild traumatic brain injury. Eur. J. Neurosci. 1997, 9, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.L.; Li, S.; Lou, J.C.; Ma, X.C.; Zhang, B. The potential roles of dopamine in traumatic brain injury: A preclinical and clinical update. Am. J. Transl. Res. 2019, 11, 2616–2631. [Google Scholar] [PubMed]
- Kang, Y.; Jamison, K.; Jaywant, A.; Dams-O’Connor, K.; Kim, N.; Karakatsanis, N.A.; Butler, T.; Schiff, N.D.; Kuceyeski, A.; Shah, S.A. Longitudinal alterations in gamma-aminobutyric acid (GABAA) receptor availability over approximately 1 year following traumatic brain injury. Brain Commun. 2022, 4, fcac159. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, X.; Shoga, M.; Li, L.; Zouridakis, G.; Tran, T.; Fonteh, A.N.; Dawlaty, J.; Goldweber, R.; Pogoda, J.M.; Harrington, M.G. Alpha desynchronization/synchronization during working memory testing is compromised in acute mild traumatic brain injury (mTBI). PLoS ONE 2018, 13, e0188101. [Google Scholar] [CrossRef]
- Tanaka, K.; Ogawa, N.; Asanuma, M.; Kondo, Y. Thyrotropin releasing hormone prevents abnormalities of cortical acetylcholine and monoamines in mice following head injury. Regul. Pept. 1997, 70, 173–178. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Grady, M.S. Loss of forebrain cholinergic neurons following fluid-percussion injury: Implications for cognitive impairment in closed head injury. J. Neurosurg. 1995, 83, 496–502. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makale, M.T.; Nybo, C.; Keifer, J.; Blum, K.; Dennen, C.A.; Baron, D.; Sunder, K.; Elman, I.; Makale, M.R.; Thanos, P.K.; et al. Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion. Brain Sci. 2023, 13, 1179. https://doi.org/10.3390/brainsci13081179
Makale MT, Nybo C, Keifer J, Blum K, Dennen CA, Baron D, Sunder K, Elman I, Makale MR, Thanos PK, et al. Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion. Brain Sciences. 2023; 13(8):1179. https://doi.org/10.3390/brainsci13081179
Chicago/Turabian StyleMakale, Milan T., Chad Nybo, Jason Keifer, Kenneth Blum, Catherine A. Dennen, David Baron, Keerthy Sunder, Igor Elman, Miles R. Makale, Panayotis K. Thanos, and et al. 2023. "Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion" Brain Sciences 13, no. 8: 1179. https://doi.org/10.3390/brainsci13081179
APA StyleMakale, M. T., Nybo, C., Keifer, J., Blum, K., Dennen, C. A., Baron, D., Sunder, K., Elman, I., Makale, M. R., Thanos, P. K., & Murphy, K. T. (2023). Preliminary Observations of Personalized Repetitive Magnetic Stimulation (PrTMS) Guided by EEG Spectra for Concussion. Brain Sciences, 13(8), 1179. https://doi.org/10.3390/brainsci13081179










