Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota
Abstract
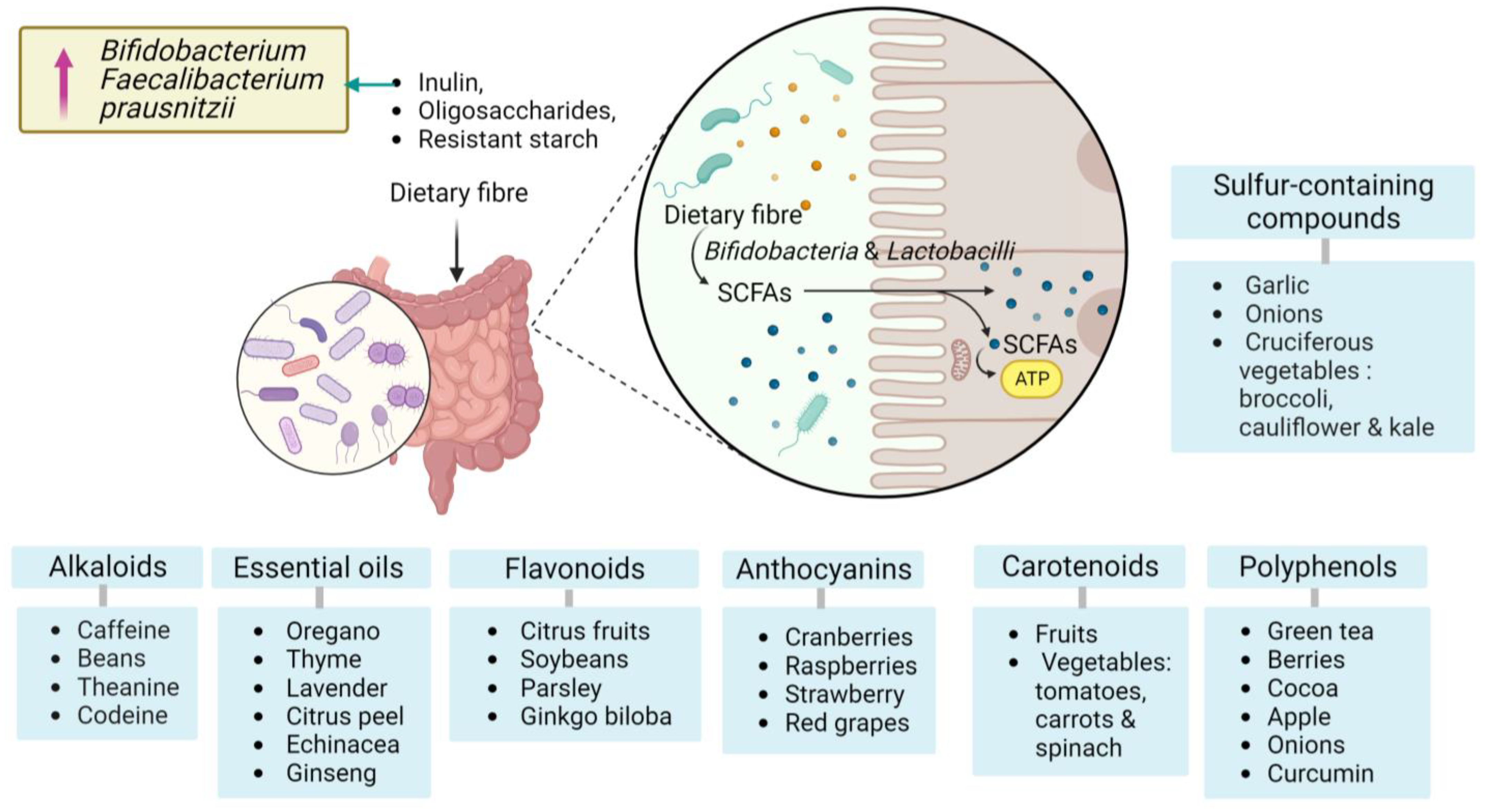
:1. Introduction
2. Pathophysiology of Inflammation
3. Inflammation Pathogenesis
4. Gut Microbiota and Its Biological Functions
5. Interplay between the ‘Gut Microbiota–Brain Axis’ and Inflammation
6. Gut Microbiota–Brain Axis and Neurodegenerative Diseases
6.1. Alterations in the Gut Microbiota in Parkinson’s Disease
6.2. Effects of Gut Microbiota on Alzheimer’s Disease
6.3. Gut Changes in Huntington’s Disease
7. The Interplay between Inflammatory Bowel Diseases and Human Gut Microbiota
8. The Role of Bioactive Compounds in Modulating Inflammatory Pathways Associated with Diseases
9. The Protective and Preventive Role of Bioactive Compounds against the Development of Inflammatory-Associated Diseases
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology, 10th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 57–95, 175–176, 234–235, 621–626. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology Functions and Disorders of the Immune System, 5th ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 27–53. [Google Scholar]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Roy, S.; Bagchi, D.; Raychaudhuri, S.P. Chronic Inflammation: Molecular Pathophysiology, Nutritional and Therapeutic Interventions, 1st ed.; Roy, S., Bagchi, D., Raychaudhuri, S.P., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alyami, B.A.; Alqahtani, Y.S.; Jan, M.S.; Rashid, U.; Sadiq, A.; Alqarni, A.O. Phytochemical Profiling of Bioactive Compounds, Anti-Inflammatory and Analgesic Potentials of Habenaria Digitata Lindl.: Molecular Docking Based Synergistic Effect of the Identified Compounds. J. Ethnopharmacol. 2021, 273, 113976. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B. Bioactive Compounds as Inhibitors of Inflammation, Oxidative Stress and Metabolic Dysfunctions via Regulation of Cellular Redox Balance and Histone Acetylation State. Foods 2023, 12, 925. [Google Scholar] [CrossRef]
- Pham, T.X.; Lee, J. Dietary Regulation of Histone Acetylases and Deacetylases for the Prevention of Metabolic Diseases. Nutrients 2012, 4, 1868–1886. [Google Scholar] [CrossRef]
- NavaneethaKrishnan, S.; Rosales, J.L.; Lee, K.-Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell. Longev. 2019, 2019, 9051542. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Noh, E.J.; Cha, K.-H.; Kim, Y.S.; Lim, S.S.; Shin, K.H.; Jung, S.H. Inhibitory Effects of Irigenin from the Rhizomes of Belamcanda Chinensis on Nitric Oxide and Prostaglandin E(2) Production in Murine Macrophage RAW 264.7 Cells. Life Sci. 2006, 78, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of Adipose Tissue Inflammation by Bioactive Food Compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds and Nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F.J., Saraiva, J.M.A., Cravotto, G., Lorenzo, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. [Google Scholar]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of Resveratrol on Gut Microbiota and Fat Storage in a Mouse Model with High-Fat- Induced Obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Xiong, H.-H.; Lin, S.-Y.; Chen, L.-L.; Ouyang, K.-H.; Wang, W.-J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.-L.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved Glucose Homeostasis in Obese Mice Treated with Resveratrol Is Associated with Alterations in the Gut Microbiome. Diabetes 2017, 66, 418–425. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols Enhanced Production of Anti-Inflammatory Substance(s) by Bifidobacterium Adolescentis: Prebiotic Actions of Galangin, Quercetin, and Fisetin: Prebiotic Actions of Flavonols to B. Adolescentis. Biofactors 2013, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, M.; Kang, G.; Huang, H. The Potential Role of Phytonutrients Flavonoids Influencing Gut Microbiota in the Prophylaxis and Treatment of Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 798038. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, Y.; Long, L.; Zhang, H. Effects of Dietary L-Theanine on Growth Performance, Antioxidation, Meat Quality, and Intestinal Microflora in White Feather Broilers with Acute Oxidative Stress. Front. Vet. Sci. 2022, 9, 889485. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.-M. Possible Benefits of Faecalibacterium Prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef]
- Sánchez-Gloria, J.L.; Rada, K.M.; Juárez-Rojas, J.G.; Sánchez-Lozada, L.G.; Rubio-Gayosso, I.; Sánchez-Muñoz, F.; Osorio-Alonso, H. Role of Sulfur Compounds in Garlic as Potential Therapeutic Option for Inflammation and Oxidative Stress in Asthma. Int. J. Mol. Sci. 2022, 23, 15599. [Google Scholar] [CrossRef]
- Wang, W.; Meng, H. Cytotoxic, Anti-Inflammatory and Hemostatic Spirostane-Steroidal Saponins from the Ethanol Extract of the Roots of Bletilla Striata. Fitoterapia 2015, 101, 12–18. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of Prebiotics, Probiotics, and Synbiotics in Management of Inflammatory Bowel Disease: Current Perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol Modulates the Gut Microbiota to Prevent Murine Colitis Development through Induction of Tregs and Suppression of Th17 Cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut Microbiota and Inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A Guiding Map for Inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Zigterman, B.G.R.; Dubois, L. Inflammation and infection: Cellular and biochemical processes. Ned. Tijdschr. Tandheelkd. 2022, 129, 125–129. [Google Scholar] [CrossRef]
- Häder, A.; Schäuble, S.; Gehlen, J.; Thielemann, N.; Buerfent, B.C.; Schüller, V.; Hess, T.; Wolf, T.; Schröder, J.; Weber, M.; et al. Pathogen-Specific Innate Immune Response Patterns Are Distinctly Affected by Genetic Diversity. Nat. Commun. 2023, 14, 3239. [Google Scholar] [CrossRef]
- Bonecchi, R. Chemokines and Chemokine Receptors: An Overview. Front. Biosci. 2009, 14, 540. [Google Scholar] [CrossRef]
- Scapini, P.; Marini, O.; Tecchio, C.; Cassatella, M.A. Human Neutrophils in the Saga of Cellular Heterogeneity: Insights and Open Questions. Immunol. Rev. 2016, 273, 48–60. [Google Scholar] [CrossRef]
- Eyerich, K.; Dimartino, V.; Cavani, A. IL-17 and IL-22 in Immunity: Driving Protection and Pathology. Eur. J. Immunol. 2017, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Bernshtein, B.; Curato, C.; Ioannou, M.; Thaiss, C.A.; Gross-Vered, M.; Kolesnikov, M.; Wang, Q.; David, E.; Chappell-Maor, L.; Harmelin, A.; et al. IL-23-Producing IL-10Rα-Deficient Gut Macrophages Elicit an IL-22-Driven Proinflammatory Epithelial Cell Response. Sci. Immunol. 2019, 4, eaau6571. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed on 8 August 2023).
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.L.; Basit, H.; Burns, B. Pathology, Inflammation; StatPearls Publishing: Orlando, FL, USA, 2022. [Google Scholar]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-Induced Cancer: Crosstalk between Tumours, Immune Cells and Microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Karunakaran, D.; Nguyen, M.-A.; Geoffrion, M.; Vreeken, D.; Lister, Z.; Cheng, H.S.; Otte, N.; Essebier, P.; Wyatt, H.; Kandiah, J.W.; et al. RIPK1 Expression Associates with Inflammation in Early Atherosclerosis in Humans and Can Be Therapeutically Silenced to Reduce NF-ΚB Activation and Atherogenesis in Mice. Circulation 2021, 143, 163–177. [Google Scholar] [CrossRef]
- Goyal, A.; Yeh, A.; Bush, B.R.; Firek, B.A.; Siebold, L.M.; Rogers, M.B.; Kufen, A.D.; Morowitz, M.J. Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 410–421. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-Biotics, and Post-Biotics. Front. Nutr. 2021, 8, 634897. [Google Scholar] [CrossRef]
- Sarmiento-Andrade, Y.; Suárez, R.; Quintero, B.; Garrochamba, K.; Chapela, S.P. Gut Microbiota and Obesity: New Insights. Front. Nutr. 2022, 9, 1018212. [Google Scholar] [CrossRef]
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The Role of Gut Microbiota in the Development of Obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, Z.; Chen, Y.; Guo, Q.; Dong, Y. Interactions between Gut Microbes and NLRP3 Inflammasome in the Gut-Brain Axis. Comput. Struct. Biotechnol. J. 2023, 21, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The Infant Microbiome Development: Mom Matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The Gut Microbiota--Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, Y.; Fuhrer, T.; Li, H.; Lawson, M.A.; Zimmermann, M.; Yilmaz, B.; Zindel, J.; Ronchi, F.; Sorribas, M.; Hapfelmeier, S.; et al. Antibodies Set Boundaries Limiting Microbial Metabolite Penetration and the Resultant Mammalian Host Response. Immunity 2018, 49, 545–559.e5. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the Immune System. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef]
- Krishnan, S.; Alden, N.; Lee, K. Pathways and Functions of Gut Microbiota Metabolism Impacting Host Physiology. Curr. Opin. Biotechnol. 2015, 36, 137–145. [Google Scholar] [CrossRef]
- Siddiqui, R.; Soopramanien, M.; Alharbi, A.M.; Alfahemi, H.; Khan, N.A. Novel Sources of Bioactive Molecules: Gut Microbiome of Species Routinely Exposed to Microorganisms. Vet. Sci. 2022, 9, 380. [Google Scholar] [CrossRef]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-Gut-Brain Axis: Enteroendocrine Cells and the Enteric Nervous System Form an Interface between the Microbiota and the Central Nervous System. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-López, G. The Microbiota-Gut-Brain Axis: Neurobehavioral Correlates, Health and Sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L., IV; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut Feelings: The Emerging Biology of Gut-Brain Communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex Control of Immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The Interplay between the Intestinal Microbiota and the Brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Bakker, G.J.; Nieuwdorp, M. Fecal Microbiota Transplantation: Therapeutic Potential for a Multitude of Diseases beyond Clostridium difficile. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qiao, Y. Fecal Microbiota Transplantation Ameliorates Gut Microbiota Imbalance and Intestinal Barrier Damage in Rats with Stress-Induced Depressive-like Behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Qiao, Y.; Xie, R.; Lin, L.; Jiang, J.; Wang, C.; Li, G. Fecal Microbiota Transplantation Ameliorates Stress-Induced Depression-like Behaviors Associated with the Inhibition of Glial and NLRP3 Inflammasome in Rat Brain. J. Psychiatr. Res. 2021, 137, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Wortelboer, K.; Nieuwdorp, M.; Herrema, H. Fecal Microbiota Transplantation beyond Clostridioides Difficile Infections. EBioMedicine 2019, 44, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27, 3402. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef]
- Zhao, Z.; Ning, J.; Bao, X.-Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal Microbiota Transplantation Protects Rotenone-Induced Parkinson’s Disease Mice via Suppressing Inflammation Mediated by the Lipopolysaccharide-TLR4 Signaling Pathway through the Microbiota-Gut-Brain Axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Anto, L.; Blesso, C.N. Interplay between Diet, the Gut Microbiome, and Atherosclerosis: Role of Dysbiosis and Microbial Metabolites on Inflammation and Disordered Lipid Metabolism. J. Nutr. Biochem. 2022, 105, 108991. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Fernández-Real, J.M. Microbiota Impacts on Chronic Inflammation and Metabolic Syndrome-Related Cognitive Dysfunction. Rev. Endocr. Metab. Disord. 2019, 20, 473–480. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Liébana-García, R.; Olivares, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Romaní-Pérez, M.; Sanz, Y. The Gut Microbiota as a Versatile Immunomodulator in Obesity and Associated Metabolic Disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101542. [Google Scholar] [CrossRef] [PubMed]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The Role of Gut and Genital Microbiota and the Estrobolome in Endometriosis, Infertility and Chronic Pelvic Pain. Hum. Reprod. Update 2021, 28, 92–131. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Fornai, M.; D’Antongiovanni, V.; Antonioli, L.; Bernardini, N.; Derkinderen, P. The Intestinal Barrier in Disorders of the Central Nervous System. Lancet Gastroenterol. Hepatol. 2023, 8, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Rossi, O.; Meijerink, M.; van Baarlen, P. Epithelial Crosstalk at the Microbiota-Mucosal Interface. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4607–4614. [Google Scholar] [CrossRef] [PubMed]
- Caron, T.J.; Scott, K.E.; Fox, J.G.; Hagen, S.J. Tight Junction Disruption: Helicobacter Pylori and Dysregulation of the Gastric Mucosal Barrier. World J. Gastroenterol. 2015, 21, 11411–11427. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Kumar, H.; Bot, A. In This Issue: Effect of Gut Microbiome on Mucosal Immunity and Enteric Diseases. Int. Rev. Immunol. 2018, 37, 77–78. [Google Scholar] [CrossRef]
- Tomkovich, S.; Jobin, C. Microbiota and Host Immune Responses: A Love-Hate Relationship. Immunology 2016, 147, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-C.; Chang, C.-C.; Huang, C.-W.; Nouchi, R.; Cheng, C.-H. Gut Microbiota in Patients with Alzheimer’s Disease Spectrum: A Systematic Review and Meta-Analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Taliyan, R.; Dubey, S.K. Comprehensive Review on Potential Signaling Pathways Involving the Transfer of α-Synuclein from the Gut to the Brain That Leads to Parkinson’s Disease. ACS Chem. Neurosci. 2023, 14, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Perottoni, S.; Izzo, L.; Giordano, C.; Albani, D. Microbiota-Host Immunity Communication in Neurodegenerative Disorders: Bioengineering Challenges for in Vitro Modeling. Adv. Healthc. Mater. 2021, 10, e2002043. [Google Scholar] [CrossRef]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada González, F.; Perrin, P.; et al. Microglia Development Follows a Stepwise Program to Regulate Brain Homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The Role of Microbial Amyloid in Neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s Disease: Possible Routes by Which Vulnerable Neuronal Types May Be Subject to Neuroinvasion by an Unknown Pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Zhu, Y.; Huan, F.; Wang, J.; Xie, X.; Yu, G.; Wang, X.; Jiang, L.; Gao, R.; Xiao, H.; Ding, H.; et al. 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Induced Parkinson’s Disease in Mouse: Potential Association between Neurotransmitter Disturbance and Gut Microbiota Dysbiosis. ACS Chem. Neurosci. 2020, 11, 3366–3376. [Google Scholar] [CrossRef]
- Nowak, J.M.; Kopczyński, M.; Friedman, A.; Koziorowski, D.; Figura, M. Microbiota Dysbiosis in Parkinson Disease-in Search of a Biomarker. Biomedicines 2022, 10, 2057. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, F.; Cao, J.; Ding, W.; Yan, S.; Shi, W.; Wen, S.; Yao, L. Alterations of Gut Microbiota and Metabolome with Parkinson’s Disease. Microb. Pathog. 2021, 160, 105187. [Google Scholar] [CrossRef]
- Sun, M.-F.; Shen, Y.-Q. Dysbiosis of Gut Microbiota and Microbial Metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Murros, K.E. Hydrogen Sulfide Produced by Gut Bacteria May Induce Parkinson’s Disease. Cells 2022, 11, 978. [Google Scholar] [CrossRef]
- Chien, C.-H.; Lee, M.-J.; Liou, H.-C.; Liou, H.-H.; Fu, W.-M. Microglia-Derived Cytokines/Chemokines Are Involved in the Enhancement of LPS-Induced Loss of Nigrostriatal Dopaminergic Neurons in DJ-1 Knockout Mice. PLoS ONE 2016, 11, e0151569. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cui, L.; Gao, J.; Zhu, M.; Zhang, Y.; Zhang, H.-L. Gut Microbial Metabolites in Parkinson’s Disease: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Mol. Neurobiol. 2021, 58, 3745–3758. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Murphy, S.; Martinson, H.A. Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front. Neurosci. 2019, 13, 369. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Grathwohl, S.; Quansah, E.; Maroof, N.; Steiner, J.A.; Spycher, L.; Benmansour, F.; Duran-Pacheco, G.; Siebourg-Polster, J.; Oroszlan-Szovik, K.; Remy, H.; et al. Specific Immune Modulation of Experimental Colitis Drives Enteric Alpha-Synuclein Accumulation and Triggers Age-Related Parkinson-like Brain Pathology. Free Neuropathol. 2021, 2, 2–13. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, S.P.; Frye, A.K.; El-Gendy, A.O.; Castejon, M.; Keshavarzian, A.; van Dijk, G.; El Aidy, S. Gut Bacterial Tyrosine Decarboxylases Restrict Levels of Levodopa in the Treatment of Parkinson’s Disease. Nat. Commun. 2019, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Wasser, C.I.; Mercieca, E.-C.; Kong, G.; Hannan, A.J.; McKeown, S.J.; Glikmann-Johnston, Y.; Stout, J.C. Gut Dysbiosis in Huntington’s Disease: Associations among Gut Microbiota, Cognitive Performance and Clinical Outcomes. Brain Commun. 2020, 2, fcaa110. [Google Scholar] [CrossRef] [PubMed]
- Park, A.-M.; Omura, S.; Fujita, M.; Sato, F.; Tsunoda, I. Helicobacter Pylori and Gut Microbiota in Multiple Sclerosis versus Alzheimer’s Disease: 10 Pitfalls of Microbiome Studies. Clin. Exp. Neuroimmunol. 2017, 8, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Shen, L.; Zhou, M.; Luo, J.; Yu, Z.; Qu, C.; Lei, R.; Lei, M.; Huang, R. Helicobacter Pylori and Alzheimer’s Disease-Related Metabolic Dysfunction: Activation of TLR4/Myd88 Inflammation Pathway from P53 Perspective and a Case Study of Low-Dose Radiation Intervention. ACS Chem. Neurosci. 2022, 13, 1065–1081. [Google Scholar] [CrossRef]
- Konjevod, M.; Nikolac Perkovic, M.; Sáiz, J.; Svob Strac, D.; Barbas, C.; Rojo, D. Metabolomics Analysis of Microbiota-Gut-Brain Axis in Neurodegenerative and Psychiatric Diseases. J. Pharm. Biomed. Anal. 2021, 194, 113681. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N.; Rashid, S.; Bano, G. Effects of Gut Microbiota on Neurodegenerative Diseases. Front. Aging Neurosci. 2023, 15, 1145241. [Google Scholar] [CrossRef]
- Kumar, P.; Clark, M. Kumar & Clark Clinical Medicine, 8th ed.; Saunders Elsevier: Edinburgh, Scotland; London, UK; New York, NY, USA; Oxford, UK; Philadelphia, PA, USA; St Louis, MO, USA; Sydney, Australia; Toronto, Japan, 2012; pp. 271–279. [Google Scholar]
- Mao, L.; Kitani, A.; Similuk, M.; Oler, A.J.; Albenberg, L.; Kelsen, J.; Aktay, A.; Quezado, M.; Yao, M.; Montgomery-Recht, K.; et al. Loss-of-Function CARD8 Mutation Causes NLRP3 Inflammasome Activation and Crohn’s Disease. J. Clin. Investig. 2018, 128, 1793–1806. [Google Scholar] [CrossRef]
- Kiesslich, R.; Duckworth, C.A.; Moussata, D.; Gloeckner, A.; Lim, L.G.; Goetz, M.; Pritchard, D.M.; Galle, P.R.; Neurath, M.F.; Watson, A.J.M. Local Barrier Dysfunction Identified by Confocal Laser Endomicroscopy Predicts Relapse in Inflammatory Bowel Disease. Gut 2012, 61, 1146–1153. [Google Scholar] [CrossRef]
- Zuo, T.; Lu, X.-J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut Mucosal Virome Alterations in Ulcerative Colitis. Gut 2019, 68, 1169–1179. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Kleiner, M.; Paez-Espino, D.; Zhu, W.; Bushnell, B.; Hassell, B.; Winter, S.E.; Kyrpides, N.C.; Hooper, L.V. Murine Colitis Reveals a Disease-Associated Bacteriophage Community. Nat. Microbiol. 2018, 3, 1023–1031. [Google Scholar] [CrossRef]
- Shan, Y.; Lee, M.; Chang, E.B. The Gut Microbiome and Inflammatory Bowel Diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.S.; Mahapatra, P.; Ghosh, A.; Basu, S. Estimation of the Reducing Power and Electrochemical Behavior of Few Flavonoids and Polyhydroxybenzophenones Substantiated by Bond Dissociation Energy: A Comparative Analysis. Mol. Divers. 2022, 26, 1101–1113. [Google Scholar] [CrossRef]
- Bukhari, I.A.; Khan, R.A.; Gilani, A.H.; Ahmed, S.; Saeed, S.A. Analgesic, Anti-Inflammatory and Anti-Platelet Activities of the Methanolic Extract of Acacia Modesta Leaves. Inflammopharmacology 2010, 18, 187–196. [Google Scholar] [CrossRef]
- Dickson, K.; Scott, C.; White, H.; Zhou, J.; Kelly, M.; Lehmann, C. Antibacterial and Analgesic Properties of Beta-Caryophyllene in a Murine Urinary Tract Infection Model. Molecules 2023, 28, 4144. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Boland Nazar, A.R. Total Phenolic, Total Flavonoids, Antioxidant and Antimicrobial Activities of Scrophularia Striata Boiss Extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 15–19. [Google Scholar] [CrossRef]
- Tsai, S.H.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of Nitric Oxide Synthase and the Down-Regulation of the Activation of NFkappaB in Macrophages by Resveratrol: Resveratrol Inhibits INOS Induction in Macrophages. Br. J. Pharmacol. 1999, 126, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive Properties of Trans-Resveratrol Are Associated with Inhibition of Activation of the IkappaB Kinase. Cancer Res. 2000, 60, 3477–3483. [Google Scholar]
- Liu, Q.; Zuo, R.; Wang, K.; Nong, F.-F.; Fu, Y.-J.; Huang, S.-W.; Pan, Z.-F.; Zhang, Y.; Luo, X.; Deng, X.-L.; et al. Oroxindin Inhibits Macrophage NLRP3 Inflammasome Activation in DSS-Induced Ulcerative Colitis in Mice via Suppressing TXNIP-Dependent NF-ΚB Pathway. Acta Pharmacol. Sin. 2020, 41, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Saadoune, Z.; Laribi, H.; Benali, Y.; Brahimi, A.; Bennani, R.; El-Hadi, D. Valorization of Algerian Grape Pomace Seeds: Extraction of Bioactive Compounds, Prevention and Treatment of Experimental Inflammatory Bowel Diseases. Waste Biomass Valorization 2021, 12, 5401–5412. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Wopperer, A.L.; Chrisfield, B.J.; Tao, L.; Cooper, T.K.; Vanamala, J.; Elias, R.J.; Hayes, J.E.; Lambert, J.D. Soy Protein Concentrate Mitigates Markers of Colonic Inflammation and Loss of Gut Barrier Function In Vitro and In Vivo. J. Nutr. Biochem. 2017, 40, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chaparala, A.; Poudyal, D.; Tashkandi, H.; Witalison, E.E.; Chumanevich, A.A.; Hofseth, J.L.; Nguyen, I.; Hardy, O.; Pittman, D.L.; Wyatt, M.D.; et al. Panaxynol, a Bioactive Component of American Ginseng, Targets Macrophages and Suppresses Colitis in Mice. Oncotarget 2020, 11, 2026–2036. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Anti-Inflammatory Effects of Baicalin, Baicalein, and Wogonin In Vitro and In Vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef]
- Clemente, A.; Gee, J.M.; Johnson, I.T.; Mackenzie, D.A.; Domoney, C. Pea (Pisum sativum L.) Protease Inhibitors from the Bowman-Birk Class Influence the Growth of Human Colorectal Adenocarcinoma HT29 Cells In Vitro. J. Agric. Food Chem. 2005, 53, 8979–8986. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Maggiano, N.; Tringali, G.; Navarra, P.; Ranelletti, F.O.; Calviello, G. Beta-Carotene Downregulates the Steady-State and Heregulin-Alpha-Induced COX-2 Pathways in Colon Cancer Cells. J. Nutr. 2005, 135, 129–136. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Oniga, O.; Vlase, A.-M.; Ielciu, I.; Toiu, A.; Oniga, I. New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum Officinale Tincture. Pharmaceuticals 2023, 16, 358. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.; El-Twab, S.M.A.; Yousef, A.I.; Reheim, E.S.A.; Ashour, M.B. Modulation of Hyperglycemia and Dyslipidemia in Experimental Type 2 Diabetes by Gallic Acid and P-Coumaric Acid: The Role of Adipocytokines and PPARγ. Biomed. Pharmacother. 2018, 105, 1091–1097. [Google Scholar] [CrossRef]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; Di Felice, M.; Scaccini, C. Inhibition of Human Low-Density Lipoprotein Oxidation by Caffeic Acid and Other Hydroxycinnamic Acid Derivatives. Free Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Jialal, I.; Grundy, S.M. Effect of Dietary Supplementation with Alpha-Tocopherol on the Oxidative Modification of Low Density Lipoprotein. J. Lipid Res. 1992, 33, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Villa-Jaimes, G.S.; Moshage, H.; Avelar-González, F.J.; González-Ponce, H.A.; Buist-Homan, M.; Guevara-Lara, F.; Sánchez-Alemán, E.; Martínez-Hernández, S.L.; Ventura-Juárez, J.; Muñoz-Ortega, M.H.; et al. Molecular and Antioxidant Characterization of Opuntia Robusta Fruit Extract and Its Protective Effect against Diclofenac-Induced Acute Liver Injury in an in Vivo Rat Model. Antioxidants 2023, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-C.; Rao, Y.K.; Whang-Peng, J.; Huang, C.-Y.F.; Shyue, S.-K.; Hsu, S.-L.; Tzeng, Y.-M. Antcin B and Its Ester Derivative from Antrodia Camphorata Induce Apoptosis in Hepatocellular Carcinoma Cells Involves Enhancing Oxidative Stress Coincident with Activation of Intrinsic and Extrinsic Apoptotic Pathway. J. Agric. Food Chem. 2011, 59, 10943–10954. [Google Scholar] [CrossRef]
- Lee, K.-J.; Inoue, M.; Otani, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S.; JPHC Study Group. Coffee Consumption and Risk of Colorectal Cancer in a Population-Based Prospective Cohort of Japanese Men and Women. Int. J. Cancer 2007, 121, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Dik, V.K.; Bueno-de-Mesquita, H.B.A.; Van Oijen, M.G.H.; Siersema, P.D.; Uiterwaal, C.S.P.M.; Van Gils, C.H.; Van Duijnhoven, F.J.B.; Cauchi, S.; Yengo, L.; Froguel, P.; et al. Coffee and Tea Consumption, Genotype-Based CYP1A2 and NAT2 Activity and Colorectal Cancer Risk-Results from the EPIC Cohort Study: Coffee and Tea Consumption and Colorectal Cancer Risk. Int. J. Cancer 2014, 135, 401–412. [Google Scholar] [CrossRef]

| Bioactive Compound | Targeted Microbiota | Effects on Microbiota | Effects on Inflammation | Reference |
|---|---|---|---|---|
| Polyphenols | Bifidobacteria, Lactobacilli Clostridia Bifidobacterium and Lactobacillus Faecalibacterium prausnitzii Roseburia species. |
|
| [35] |
| Prebiotics | Bifidobacteria, Lactobacilli |
|
| [36] |
| Probiotics | Lactobacillus species, and Bifidobacterium species |
|
| [36] |
| Resveratrol | Bacillus species, Lactobacillus species, Bifidobacterium species, Ackermania species. |
|
| [24,37] |
| Quercetin | Bifidobacterium and Akkermansia |
|
| [25] |
| Dietary fibres | Bifidobacterium and Faecalibacterium prausnitzii |
|
| [31,32,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benameur, T.; Porro, C.; Twfieg, M.-E.; Benameur, N.; Panaro, M.A.; Filannino, F.M.; Hasan, A. Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota. Brain Sci. 2023, 13, 1226. https://doi.org/10.3390/brainsci13081226
Benameur T, Porro C, Twfieg M-E, Benameur N, Panaro MA, Filannino FM, Hasan A. Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota. Brain Sciences. 2023; 13(8):1226. https://doi.org/10.3390/brainsci13081226
Chicago/Turabian StyleBenameur, Tarek, Chiara Porro, Mohammed-Elfatih Twfieg, Nassima Benameur, Maria Antonietta Panaro, Francesca Martina Filannino, and Abeir Hasan. 2023. "Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota" Brain Sciences 13, no. 8: 1226. https://doi.org/10.3390/brainsci13081226
APA StyleBenameur, T., Porro, C., Twfieg, M.-E., Benameur, N., Panaro, M. A., Filannino, F. M., & Hasan, A. (2023). Emerging Paradigms in Inflammatory Disease Management: Exploring Bioactive Compounds and the Gut Microbiota. Brain Sciences, 13(8), 1226. https://doi.org/10.3390/brainsci13081226






