Metabolite Variations in the Hippocampus and Corpus Callosum of Patients with Mild Cognitive Impairment Using Magnetic Resonance Spectroscopy with Three-Dimensional Chemical Shift Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
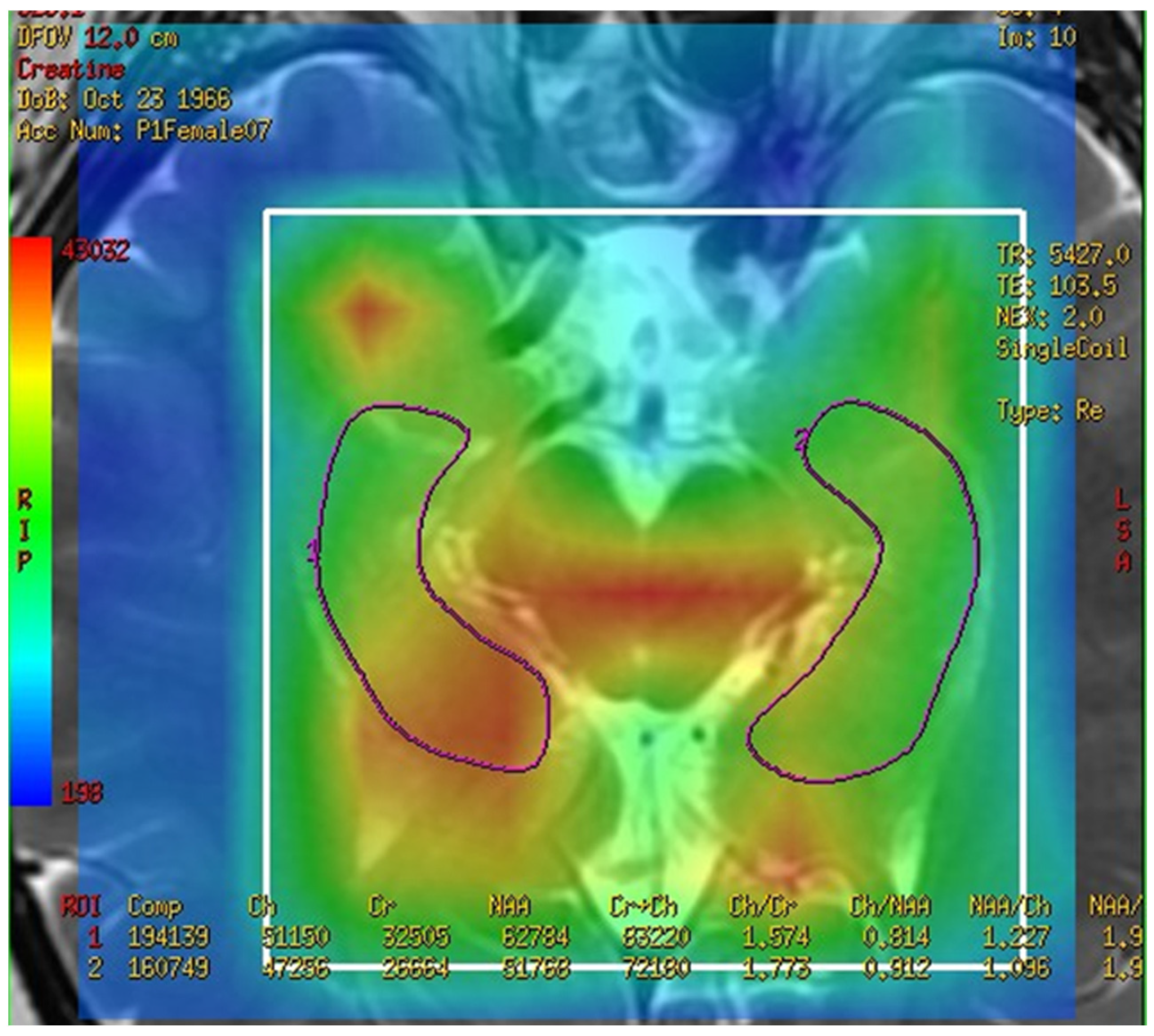
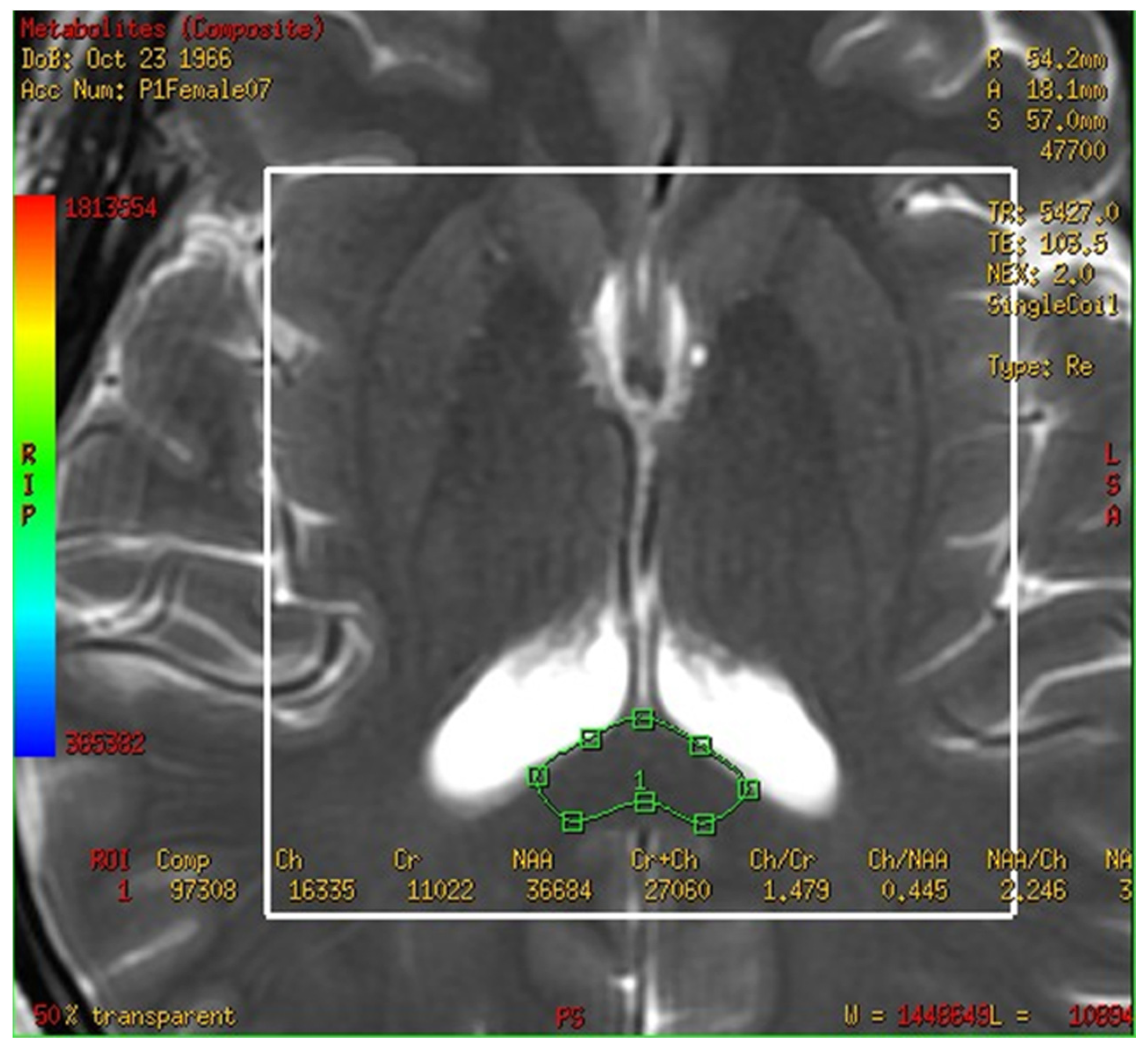
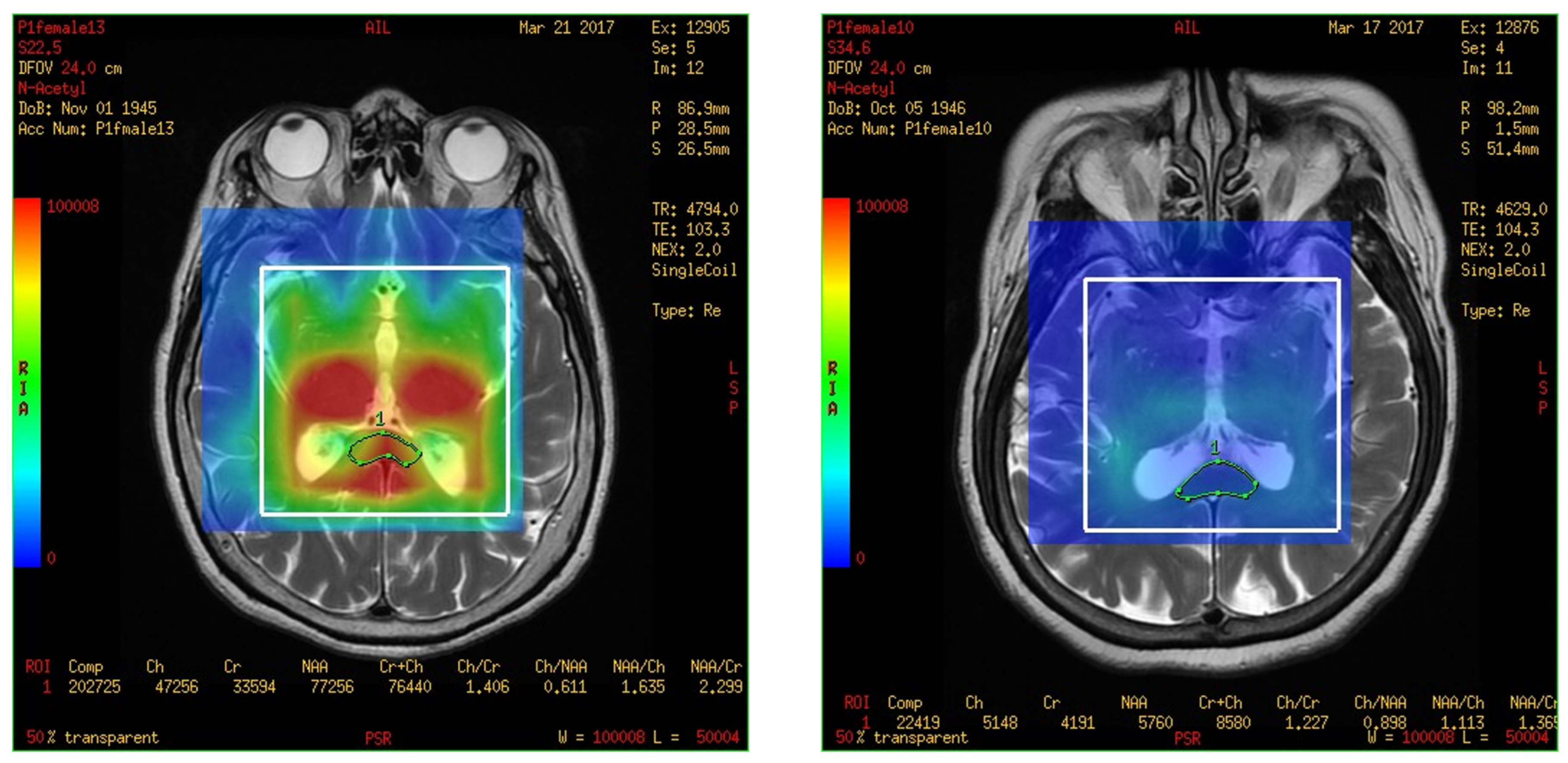
3.2. The Spectra of MRS Analysis in Brain and Fusion Map the 2-D Multi-Voxel Spectrum and Image
3.3. Metabolites in the Total Hippocampus, Left Hippocampus, and Right Hippocampus in the MCI and Control Groups
3.4. Metabolites in the Corpus Callosum in the MCI and Control Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Usarel, C.; Dokuzlar, O.; Aydin, A.E.; Soysal, P.; Isik, A.T. The AD8 (Dementia Screening Interview) is a valid and reliable screening scale not only for dementia but also for mild cognitive impairment in the Turkish geriatric outpatients. Int. Psychogeriatr. 2019, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Crescioli, G.; Cavedo, E.; Lucenteforte, E.; Casazza, G.; Bellatorre, A.G.; Lista, C.; Costantino, G.; Frisoni, G.; Virgili, G.; et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer’s disease in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2020, 3, Cd009628. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Isoda, H.; Togashi, K. 31P MR Spectroscopy with 3D chemical-shift imaging detects changes in levels of phosphorus metabolites due to saliva secretion in human parotid glands. Open J. Med. Imaging 2020, 10, 42. [Google Scholar] [CrossRef]
- Chang, L.; Ernst, T.; Witt, M.D.; Ames, N.; Gaiefsky, M.; Miller, E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage 2002, 17, 1638–1648. [Google Scholar] [CrossRef]
- Kim, D.H. Three-dimensional chemical shift imaging with PRESS excitation and spiral readouts. J. Korean Soc. Magn. Reson. Med. 2008, 12, 27–32. [Google Scholar]
- Chmelík, M.; Schmid, A.I.; Gruber, S.; Szendroedi, J.; Krssák, M.; Trattnig, S.; Moser, E.; Roden, M. Three-dimensional high-resolution magnetic resonance spectroscopic imaging for absolute quantification of 31P metabolites in human liver. Magn. Reson. Med. 2008, 60, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Chmelik, M.; Schmid, A.I.; Nowotny, P.; Brehm, A.; Krssak, M.; Moser, E.; Roden, M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 2009, 50, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, M.H.; Maddock, R.J. Magnetic resonance spectroscopy of the brain: A review of physical principles and technical methods. Rev. Neurosci. 2015, 26, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, J.M.; Dawood, M.; Shariff, M.I.; Grover, V.P.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic resonance spectroscopy: Principles and techniques: Lessons for clinicians. J. Clin. Exp. Hepatol. 2015, 5, 320–328. [Google Scholar] [CrossRef]
- Namboodiri, A.M.; Peethambaran, A.; Mathew, R.; Sambhu, P.A.; Hershfield, J.; Moffett, J.R.; Madhavarao, C.N. Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol. Cell. Endocrinol. 2006, 252, 216–223. [Google Scholar] [CrossRef]
- Bitsch, A.; Bruhn, H.; Vougioukas, V.; Stringaris, A.; Lassmann, H.; Frahm, J.; Brück, W. Inflammatory CNS demyelination: Histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am. J. Neuroradiol. 1999, 20, 1619–1627. [Google Scholar]
- Davie, C.A.; Hawkins, C.P.; Barker, G.J.; Brennan, A.; Tofts, P.S.; Miller, D.H.; McDonald, W.I. Detection of myelin breakdown products by proton magnetic resonance spectroscopy. Lancet 1993, 341, 630–631. [Google Scholar] [CrossRef]
- Miller, B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991, 4, 47–52. [Google Scholar] [CrossRef]
- Su, L.; Balmire, A.M.; Watson, R.; He, J.; O’Brien, J.T. Whole-brain patterns of (1)H-magnetic resonance spectroscopy imaging in Alzheimer’s disease and dementia with Lewy bodies. Transl. Psychiatry 2016, 30, e877. [Google Scholar] [CrossRef]
- Jack, C.R., Jr. Alzheimer disease: New concepts on its neurobiology and the clinical role imaging will play. Radiology 2012, 263, 344–361. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N. Intra- and inter-hemispheric connectivity supporting hemispheric specialization. In Micro-, Meso- and Macro-Connectomics of the Brain; Kennedy, H., Van Essen, D.C., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 129–146. [Google Scholar]
- Wang, X.D.; Ren, M.; Zhu, M.W.; Gao, W.P.; Zhang, J.; Shen, H.; Lin, Z.G.; Feng, H.L.; Zhao, C.J.; Gao, K. Corpus callosum atrophy associated with the degree of cognitive decline in patients with Alzheimer’s dementia or mild cognitive impairment: A meta-analysis of the region of interest structural imaging studies. J. Psychiatry Res. 2015, 63, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Stricker, N.H.; Salat, D.H.; Kuhn, T.P.; Foley, J.M.; Price, J.S.; Westlye, L.T.; Esterman, M.S.; McGlinchey, R.E.; Milberg, W.P.; Leritz, E.C. Mild cognitive impairment is associated with white matter integrity changes in late-myelinating regions within the corpus callosum. Am. J. Alzheimers Dis. Other Dement. 2016, 31, 68–75. [Google Scholar] [CrossRef]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef]
- Vion-Dury, J.; Meyerhoff, D.J.; Cozzone, P.J.; Weiner, M.W. What might be the impact on neurology of the analysis of brain metabolism by in vivo magnetic resonance spectroscopy? J. Neurol. 1994, 241, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wan, X.; Zhao, X.; Rong, Y.; Wu, Y.; Cao, Z.; Xie, Q.; Luo, M.; Liu, Y. Brain neurometabolites differences in individuals with subjective cognitive decline plus: A quantitative single- and multi-voxel proton magnetic resonance spectroscopy study. Quant. Imaging Med. Surg. 2021, 11, 4074–4096. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, C.; Prestopnik, J.; Thompson, J.; Taheri, S.; Huisa, B.; Schrader, R.; Adair, J.C.; Rosenberg, G.A. 1H-MR spectroscopy metabolite levels correlate with executive function in vascular cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2013, 84, 715–721. [Google Scholar] [CrossRef]
- Kotb, M.A.; Kamal, A.M.; Aldossary, N.M.; Alsify, A.A.; Ahmed, Y.M. Value of magnetic resonance spectroscopy in geriatric patients with cognitive impairment. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 10. [Google Scholar] [CrossRef]
- Glodzik, L.; Sollberger, M.; Gass, A.; Gokhale, A.; Rusinek, H.; Babb, J.S.; Hirsch, J.G.; Amann, M.; Monsch, A.U.; Gonen, O. Global N-acetylaspartate in normal subjects, mild cognitive impairment and Alzheimer’s disease patients. J. Alzheimers Dis. 2015, 43, 939–947. [Google Scholar] [CrossRef]
- Klein, J. Membrane breakdown in acute and chronic neurodegeneration: Focus on choline-containing phospholipids. J. Neural Transm. 2000, 107, 1027–1063. [Google Scholar] [CrossRef]
- Lehto, L.J.; Albors, A.A.; Sierra, A.; Tolppanen, L.; Eberly, L.E.; Mangia, S.; Nurmi, A.; Michaeli, S.; Gröhn, O. Lysophosphatidyl choline induced demyelination in rat probed by relaxation along a fictitious field in high rank rotating frame. Front. Neurosci. 2017, 11, 433. [Google Scholar] [CrossRef]
- Mathews, P.M.; Andermann, F.; Silver, K.; Karpati, G.; Arnold, D.L. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology 1993, 43, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Bouhrara, M.; Reiter, D.A.; Bergeron, C.M.; Zukley, L.M.; Ferrucci, L.; Resnick, S.M.; Spencer, R.G. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement. 2018, 14, 998–1004. [Google Scholar] [CrossRef]
- Haroutunian, V.; Hoffman, L.B.; Beeri, M.S. Is there a neuropathology difference between mild cognitive impairment and dementia? Dialogues Clin. Neurosci. 2009, 11, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Kelty, T.J.; Kerr, N.R.; Childs, T.E.; Roberts, M.D.; Booth, F.W. Creatine supplementation upregulates mTORC1 signaling and markers of synaptic plasticity in the dentate gyrus while ameliorating LPS-induced cognitive impairment in female rats. Nutrients 2021, 13, 2758. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Pilatus, U.; Lais, C.; Rochmont Adu, M.; Kratzsch, T.; Frölich, L.; Maurer, K.; Zanella, F.E.; Lanfermann, H.; Pantel, J. Conversion to dementia in mild cognitive impairment is associated with decline of N-actylaspartate and creatine as revealed by magnetic resonance spectroscopy. Psychiatry Res. 2009, 173, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Foy, C.M.; Daly, E.M.; Glover, A.; O’Gorman, R.; Simmons, A.; Murphy, D.G.; Lovestone, S. Hippocampal proton MR spectroscopy in early Alzheimer’s disease and mild cognitive impairment. Brain Topogr. 2011, 24, 316–322. [Google Scholar] [CrossRef]
- Häussinger, D.; Laubenberger, J.; vom Dahl, S.; Ernst, T.; Bayer, S.; Langer, M.; Gerok, W.; Hennig, J. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology 1994, 107, 1475–1480. [Google Scholar] [CrossRef]
- Siger, M.; Schuff, N.; Zhu, X.; Miller, B.L.; Weiner, M.W. Regional myo-inositol concentration in mild cognitive impairment Using 1H magnetic resonance spectroscopic imaging. Alzheimer Dis. Assoc. Disord. 2009, 23, 57–62. [Google Scholar] [CrossRef]

| Control Group | MCI Group | p Value | |
|---|---|---|---|
| Age (years) | 61.3 ± 5.4 | 60.0 ± 3.7 | 0.476 |
| Weight (Kilogram) | 60.8 ± 9.1 | 66.4 ± 4.7 | 0.519 |
| Metabolite | Control Group | MCI Group | p Value |
|---|---|---|---|
| Total hippocampus | |||
| NAA/Cr | 1.714 ± 0.752 | 0.569 ± 0.341 | 0.001 * |
| Cho/Cr | 1.317 ± 0.614 | 0.410 ± 0.148 | 0.001 * |
| MI/Cr | 0.313 ± 0.131 | 0.276 ± 0.262 | 0.475 |
| Cr | 29,000 ± 12,700 | 8900 ± 2400 | 0.001 * |
| Left hippocampus | |||
| NAA/Cr | 1.663 ± 0.701 | 0.742 ± 0.621 | 0.030 * |
| Cho/Cr | 1.413 ± 0.644 | 0.443 ± 0.148 | 0.010 ** |
| MI/Cr | 0.333 ± 0.140 | 0.239 ± 0.186 | 0.320 |
| Cr | 26,400 ± 11,500 | 9500 ± 2800 | 0.010 ** |
| Right hippocampus | |||
| NAA/Cr | 1.759 ± 0.835 | 0.427 ± 0.212 | 0.001 ** |
| Cho/Cr | 1.234 ± 0.604 | 0.383 ± 0.161 | 0.001 ** |
| MI/Cr | 0.297 ± 0.136 | 0.307 ± 0.041 | 0.320 |
| Cr | 31,600 ± 14,100 | 8300 ± 2900 | 0.001 ** |
| Metabolite | Control Group | MCI Group | p Value |
|---|---|---|---|
| NAA/Cr | 2.506 ± 1.648 | 0.778 ± 0.333 | 0.020 * |
| Cho/Cr | 1.327 ± 1.062 | 0.321 ± 0.204 | 0.113 |
| MI/Cr | 0.222 ± 0.136 | 0.062 ± 0.056 | 0.001 ** |
| Cr | 16,200 ± 11800 | 4200 ± 2100 | 0.020 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kau, Y.-L.; Lin, I.-H.; Juang, C.-L.; Chang, C.-K.; Ho, W.-H.; Wen, H.-C. Metabolite Variations in the Hippocampus and Corpus Callosum of Patients with Mild Cognitive Impairment Using Magnetic Resonance Spectroscopy with Three-Dimensional Chemical Shift Images. Brain Sci. 2023, 13, 1244. https://doi.org/10.3390/brainsci13091244
Kau Y-L, Lin I-H, Juang C-L, Chang C-K, Ho W-H, Wen H-C. Metabolite Variations in the Hippocampus and Corpus Callosum of Patients with Mild Cognitive Impairment Using Magnetic Resonance Spectroscopy with Three-Dimensional Chemical Shift Images. Brain Sciences. 2023; 13(9):1244. https://doi.org/10.3390/brainsci13091244
Chicago/Turabian StyleKau, Yen-Lon, I-Hung Lin, Chi-Long Juang, Chao-Kai Chang, Wen-Hsiang Ho, and Hsiao-Chuan Wen. 2023. "Metabolite Variations in the Hippocampus and Corpus Callosum of Patients with Mild Cognitive Impairment Using Magnetic Resonance Spectroscopy with Three-Dimensional Chemical Shift Images" Brain Sciences 13, no. 9: 1244. https://doi.org/10.3390/brainsci13091244
APA StyleKau, Y.-L., Lin, I.-H., Juang, C.-L., Chang, C.-K., Ho, W.-H., & Wen, H.-C. (2023). Metabolite Variations in the Hippocampus and Corpus Callosum of Patients with Mild Cognitive Impairment Using Magnetic Resonance Spectroscopy with Three-Dimensional Chemical Shift Images. Brain Sciences, 13(9), 1244. https://doi.org/10.3390/brainsci13091244






