Transcranial Direct-Current Stimulation Improves Verbal Fluency in Children with Attention Deficit Hyperactivity Disorder (ADHD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Swanson, Nolan, and Pelham Scale (SNAP-IV)
2.3. Semantic Verbal Fluency Test (SVFT)
2.4. Phonemic Verbal Fluency Test (PVFT)
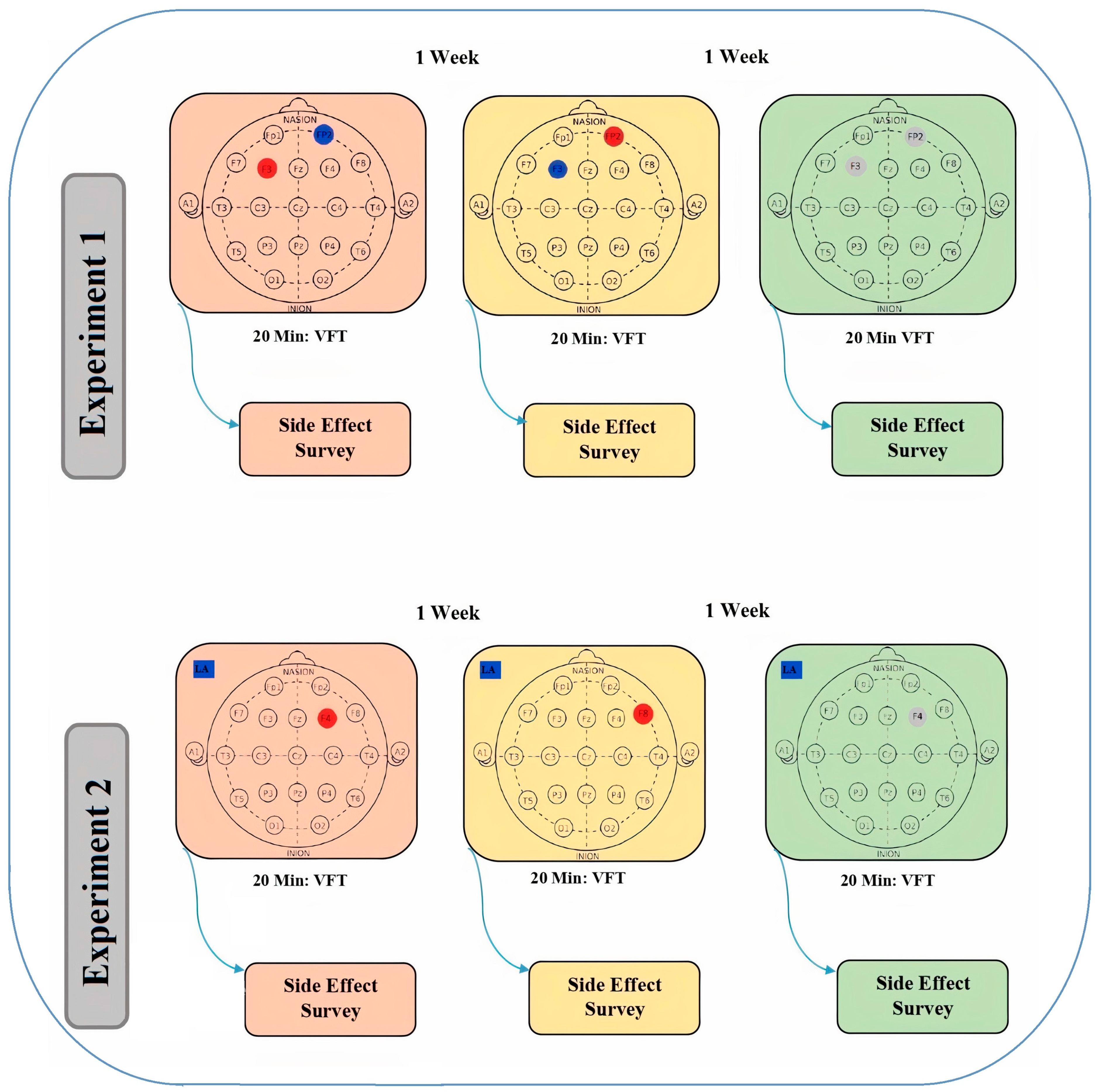
2.5. Protocols for the tDCS
2.6. Experimental Procedures
2.7. Data Analysis
3. Results
4. Discussion
4.1. Improved Phonemic Verbal Fluency
4.2. Improved Semantic Verbal Fluency
4.3. Domain-Specific Improvement
4.4. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- APA American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA American Psychiatric Association: Washington, DC, USA, 2013; ISBN 9780890425541. [Google Scholar]
- Soluki, S.; Nejati, V.; Fathabadi, J. Spatial Ability in children with Attention-Deficit/Hyperactivity Disorder (ADHD) and its Impact on Executive Functions. Res. Sq. 2020, 1–26. [Google Scholar] [CrossRef]
- Nejati, V.; Khoshroo, S.; Mirikaram, F. Review of spatial disability in individuals with attention deficit-hyperactivity disorder: Toward spatial cognition theory. Clin. Child Psychol. Psychiatry 2023, 13591045231176708. [Google Scholar] [CrossRef]
- Nejati, V.; Abadi, F.; Ramezannia, Z.; Najian, A. The study of sustained attention of children with attention deficit-hyperactivity disorder and typical children. J. Psychol. 2016, 15, 276–288. [Google Scholar]
- Nejati, V.; Yazdani, S. Time perception in children with attention deficit–hyperactivity disorder (ADHD): Does task matter? A meta-analysis study. Child Neuropsychol. 2020, 26, 900–916. [Google Scholar] [CrossRef]
- Borhani, K.; Nejati, V. Emotional face recognition in individuals withattention-deficit/hyperactivity disorder: A review article. Dev. Neuropsychol. 2018, 43, 256–277. [Google Scholar] [CrossRef]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65. [Google Scholar] [CrossRef]
- Nejati, V. Reading mind from the eyes in attention deficit-hyperactivity disorder (ADHD): A meta-analysis. Expert Rev. Neurother. 2022, 22, 889–896. [Google Scholar] [CrossRef]
- Stoy, M.; Schlagenhauf, F.; Schlochtermeier, L.; Wrase, J.; Knutson, B.; Lehmkuhl, U.; Huss, M.; Heinz, A.; Ströhle, A. Reward processing in male adults with childhood ADHD—A comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology 2011, 215, 467–481. [Google Scholar]
- Sonuga-Barke, E.J.S.; Taylor, E.; Sembi, S.; Smith, J. Hyperactivity and delay aversion—I. The effect of delay on choice. J. Child Psychol. Psychiatry 1992, 33, 387–398. [Google Scholar] [CrossRef]
- Wodka, E.L.; Mark Mahone, E.; Blankner, J.G.; Gidley Larson, J.C.; Fotedar, S.; Denckla, M.B.; Mostofsky, S.H. Evidence that response inhibition is a primary deficit in ADHD. J. Clin. Exp. Neuropsychol. 2007, 29, 345–356. [Google Scholar] [CrossRef]
- Bálint, S.; Bitter, I.; Czobor, P. Neurobiological correlates of cognitive flexibility in ADHD-A systematic review of the literature. Psychiatr. Hung. 2015, 30, 363–371. [Google Scholar] [PubMed]
- Ramos, A.A.; Hamdan, A.C.; Machado, L. A meta-analysis on verbal working memory in children and adolescents with ADHD. Clin. Neuropsychol. 2020, 34, 873–898. [Google Scholar]
- Goldstein, S.; Naglieri, J.A. Executive Functioning; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar]
- Diamond, A. The early development of executive functions. In Lifespan Cognition: Mechanisms of Change; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Christiansen, H.; Hirsch, O.; Albrecht, B.; Chavanon, M.-L. Attention-deficit/hyperactivity disorder (ADHD) and emotion regulation over the life span. Curr. Psychiatry Rep. 2019, 21, 17. [Google Scholar] [PubMed]
- Charman, T.; Carroll, F.; Sturge, C. Theory of mind, executive function and social competence in boys with ADHD. Emot. Behav. Diffic. 2001, 6, 31–49. [Google Scholar]
- Scheres, A.; Oosterlaan, J.; Geurts, H.; Morein-Zamir, S.; Meiran, N.; Schut, H.; Vlasveld, L.; Sergeant, J.A. Executive functioning in boys with ADHD: Primarily an inhibition deficit? Arch. Clin. Neuropsychol. 2004, 19, 569–594. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Fischer, J.S. Neuropsychological Assessment; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Ardila, A.; Ostrosky-Solís, F.; Bernal, B. Cognitive testing toward the future: The example of Semantic Verbal Fluency (ANIMALS). Int. J. Psychol. 2006, 41, 324–332. [Google Scholar]
- Reuter, I.; Mehnert, S.; Sammer, G.; Oechsner, M.; Engelhardt, M. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in parkinsons disease. J. Aging Res. 2012, 2012, 235765. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V. Correlation between working memory and verbal fluency among the elderly. J. Res. Rehabil. Sci. 2012, 8, 412–418. [Google Scholar]
- Hirshorn, E.A.; Thompson-Schill, S.L. Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia 2006, 44, 2547–2557. [Google Scholar]
- Abwender, D.A.; Swan, J.G.; Bowerman, J.T.; Connolly, S.W. Qualitative analysis of verbal fluency output: Review and comparison of several scoring methods. Assessment 2001, 8, 323–338. [Google Scholar] [PubMed]
- Dromey, C.; Shim, E. The effects of divided attention on speech motor, verbal fluency, and manual task performance. J. Speech Lang. Hear. Res. 2008, 51, 1171–1182. [Google Scholar]
- Whiteside, D.M.; Kealey, T.; Semla, M.; Luu, H.; Rice, L.; Basso, M.R.; Roper, B. Verbal fluency: Language or executive function measure? Appl. Neuropsychol. Adult 2016, 23, 29–34. [Google Scholar] [PubMed]
- Kavé, G.; Sapir-Yogev, S. Associations between memory and verbal fluency tasks. J. Commun. Disord. 2020, 83, 105968. [Google Scholar] [PubMed]
- Hurks, P.P.M.; Hendriksen, J.G.M.; Vles, J.S.H.; Kalff, A.C.; Feron, F.J.M.; Kroes, M.; Van Zeben, T.; Steyaert, J.; Jolles, J. Verbal fluency over time as a measure of automatic and controlled processing in children with ADHD. Brain Cogn. 2004, 55, 535–544. [Google Scholar] [PubMed]
- Andreou, G.; Trott, K. Verbal fluency in adults diagnosed with attention-deficit hyperactivity disorder (ADHD) in childhood. ADHD Atten. Deficit Hyperact. Disord. 2013, 5, 343–351. [Google Scholar]
- Marchetta, N.D.J.; Hurks, P.P.M.; Krabbendam, L.; Jolles, J. Interference control, working memory, concept shifting, and verbal fluency in adults with attention-deficit/hyperactivity disorder (ADHD). Neuropsychology 2008, 22, 74. [Google Scholar] [PubMed]
- Abreu, N.; Argollo, N.; Oliveira, F.; Cardoso, A.L.; Bueno, J.L.O.; Xavier, G.F. Semantic and phonologic verbal fluency tests for adolescents with ADHD. Clin. Neuropsychiatry 2013, 10, 63–71. [Google Scholar]
- Koziol, L.F.; Stout, C.E. Use of a verbal fluency measure in understanding and evaluating ADHD as an executive function disorder. Percept. Mot. Skills 1992, 75, 1187–1192. [Google Scholar]
- Asefi, M.; Nejati, V.; Sharifi, M. The Effect of Cognitive Rehabilitation on the Improvement of Language Skills in 9–12 Years Old Children with Attention Deficit/Hyperactivity Disorder. J. Rehabil. Sci. Res. 2017, 4, 89–96. [Google Scholar]
- Boggio, P.S.; Rigonatti, S.P.; Ribeiro, R.B.; Myczkowski, M.L.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 2008, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nejati, V.; Rasanan, A.H.H.; Rad, J.A.; Alavi, M.M.; Haghi, S.; Nitsche, M.A. Transcranial direct current stimulation (tDCS) alters the pattern of information processing in children with ADHD: Evidence from drift diffusion modeling. Neurophysiol. Clin. 2022, 52, 17–27. [Google Scholar] [CrossRef]
- Nejati, V.; Mirikaram, F.; Rad, J.A. Transcranial direct current stimulation alters the process of reward processing in children with ADHD: Evidence from cognitive modeling. Neurophysiol. Clin. 2023, 53, 102884. [Google Scholar] [PubMed]
- Berger, I.; Dakwar-Kawar, O.; Grossman, E.S.; Nahum, M.; Kadosh, R.C. Scaffolding the attention-deficit/hyperactivity disorder brain using transcranial direct current and random noise stimulation: A randomized controlled trial. Clin. Neurophysiol. 2021, 132, 699–707. [Google Scholar] [CrossRef]
- Nejati, V.; Salehinejad, M.A.; Nitsche, M.A.; Najian, A.; Javadi, A.-H. Transcranial Direct Current Stimulation Improves Executive Dysfunctions in ADHD: Implications for Inhibitory Control, Interference Control, Working Memory, and Cognitive Flexibility. J. Atten. Disord. 2017, 24, 1928–1943. [Google Scholar] [CrossRef]
- Nejati, V.; Alavi, M.M.; Nitsche, M.A. The Impact of Attention Deficit-hyperactivity Disorder Symptom Severity on the Effectiveness of Transcranial Direct Current Stimulation (tDCS) on Inhibitory Control. Neuroscience 2021, 466, 248–257. [Google Scholar] [CrossRef]
- Klomjai, W.; Siripornpanich, V.; Aneksan, B.; Vimolratana, O.; Permpoonputtana, K.; Tretriluxana, J.; Thichanpiang, P. Effects of cathodal transcranial direct current stimulation on inhibitory and attention control in children and adolescents with attention-deficit hyperactivity disorder: A pilot randomized sham-controlled crossover study. J. Psychiatr. Res. 2022, 150, 130–141. [Google Scholar] [CrossRef]
- Soltaninejad, Z.; Nejati, V.; Ekhtiari, H. Effect of Anodal and Cathodal Transcranial Direct Current Stimulation on DLPFC on Modulation of Inhibitory Control in ADHD. J. Atten. Disord. 2019, 23, 325–332. [Google Scholar] [CrossRef]
- Soltaninejad, Z.; Nejati, V.; Ekhtiari, H. Effect of transcranial direct current stimulation on remediation of inhibitory control on right inferio frontal gyrus in attention deficit and hyperactivity symptoms. Rehabil. Med. 2015, 3, 1–9. [Google Scholar]
- Salehinejad, M.A.; Nejati, V.; Mosayebi-Samani, M.; Mohammadi, A.; Wischnewski, M.; Kuo, M.-F.; Avenanti, A.; Vicario, C.M.; Nitsche, M.A. Transcranial direct current stimulation in ADHD: A systematic review of efficacy, safety, and protocol-induced electrical field modeling results. Neurosci. Bull. 2020, 36, 1191–1212. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Wischnewski, M.; Nejati, V.; Vicario, C.M.; Nitsche, M.A. Transcranial direct current stimulation in attention-deficit hyperactivity disorder: A meta-analysis of neuropsychological deficits. PLoS ONE 2019, 14, e0215095. [Google Scholar]
- Westwood, S.J.; Radua, J.; Rubia, K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2021, 46, E14–E33. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Vosough, Y.; Nejati, V. The impact of bilateral anodal tDCS over left and right DLPFC on executive functions in children with ADHD. Brain Sci. 2022, 12, 1098. [Google Scholar] [CrossRef]
- Cattaneo, Z.; Pisoni, A.; Papagno, C. Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience 2011, 183, 64–70. [Google Scholar] [CrossRef]
- Mayseless, N.; Shamay-Tsoory, S.G. Enhancing verbal creativity: Modulating creativity by altering the balance between right and left inferior frontal gyrus with tDCS. Neuroscience 2015, 291, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, E.; Salehinejad, M.A.; Nejati, V.; Nitsche, M.A. Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: Implications for the supramodal contribution of executive functions. Sci. Rep. 2019, 9, 3700. [Google Scholar] [CrossRef] [PubMed]
- Smirni, D.; Oliveri, M.; Misuraca, E.; Catania, A.; Vernuccio, L.; Picciolo, V.; Inzerillo, F.; Barbagallo, M.; Cipolotti, L.; Turriziani, P. Verbal fluency in mild Alzheimer’s disease: Transcranial direct current stimulation over the dorsolateral prefrontal cortex. J. Alzheimers Dis. 2021, 81, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J. School-Based Assessments and Interventions for ADD Students; KC Publishing: Las Vegas, NV, USA, 1992. [Google Scholar]
- Kiani, B.; Hadianfard, H. Psychometric properties of a persian self-report version of Swanson, Nolan and Pelham rating scale (version IV) for screening attention-deficit/hyperactivity disorder in adolescents. Iran. J. Psychiatry Clin. Psychol. 2016, 21, 317–326. [Google Scholar]
- Nejati, V.; Asadi, A. Semantic and phonemic verbal fluency in blinds. J. Psycholinguist. Res. 2010, 39, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, C.; Mondino, M.; Arns, M.; Baeken, C.; Bikson, M.; Brunoni, A.R.; Burke, M.J.; Neuvonen, T.; Padberg, F.; Pascual-Leone, A. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 2019, 12, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Nejati, V.; Najian, A.; Akbarpour, F. The effectiveness of motor based cognitive rehabilitation on improvement of working memory of children with ADHD. J. Psychol. 2017, 15, 504–517. [Google Scholar]
- Najian, A.; Nejati, V. Effectiveness of motor based cognitive rehabilitation on improvement of sustained attention and cognitive flexibility of children with ADHD. Sci. J. Rehabil. Med. 2017, 6, 1–12. [Google Scholar]
- Rothärmel, M.; Moulier, V.; Vasse, M.; Isaac, C.; Faerber, M.; Bendib, B.; Mirea-Grivel, I.; Opolczynski, G.; Rosier, A.; Guillin, O. A prospective open-label pilot study of transcranial direct current stimulation in high-functioning autistic patients with a dysexecutive syndrome. Neuropsychobiology 2019, 78, 189–199. [Google Scholar] [CrossRef]
- Manenti, R.; Cotelli, M.S.; Cobelli, C.; Gobbi, E.; Brambilla, M.; Rusich, D.; Alberici, A.; Padovani, A.; Borroni, B.; Cotelli, M. Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: A randomized, placebo-controlled study. Brain Stimul. 2018, 11, 1251–1262. [Google Scholar] [CrossRef]
- Pereira, J.B.; Junqué, C.; Bartrés-Faz, D.; Martí, M.J.; Sala-Llonch, R.; Compta, Y.; Falcón, C.; Vendrell, P.; Pascual-Leone, Á.; Valls-Solé, J. Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson’s disease. Brain Stimul. 2013, 6, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pestalozzi, M.I.; Di Pietro, M.; Martins Gaytanidis, C.; Spierer, L.; Schnider, A.; Chouiter, L.; Colombo, F.; Annoni, J.-M.; Jost, L.B. Effects of prefrontal transcranial direct current stimulation on lexical access in chronic poststroke aphasia. Neurorehabil. Neural Repair 2018, 32, 913–923. [Google Scholar] [CrossRef]
- Hart, H.; Radua, J.; Nakao, T.; Mataix-Cols, D.; Rubia, K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: Exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 2013, 70, 185–198. [Google Scholar] [CrossRef]
- Lukito, S.; Norman, L.; Carlisi, C.; Radua, J.; Hart, H.; Simonoff, E.; Rubia, K. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol. Med. 2020, 50, 894–919. [Google Scholar] [CrossRef] [PubMed]
- Norman, L.J.; Carlisi, C.; Lukito, S.; Hart, H.; Mataix-Cols, D.; Radua, J.; Rubia, K. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: A comparative meta-analysis. JAMA Psychiatry 2016, 73, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Koeda, M.; Ikeda, Y.; Hama, T.; Funayama, T.; Akiyama, T.; Arakawa, R.; Tateno, A.; Suzuki, H.; Okubo, Y. Effects of anodal transcranial direct current stimulation on implicit motor learning and language-related brain function: An fMRI study. Psychiatry Clin. Neurosci. 2021, 75, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Gourovitch, M.L.; Kirkby, B.S.; Goldberg, T.E.; Weinberger, D.R.; Gold, J.M.; Esposito, G.; Van Horn, J.D.; Berman, K.F. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 2000, 14, 353. [Google Scholar] [CrossRef]
- Perani, D.; Abutalebi, J.; Paulesu, E.; Brambati, S.; Scifo, P.; Cappa, S.F.; Fazio, F. The role of age of acquisition and language usage in early, high-proficient bilinguals: An fMRI study during verbal fluency. Hum. Brain Mapp. 2003, 19, 170–182. [Google Scholar] [CrossRef]
- Birn, R.M.; Kenworthy, L.; Case, L.; Caravella, R.; Jones, T.B.; Bandettini, P.A.; Martin, A. Neural systems supporting lexical search guided by letter and semantic category cues: A self-paced overt response fMRI study of verbal fluency. Neuroimage 2010, 49, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Gollan, T.H.; Ferreira, V.S. Should I stay or should I switch? A cost–benefit analysis of voluntary language switching in young and aging bilinguals. J. Exp. Psychol. Learn. Mem. Cogn. 2009, 35, 640. [Google Scholar] [CrossRef]
- Esse Wilson, J.; Trumbo, M.C.; Wilson, J.K.; Tesche, C.D. Transcranial direct current stimulation (tDCS) over right temporoparietal junction (rTPJ) for social cognition and social skills in adults with autism spectrum disorder (ASD). J. Neural Transm. 2018, 125, 1857–1866. [Google Scholar] [CrossRef] [PubMed]


| Variables | M (SD) | |
|---|---|---|
| Experiment 1 | Experiment 2 | |
| Age (Years) | 8.83 (1.50) | 9.57 (1.38) |
| Education (Years *) | 2.89 (1.45) | 3.42 (1.60) |
| Gender (Male/Female) | 14/4 | 18/1 |
| SNAP-IV | 29.04 (8.63) | 23.32 (11.41) |
| Conditions, M (SD) | Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Experiment 1 | F3/FP2 | FP2/F3 | Sham | df | F | p | ηp2 |
| Pain | 0.11 (0.32) | 0.06 (0.23) | 0.00 (0.00) | 1.45 | 1.00 | 0.35 | 0.05 |
| Vertigo | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 2 | |||
| Burning | 1.17 (1.04) | 1.33 (1.28) | 0.44 (0.61) | 2 | 5.40 | 0.01 | 0.24 |
| Tingling | 0.61 (0.77) | 0.89 (1.02) | 0.56 (0.92) | 2 | 1.11 | 0.34 | 0.06 |
| Confusion | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 2 | |||
| Drowsiness | 0.33 (0.76) | 0.06 (0.23) | 0.22 (0.73) | 2 | 1.12 | 0.33 | 0.06 |
| Experiment 2 | F4/LA | F8/LA | Sham | df | F | p | ηp2 |
| Pain | 1.11 (1.76) | 0.89 (1.41) | 0.21 (0.71) | 2 | 3.42 | 0.04 | 0.16 |
| Vertigo | 0.16 (0.37) | 0.32 (0.74) | 0.05 (0.22) | 1.47 | 1.28 | 0.28 | 0.06 |
| Burning | 1.00 (1.82) | 0.89 (1.24) | 0.37 (1.16) | 1.47 | 1.28 | 0.28 | 0.06 |
| Tingling | 1.42 (1.80) | 0.89 (1.32) | 0.26 (0.73) | 2 | 7.06 | <0.01 | 0.28 |
| Confusion | 0.53 (0.90) | 0.63 (1.11) | 0.11 (0.45) | 1.25 | 3.68 | 0.05 | 0.17 |
| Drowsiness | 0.74 (1.28) | 0.58 (0.90) | 0.37 (1.16) | 2 | 0.53 | 0.59 | 0.02 |
| Conditions, M (SD) | Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Experiment 1 | F3/FP2 | FP2/F3 | Sham | df | F | p | ηp2 |
| Phonemic | 14.11 (7.59) | 12.22 (7.98) | 11.00 (7.12) | 1.23 | 4.63 | 0.03 | 0.21 |
| Semantic | 25.17 (7.80) | 23.39 (7.04) | 24.50 (6.99) | 2 | 1.15 | 0.32 | 0.06 |
| Experiment 2 | F4/LA | F8/LA | Sham | df | F | p | ηp2 |
| Phonemic | 12.00 (5.09) | 10.63 (3.04) | 10.16 (4.74) | 2 | 1.50 | 0.23 | 0.07 |
| Semantic | 25.26 (5.94) | 24.95 (5.08) | 21.89 (5.01) | 2 | 3.97 | 0.02 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejati, V.; Estaji, R.; Helisaz, Z. Transcranial Direct-Current Stimulation Improves Verbal Fluency in Children with Attention Deficit Hyperactivity Disorder (ADHD). Brain Sci. 2023, 13, 1257. https://doi.org/10.3390/brainsci13091257
Nejati V, Estaji R, Helisaz Z. Transcranial Direct-Current Stimulation Improves Verbal Fluency in Children with Attention Deficit Hyperactivity Disorder (ADHD). Brain Sciences. 2023; 13(9):1257. https://doi.org/10.3390/brainsci13091257
Chicago/Turabian StyleNejati, Vahid, Reza Estaji, and Zahra Helisaz. 2023. "Transcranial Direct-Current Stimulation Improves Verbal Fluency in Children with Attention Deficit Hyperactivity Disorder (ADHD)" Brain Sciences 13, no. 9: 1257. https://doi.org/10.3390/brainsci13091257
APA StyleNejati, V., Estaji, R., & Helisaz, Z. (2023). Transcranial Direct-Current Stimulation Improves Verbal Fluency in Children with Attention Deficit Hyperactivity Disorder (ADHD). Brain Sciences, 13(9), 1257. https://doi.org/10.3390/brainsci13091257





