Non-Opioid Anesthetics Addiction: A Review of Current Situation and Mechanism
Abstract
1. Introduction
2. Methods
3. The Current Situation of Non-Opioid Anesthetics Addiction
4. Molecular Mechanism of Non-Opioid Anesthetic Drug Addiction
4.1. Propofol
4.1.1. Propofol Addiction and DAergic System
4.1.2. Propofol Addiction and Stress/Anti-Reward System
4.1.3. Propofol Addiction and Nitrergic System
4.2. Ketamine
4.2.1. Ketamine Addiction and GLUergic System
4.2.2. Ketamine Addiction and DAergic System
4.2.3. Ketamine Addiction and Changes in Brain Structure and Functional Brain Network Integrity
4.3. Benzodiazepines
4.3.1. BDZs Addiction and α1-GABAARs
4.3.2. BDZ Addiction and Other Subtypes of GABAARs
4.4. Inhalational Anesthetics
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volkow, N.D.; Boyle, M. Neuroscience of Addiction: Relevance to Prevention and Treatment. Am. J. Psychiatry 2018, 175, 729–740. [Google Scholar] [CrossRef]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Veerappa, A.; Pendyala, G.; Guda, C. A systems omics-based approach to decode substance use disorders and neuroadaptations. Neurosci. Biobehav. Rev. 2021, 130, 61–80. [Google Scholar] [CrossRef]
- Misra, U.; Gilvarry, E.; Marshall, J.; Hall, R.; McLure, H.; Mayall, R.; El-Ghazali, S.; Redfern, N.; McGrady, E.; Gerada, C. Substance use disorder in the anaesthetist: Guidelines from the Association of Anaesthetists: Guidelines from the Association of Anaesthetists. Anaesthesia 2022, 77, 691–699. [Google Scholar] [CrossRef]
- Zuleta-Alarcón, A.; Coffman, J.C.; Soghomonyan, S.; Papadimos, T.J.; Bergese, S.D.; Moran, K.R. Non-opioid anesthetic drug abuse among anesthesia care providers: A narrative review. Can. J. Anaesth. 2017, 64, 169–184. [Google Scholar] [CrossRef][Green Version]
- Fry, R.A.; Fry, L.E.; Castanelli, D.J. A retrospective survey of substance abuse in anaesthetists in Australia and New Zealand from 2004 to 2013. Anaesth. Intensiv. Care 2015, 43, 111–117. [Google Scholar] [CrossRef]
- Samuelson, S.T.; Bryson, E.O. The impaired anesthesiologist: What you should know about substance abuse. Can. J. Anaesth. 2017, 64, 219–235. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Kiely, J.; Luxton, R. Propofol detection for monitoring of intravenous anaesthesia: A review. J. Clin. Monit. Comput. 2022, 36, 315–323. [Google Scholar] [CrossRef]
- Sahinovic, M.M.; Struys, M.M.R.F.; Absalom, A.R. Clinical Pharmacokinetics and Pharmacodynamics of Propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef]
- Hewson, D.W.; Hardman, J.G.; Bedforth, N.M. Patient-maintained propofol sedation for adult patients undergoing surgical or medical procedures: A scoping review of current evidence and technology. Br. J. Anaesth. 2021, 126, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Shiwalkar, N.; Reddy, K.; Shin, P.; Bekker, A. Neurobiology of Propofol Addiction and Supportive Evidence: What Is the New Development? Brain Sci. 2018, 8, 36. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Johnson, B.R.; Wilson, J.E.; Dingmann, C.; Bachman, H.M.; Roller, E.; Tran, Z.V.; Henthorn, T.K. A survey of propofol abuse in academic anesthesia programs. Obstet. Anesthesia Dig. 2007, 105, 1066–1071. [Google Scholar] [CrossRef]
- Maier, C.; Iwunna, J.; Tsokos, M.; Mußhoff, F. Deaths from propofol abuse: Survey of institutes of forensic medicine in Germany, Austria and Switzerland. Anaesthesist 2017, 66, 109–114. [Google Scholar] [CrossRef]
- Hess, E.M.; Riggs, L.M.; Michaelides, M.; Gould, T.D. Mechanisms of ketamine and its metabolites as antidepressants. Biochem. Pharmacol. 2022, 197, 114892. [Google Scholar] [CrossRef]
- Marland, S.; Ellerton, J.; Andolfatto, G.; Strapazzon, G.; Thomassen, O.; Brandner, B.; Weatherall, A.; Paal, P. Ketamine: Use in anesthesia. CNS Neurosci. Ther. 2013, 19, 381–389. [Google Scholar] [CrossRef]
- Trujillo, K.A.; Heller, C.Y. Ketamine sensitization: Influence of dose, environment, social isolation and treatment interval. Behav. Brain Res. 2020, 378, 112271. [Google Scholar] [CrossRef] [PubMed]
- Menk, E.J.; Baumgarten, R.K.; Kingsley, C.P.; Culling, R.D.; Middaugh, R. Success of reentry into anesthesiology training programs by residents with a history of substance abuse. JAMA 1990, 263, 3060–3062. [Google Scholar] [CrossRef] [PubMed]
- Warner, D.O.; Berge, K.; Sun, H.; Harman, A.; Wang, T. Substance Use Disorder in Physicians after Completion of Training in Anesthesiology in the United States from 1977 to 2013. Anesthesiology 2020, 133, 342–349. [Google Scholar] [CrossRef]
- Pan, W.-H.; Wu, K.C.-C.; Chen, C.-Y.; Chu, Y.-R.; Wu, S.-C.; Jou, S.; Lu, T.-P.; Tung, Y.-C.; Hsu, J.; Chen, W.J. First-time offenders for recreational ketamine use under a new penalty system in Taiwan: Incidence, recidivism and mortality in national cohorts from 2009 to 2017. Addiction 2021, 116, 1770–1781. [Google Scholar] [CrossRef]
- Bates, M.L.S.; Trujillo, K.A. Long-lasting effects of repeated ketamine administration in adult and adolescent rats. Behav. Brain Res. 2019, 369, 111928. [Google Scholar] [CrossRef]
- Tang, W.K.; Lau, C.G.; Ungvari, G.S.; Lin, S.-K.; Lane, H.-Y. Recovery of cognitive functioning following abstinence from ketamine. Addict. Behav. 2019, 99, 106081. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-M.; Chen, P.-Y.; Fang, C.-P.; Liu, T.-H.; Wu, C.-T.; Hsu, Y.-C.; Kuo, H.-W.; Liu, Y.-L.; Huang, M.-C. Increased Nectin-4 levels in chronic ketamine abusers and the relationship with lower urinary tract symptoms. Environ. Toxicol. Pharmacol. 2021, 87, 103714. [Google Scholar] [CrossRef]
- Liu, L.; Huang, H.; Li, Y.; Zhang, R.; Wei, Y.; Wu, W. Severe Encephalatrophy and Related Disorders From Long-Term Ketamine Abuse: A Case Report and Literature Review. Front. Psychiatry 2021, 12, 707326. [Google Scholar] [CrossRef]
- Sneyd, J.R.; Gambus, P.L.; Rigby-Jones, A.E. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: A narrative review. Br. J. Anaesth. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Saari, T.I.; Uusi-Oukari, M.; Ahonen, J.; Olkkola, K.T. Enhancement of GABAergic activity: Neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol. Rev. 2011, 63, 243–267. [Google Scholar] [CrossRef]
- Airagnes, G.; Pelissolo, A.; Lavallée, M.; Flament, M.; Limosin, F. Benzodiazepine Misuse in the Elderly: Risk Factors, Consequences, and Management. Curr. Psychiatry Rep. 2016, 18, 89. [Google Scholar] [CrossRef]
- Jobert, A.; Laforgue, E.-J.; Grall-Bronnec, M.; Rousselet, M.; Péré, M.; Jolliet, P.; Feuillet, F.; Victorri-Vigneau, C. Benzodiazepine withdrawal in older people: What is the prevalence, what are the signs, and which patients? Eur. J. Clin. Pharmacol. 2021, 77, 171–177. [Google Scholar] [CrossRef]
- Warner, D.O.; Berge, K.; Sun, H.; Harman, A.; Hanson, A.; Schroeder, D.R. Substance use disorder among anesthesiology residents, 1975–2009. JAMA 2013, 310, 2289–2296. [Google Scholar] [CrossRef]
- Luo, A.; Zhang, X.; Li, S.; Zhao, Y. Sevoflurane addiction due to workplace exposure: A case report and literature review. Medicine 2018, 97, e12454. [Google Scholar] [CrossRef]
- Radparvar, S. The Clinical Assessment and Treatment of Inhalant Abuse. Perm. J. 2023, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, M.; Prud’homme, T.; Allio, A.; Bronnec, M.; Bulteau, S.; Jolliet, P.; Victorri-Vigneau, C. Nitrous oxide: What do we know about its use disorder potential? Results of the French Monitoring Centre for Addiction network survey and literature review. Subst. Abus. 2019, 40, 33–42. [Google Scholar] [CrossRef]
- Forrester, M.B. Nitrous oxide misuse reported to two United States data systems during 2000–2019. J. Addict. Dis. 2021, 39, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.-H.; Huang, M.-C.; Chen, L.-Y. Psychiatric and Other Medical Manifestations of Nitrous Oxide Abuse: Implications From Case Series. J. Clin. Psychopharmacol. 2020, 40, 80–83. [Google Scholar] [CrossRef]
- Caré, W.; Dufayet, L.; Piot, M.A.; Crassard, I.; Manceau, P.; Niclot, P.; Batisse, A.; Vodovar, D. Acute and chronic toxicities associated with the use and misuse of nitrous oxide: An update. Rev. Med. Interne 2022, 43, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Farahmand, P.; Wolkin, A. Nitrous Oxide Inhalant Use Disorder Preceding Symptoms Concerning for Primary Psychotic Illness. Am. J. Addict. 2020, 29, 525–527. [Google Scholar] [CrossRef]
- Einsiedler, M.; Voulleminot, P.; Demuth, S.; Kalaaji, P.; Bogdan, T.; Gauer, L.; Reschwein, C.; Nadaj-Pakleza, A.; de Sèze, J.; Kremer, L.; et al. A rise in cases of nitrous oxide abuse: Neurological complications and biological findings. J. Neurol. 2022, 269, 577–582. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, L.; Ma, X.; Li, S.; Xue, Y.; Yan, P.; Chen, M.; Wu, J. Recreational Nitrous Oxide Abuse: Prevalence, Neurotoxicity, and Treatment. Neurotox. Res. 2021, 39, 975–985. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Nabben, T.; van den Brink, W. Recreational nitrous oxide use: Prevalence and risks. Regul. Toxicol. Pharmacol. 2015, 73, 790–796. [Google Scholar] [CrossRef]
- Garakani, A.; Jaffe, R.J.; Savla, D.; Welch, A.K.; Protin, C.A.; Bryson, E.O.; McDowell, D.M. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: A systematic review of the case literature. Am. J. Addict. 2016, 25, 358–369. [Google Scholar] [CrossRef]
- Buizert, A.; Sharma, R.; Koppen, H. When the Laughing Stops: Subacute Combined Spinal Cord Degeneration Caused by Laughing Gas Use. J. Addict. Med. 2017, 11, 235–236. [Google Scholar] [CrossRef]
- Vollenbrock, S.E.; Fokkema, T.M.; Leijdekkers, V.J.; Vahl, A.C.; Konings, R.; van Nieuwenhuizen, R.C. Nitrous Oxide Abuse Associated with Severe Thromboembolic Complications. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 656–657. [Google Scholar] [CrossRef]
- Liu, J.-F.; Li, J.-X. Drug addiction: A curable mental disorder? Acta Pharmacol. Sin. 2018, 39, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Kauer, J.A.; Malenka, R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007, 8, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Bocklisch, C.; Pascoli, V.; Wong, J.C.Y.; House, D.R.C.; Yvon, C.; de Roo, M.; Tan, K.R.; Lüscher, C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 2013, 341, 1521–1525. [Google Scholar] [CrossRef]
- Le Merrer, J.; Becker, J.A.J.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef]
- Solinas, M.; Yasar, S.; Goldberg, S.R. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol. Res. 2007, 56, 393–405. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Huang, B.; Jiang, C.; Chen, J.; Lin, H.; Lian, Q.; Wu, B. The Adenosine A2A Receptor Activation in Nucleus Accumbens Suppress Cue-Induced Reinstatement of Propofol Self-administration in Rats. Neurochem. Res. 2021, 46, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, G.; Xiang, S.; Jiang, C.; Chen, Z.; Li, Y.; Huang, B.; Zhou, W.; Lian, Q.; Wu, B. The Antagonism of Corticotropin-Releasing Factor Receptor-1 in Brain Suppress Stress-Induced Propofol Self-Administration in Rats. Front. Behav. Neurosci. 2021, 15, 775209. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Sun, A.; Xu, L.; Wang, S.; Lin, W.; Lai, M.; Zhu, H.; Zhou, W.; Lian, Q. Extracellular Signal-Regulated Kinase in Nucleus Accumbens Mediates Propofol Self-Administration in Rats. Neurosci. Bull. 2016, 32, 531–537. [Google Scholar]
- Shahzadi, A.; Uskur, T.; Akkan, A.G.; Çevreli, B.; Uzbay, T. Effects of propofol on conditioned place preference in male rats: Involvement of nitrergic system. Am. J. Drug Alcohol. Abuse 2018, 44, 167–174. [Google Scholar] [CrossRef]
- Chen, H.; Xu, D.; Zhang, Y.; Yan, Y.; Liu, J.; Liu, C.; Shen, W.; Yu, T.; Liu, J. Neurons in the Locus Coeruleus Modulate the Hedonic Effects of Sub-Anesthetic Dose of Propofol. Front. Neurosci. 2021, 15, 636901. [Google Scholar] [CrossRef]
- Nagata, I.; Sasaki, M.; Miyazaki, T.; Saeki, K.; Ogawa, K.-I.; Kamiya, Y. Subanesthetic Dose of Propofol Activates the Reward System in Rats. Anesth. Analg. 2022, 135, 414–426. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Zhou, W.; Zhu, H.; Lian, Q.; Yang, J. Involvement of the ERK signaling pathways in the NAc in propofol-seeking behavior induced by cues in rats. Pharmacol. Biochem. Behav. 2022, 219, 173447. [Google Scholar] [CrossRef]
- Xiong, M.; Li, J.; Ye, J.H.; Zhang, C. Upregulation of DeltaFosB by propofol in rat nucleus accumbens. Anesth. Analg. 2011, 113, 259–264. [Google Scholar] [CrossRef]
- Wu, B.; Liang, Y.; Dong, Z.; Chen, Z.; Zhang, G.; Lin, W.; Wang, S.; Wang, B.; Ge, R.-S.; Lian, Q. Glucocorticoid receptor mediated the propofol self-administration by dopamine D1 receptor in nucleus accumbens. Neuroscience 2016, 328, 184–193. [Google Scholar] [CrossRef]
- Wu, B.; Lin, W.; Wang, H.; Abdullah, T.; Wang, B.; Su, Y.; Ge, R.-S.; Lian, Q. Glucocorticoid receptor in rat nucleus accumbens: Its roles in propofol addictions. Neurosci. Lett. 2018, 662, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Uskur, T.; Şenöz, A.Ö.; Çevreli, B.; Barlas, A.; Uzbay, T. Propofol but not dexmedetomidine produce locomotor sensitization via nitric oxide in rats. Psychopharmacology 2021, 238, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, A.H.; Özçetin, A.; Özlü, O.; Çevreli, B.; Uzbay, T. Locomotor stimulation by acute propofol administration in rats: Role of the nitrergic system. Pharmacol. Rep. 2015, 67, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, K.; Liu, H.; Su, Y.; Wang, H.; Wang, S.; Bao, S.; Zhou, W.-H.; Lian, Q.-Q. Contribution of the α5 GABAA receptor to the discriminative stimulus effects of propofol in rat. Neuroreport 2018, 29, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-P.; Huang, X.-X.; Dong, D.-M.; Wu, H.; Zhu, T.-Q.; Wang, B.-F. The role of NMDA receptors in rat propofol self-administration. BMC Anesthesiol. 2020, 20, 149. [Google Scholar] [CrossRef]
- Lüscher, C. The Emergence of a Circuit Model for Addiction. Annu. Rev. Neurosci. 2016, 39, 257–276. [Google Scholar] [CrossRef]
- Baik, J.-H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef]
- Nutt, D.J.; Lingford-Hughes, A.; Erritzoe, D.; Stokes, P.R.A. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015, 16, 305–312. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xu, P.; Mao, C.; Wang, L.; Krumm, B.; Zhou, X.E.; Huang, S.; Liu, H.; Cheng, X.; Huang, X.-P.; et al. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell 2021, 184, 931–942.e18. [Google Scholar] [CrossRef]
- Hikida, T.; Kimura, K.; Wada, N.; Funabiki, K.; Nakanishi, S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 2010, 66, 896–907. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A.; Baler, R. The dopamine motive system: Implications for drug and food addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef]
- Shen, W.; Flajolet, M.; Greengard, P.; Surmeier, D.J. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008, 321, 848–851. [Google Scholar] [CrossRef] [PubMed]
- García-Pardo, M.P.; Roger-Sanchez, C.; Rodríguez-Arias, M.; Miñarro, J.; Aguilar, M.A. Pharmacological modulation of protein kinases as a new approach to treat addiction to cocaine and opiates. Eur. J. Pharmacol. 2016, 781, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Giese, K.P.; Mizuno, K. The roles of protein kinases in learning and memory. Learn. Mem. 2013, 20, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Pain, L.; Gobaille, S.; Schleef, C.; Aunis, D.; Oberling, P. In vivo dopamine measurements in the nucleus accumbens after nonanesthetic and anesthetic doses of propofol in rats. Anesth. Analg. 2002, 95, 915–919. [Google Scholar]
- Pavković, Ž.; Smiljanić, K.; Kanazir, S.; Milanović, D.; Pešić, V.; Ruždijić, S. Brain molecular changes and behavioral alterations induced by propofol anesthesia exposure in peripubertal rats. Paediatr. Anaesth. 2017, 27, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Antireward, compulsivity, and addiction: Seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology 2017, 234, 1315–1332. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.J.; Schwaber, J.S. Similarities in alcohol and opioid withdrawal syndromes suggest common negative reinforcement mechanisms involving the interoceptive antireward pathway. Neurosci. Biobehav. Rev. 2021, 125, 355–364. [Google Scholar] [CrossRef]
- Koob, G.F.; Buck, C.L.; Cohen, A.; Edwards, S.; Park, P.E.; Schlosburg, J.E.; Schmeichel, B.; Vendruscolo, L.F.; Wade, C.L.; Whitfield, T.W.; et al. Addiction as a stress surfeit disorder. Neuropharmacology 2014, 76 Pt B, 370–382. [Google Scholar] [CrossRef]
- Koob, G.F. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front. Psychiatry 2013, 4, 72. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Hundal, H.S. The endocannabinoid system: ‘NO’ longer anonymous in the control of nitrergic signalling? J. Mol. Cell Biol. 2017, 9, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Tayfun Uzbay, I.; Oglesby, M.W. Nitric oxide and substance dependence. Neurosci. Biobehav. Rev. 2001, 25, 43–52. [Google Scholar] [CrossRef]
- Caffino, L.; Piva, A.; Mottarlini, F.; Di Chio, M.; Giannotti, G.; Chiamulera, C.; Fumagalli, F. Ketamine Self-Administration Elevates αCaMKII Autophosphorylation in Mood and Reward-Related Brain Regions in Rats. Mol. Neurobiol. 2018, 55, 5453–5461. [Google Scholar] [CrossRef]
- Cui, J.; Ju, X.; Lee, Y.; Hong, B.; Kang, H.; Han, K.; Shin, W.-H.; Park, J.; Lee, M.J.; Kim, Y.H.; et al. Repeated ketamine anesthesia during neurodevelopment upregulates hippocampal activity and enhances drug reward in male mice. Commun. Biol. 2022, 5, 709. [Google Scholar] [CrossRef]
- Strong, C.E.; Schoepfer, K.J.; Dossat, A.M.; Saland, S.K.; Wright, K.N.; Kabbaj, M. Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology 2017, 121, 195–203. [Google Scholar] [CrossRef]
- Simmler, L.D.; Li, Y.; Hadjas, L.C.; Hiver, A.; van Zessen, R.; Lüscher, C. Dual action of ketamine confines addiction liability. Nature 2022, 608, 368–373. [Google Scholar] [CrossRef]
- Han, D.H.; Hong, I.; Choi, J.E.; Park, P.; Baek, J.-Y.; Park, H.; Ide, S.; Mishina, M.; Ikeda, K.; Kaang, B.-K. Abolished ketamine effects on the spontaneous excitatory postsynaptic current of medial prefrontal cortex neurons in GluN2D knockout mice. Mol. Brain 2021, 14, 174. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Chen, J.; Zhou, S.-W.; Mo, Z.-X. Individual and combined effects of rhynchophylline and ketamine on proliferation, NMDAR1 and GluA2/3 protein expression in PC12 cells. Fitoterapia 2013, 85, 125–129. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakayama, T.; Yamaguchi, J.; Matsuzawa, M.; Mishina, M.; Ikeda, K.; Yamamoto, H. Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci. Lett. 2016, 610, 48–53. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, Y.; Xie, L.; Huang, J.; Duan, S.; Guo, R.; Xie, Y.; Lv, J.; Lin, Z.; Ma, S. Behavioral Changes and Neuronal Damage in Rhesus Monkeys after 10 Weeks of Ketamine Administration Involve Prefrontal Cortex Dopamine D2 Receptor and Dopamine Transporter. Neuroscience 2019, 415, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Galvanho, J.P.; Manhães, A.C.; Carvalho-Nogueira, A.C.C.; Silva, J.d.M.; Filgueiras, C.C.; Abreu-Villaça, Y. Profiling of behavioral effects evoked by ketamine and the role of 5HT and D receptors in ketamine-induced locomotor sensitization in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 97, 109775. [Google Scholar] [CrossRef]
- Masuzawa, M.; Nakao, S.; Miyamoto, E.; Yamada, M.; Murao, K.; Nishi, K.; Shingu, K. Pentobarbital inhibits ketamine-induced dopamine release in the rat nucleus accumbens: A microdialysis study. Anesth. Analg. 2003, 96, 148–152. [Google Scholar] [CrossRef]
- Du, Y.; Du, L.; Cao, J.; Hölscher, C.; Feng, Y.; Su, H.; Wang, Y.; Yun, K.-M. Levo-tetrahydropalmatine inhibits the acquisition of ketamine-induced conditioned place preference by regulating the expression of ERK and CREB phosphorylation in rats. Behav. Brain Res. 2017, 317, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, C.; Tu, G.; Li, C.; Liu, Y.; Liu, W.; Lam Yung, K.K.; Mo, Z. Rhynchophylline Downregulates Phosphorylated cAMP Response Element Binding Protein, Nuclear Receptor-related-1, and Brain-derived Neurotrophic Factor Expression in the Hippocampus of Ketamine-induced Conditioned Place Preference Rats. Pharmacogn. Mag. 2018, 14, 81–86. [Google Scholar] [CrossRef]
- Liao, Y.; Tang, J.; Liu, J.; Xie, A.; Yang, M.; Johnson, M.; Wang, X.; Deng, Q.; Chen, H.; Xiang, X.; et al. Decreased Thalamocortical Connectivity in Chronic Ketamine Users. PLoS ONE 2016, 11, e0167381. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, D.; Xu, J.; Lam, W.; Yew, D.T. Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front. Neuroanat. 2013, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tang, J.; Qi, C.; Xie, A.; Liu, J.; O’Neill, J.; Liu, T.; Hao, W.; Liao, Y. Higher glutamatergic activity in the medial prefrontal cortex in chronic ketamine users. J. Psychiatry Neurosci. 2022, 47, E263–E271. [Google Scholar] [CrossRef]
- Liao, Y.; Johnson, M.; Qi, C.; Wu, Q.; Xie, A.; Liu, J.; Yang, M.; Huang, M.; Zhang, Y.; Liu, T.; et al. Cue-Induced Brain Activation in Chronic Ketamine-Dependent Subjects, Cigarette Smokers, and Healthy Controls: A Task Functional Magnetic Resonance Imaging Study. Front. Psychiatry 2018, 9, 88. [Google Scholar] [CrossRef]
- Liao, Y.; Tang, J.; Fornito, A.; Liu, T.; Chen, X.; Chen, H.; Xiang, X.; Wang, X.; Hao, W. Alterations in regional homogeneity of resting-state brain activity in ketamine addicts. Neurosci. Lett. 2012, 522, 36–40. [Google Scholar] [CrossRef]
- Hung, C.-C.; Zhang, S.; Chen, C.-M.; Duann, J.-R.; Lin, C.-P.; Lee, T.S.-H.; Li, C.-S.R. Striatal functional connectivity in chronic ketamine users: A pilot study. Am. J. Drug Alcohol. Abuse 2020, 46, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tu, G.; Luo, C.; Guo, Y.; Fang, M.; Zhu, C.; Li, H.; Ou, J.; Zhou, Y.; Liu, W.; et al. Effects of rhynchophylline on the hippocampal miRNA expression profile in ketamine-addicted rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, K.; Zheng, W.; Beveridge, T.J.R.; Yang, S.; Li, X.; Li, P.; Zhou, W.; Liu, Y. The effects of GSK-3β blockade on ketamine self-administration and relapse to drug-seeking behavior in rats. Drug Alcohol. Depend. 2015, 147, 257–265. [Google Scholar] [CrossRef]
- Giacometti, L.L.; Barker, J.M. Sex differences in the glutamate system: Implications for addiction. Neurosci. Biobehav. Rev. 2020, 113, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.D.; Knackstedt, L.A.; Rosenberg, P.A. Glutamate homeostasis and dopamine signaling: Implications for psychostimulant addiction behavior. Neurochem. Int. 2021, 144, 104896. [Google Scholar] [CrossRef]
- Márquez, J.; Campos-Sandoval, J.A.; Peñalver, A.; Matés, J.M.; Segura, J.A.; Blanco, E.; Alonso, F.J.; de Fonseca, F.R. Glutamate and Brain Glutaminases in Drug Addiction. Neurochem. Res. 2017, 42, 846–857. [Google Scholar] [CrossRef]
- Heinsbroek, J.A.; De Vries, T.J.; Peters, J. Glutamatergic Systems and Memory Mechanisms Underlying Opioid Addiction. Cold Spring Harb. Perspect. Med. 2021, 11, a039602. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Cassell, G.H. Genital Mycoplasma infections in neonates. J. Pediatr. 1988, 112, 167–168. [Google Scholar] [CrossRef]
- Chiamulera, C.; Piva, A.; Abraham, W.C. Glutamate receptors and metaplasticity in addiction. Curr. Opin. Pharmacol. 2021, 56, 39–45. [Google Scholar] [CrossRef]
- Gipson, C.D.; Rawls, S.; Scofield, M.D.; Siemsen, B.M.; Bondy, E.O.; Maher, E.E. Interactions of neuroimmune signaling and glutamate plasticity in addiction. J. Neuroinflamm. 2021, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Ogden, K.K.; Traynelis, S.F. New advances in NMDA receptor pharmacology. Trends Pharmacol. Sci. 2011, 32, 726–733. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Wu, B.; Zhou, W. Ketamine abuse potential and use disorder. Brain Res. Bull. 2016, 126, 68–73. [Google Scholar] [CrossRef]
- Fan, N.; An, L.; Zhang, M.; He, H.; Zhou, Y.; Ou, Y. GRIN2B Gene Polymorphism in Chronic Ketamine Users. Am. J. Addict. 2020, 29, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lipsky, R.H. Repeated ketamine administration alters N-methyl-D-aspartic acid receptor subunit gene expression: Implication of genetic vulnerability for ketamine abuse and ketamine psychosis in humans. Exp. Biol. Med. 2015, 240, 145–155. [Google Scholar] [CrossRef]
- Tan, S.; Lam, W.P.; Wai, M.S.M.; Yu, W.-H.A.; Yew, D.T. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS ONE 2012, 7, e43947. [Google Scholar] [CrossRef] [PubMed]
- Kokkinou, M.; Ashok, A.H.; Howes, O.D. The effects of ketamine on dopaminergic function: Meta-analysis and review of the implications for neuropsychiatric disorders. Mol. Psychiatry 2018, 23, 59–69. [Google Scholar] [CrossRef]
- Wang, C.; Inselman, A.; Liu, S.; Liu, F. Potential mechanisms for phencyclidine/ketamine-induced brain structural alterations and behavioral consequences. Neurotoxicology 2020, 76, 213–219. [Google Scholar] [CrossRef]
- Zhang, R.; Volkow, N.D. Brain default-mode network dysfunction in addiction. Neuroimage 2019, 200, 313–331. [Google Scholar] [CrossRef]
- Sutherland, M.T.; McHugh, M.J.; Pariyadath, V.; Stein, E.A. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 2012, 62, 2281–2295. [Google Scholar] [CrossRef]
- Ernst, M.; Torrisi, S.; Balderston, N.; Grillon, C.; Hale, E.A. fMRI functional connectivity applied to adolescent neurodevelopment. Annu. Rev. Clin. Psychol. 2015, 11, 361–377. [Google Scholar] [CrossRef]
- Pariyadath, V.; Gowin, J.L.; Stein, E.A. Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks. Prog. Brain Res. 2016, 224, 155–173. [Google Scholar] [CrossRef]
- Wilcox, C.E.; Calhoun, V.D.; Rachakonda, S.; Claus, E.D.; Littlewood, R.A.; Mickey, J.; Arenella, P.B.; Hutchison, K.E. Functional network connectivity predicts treatment outcome during treatment of nicotine use disorder. Psychiatry Res. Neuroimaging 2017, 265, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Demenescu, L.R.; Sweeney-Reed, C.M.; Krause, A.L.; Metzger, C.D.; Walter, M. Novelty seeking and reward dependence-related large-scale brain networks functional connectivity variation during salience expectancy. Hum. Brain Mapp. 2017, 38, 4064–4077. [Google Scholar] [CrossRef]
- Hänggi, J.; Lohrey, C.; Drobetz, R.; Baetschmann, H.; Forstmeier, S.; Maercker, A.; Jäncke, L. Strength of Structural and Functional Frontostriatal Connectivity Predicts Self-Control in the Healthy Elderly. Front. Aging Neurosci. 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Angelides, N.H.; Gupta, J.; Vickery, T.J. Associating resting-state connectivity with trait impulsivity. Soc. Cogn. Affect. Neurosci. 2017, 12, 1001–1008. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W.; Scofield, M.D. Astrocyte regulation of synaptic signaling in psychiatric disorders. Neuropsychopharmacology 2023, 48, 21–36. [Google Scholar] [CrossRef]
- Lüscher, C.; Ungless, M.A. The mechanistic classification of addictive drugs. PLoS Med. 2006, 3, e437. [Google Scholar] [CrossRef]
- Engin, E.; Benham, R.S.; Rudolph, U. An Emerging Circuit Pharmacology of GABA Receptors. Trends Pharmacol. Sci. 2018, 39, 710–732. [Google Scholar] [CrossRef]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef]
- Tan, K.R.; Rudolph, U.; Lüscher, C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011, 34, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Ernst, M. The Benzodiazepine Binding Sites of GABA Receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef]
- Wafford, K.A. GABAA receptor subtypes: Any clues to the mechanism of benzodiazepine dependence? Curr. Opin. Pharmacol. 2005, 5, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.R.; Brown, M.; Labouèbe, G.; Yvon, C.; Creton, C.; Fritschy, J.-M.; Rudolph, U.; Lüscher, C. Neural bases for addictive properties of benzodiazepines. Nature 2010, 463, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.D.; Teixeira, L.P.; van Linn, M.L.; Namjoshi, O.A.; Cook, J.M.; Rowlett, J.K. Role of gamma-aminobutyric acid type A (GABAA) receptor subtypes in acute benzodiazepine physical dependence-like effects: Evidence from squirrel monkeys responding under a schedule of food presentation. Psychopharmacology 2013, 227, 347–354. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Engin, E.; Tantillo, G.; Lau, H.M.; Muschamp, J.W.; Carlezon, W.A.; Rudolph, U. Differential roles of GABA(A) receptor subtypes in benzodiazepine-induced enhancement of brain-stimulation reward. Neuropsychopharmacology 2012, 37, 2531–2540. [Google Scholar] [CrossRef]
- Engin, E.; Bakhurin, K.I.; Smith, K.S.; Hines, R.M.; Reynolds, L.M.; Tang, W.; Sprengel, R.; Moss, S.J.; Rudolph, U. Neural basis of benzodiazepine reward: Requirement for α2 containing GABAA receptors in the nucleus accumbens. Neuropsychopharmacology 2014, 39, 1805–1815. [Google Scholar] [CrossRef]
- Shinday, N.M.; Sawyer, E.K.; Fischer, B.D.; Platt, D.M.; Licata, S.C.; Atack, J.R.; Dawson, G.R.; Reynolds, D.S.; Rowlett, J.K. Reinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology 2013, 38, 1006–1014. [Google Scholar] [CrossRef]
- Rowlett, J.K.; Platt, D.M.; Lelas, S.; Atack, J.R.; Dawson, G.R. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 915–920. [Google Scholar] [CrossRef]
- Ator, N.A.; Atack, J.R.; Hargreaves, R.J.; Burns, H.D.; Dawson, G.R. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J. Pharmacol. Exp. Ther. 2010, 332, 4–16. [Google Scholar] [CrossRef] [PubMed]
- van Rijnsoever, C.; Täuber, M.; Choulli, M.K.; Keist, R.; Rudolph, U.; Mohler, H.; Fritschy, J.M.; Crestani, F. Requirement of alpha5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J. Neurosci. 2004, 24, 6785–6790. [Google Scholar] [CrossRef] [PubMed]
- Foitzick, M.F.; Medina, N.B.; Iglesias García, L.C.; Gravielle, M.C. Benzodiazepine exposure induces transcriptional down-regulation of GABA receptor α1 subunit gene via L-type voltage-gated calcium channel activation in rat cerebrocortical neurons. Neurosci. Lett. 2020, 721, 134801. [Google Scholar] [CrossRef] [PubMed]
- Eshel, N.; Bukwich, M.; Rao, V.; Hemmelder, V.; Tian, J.; Uchida, N. Arithmetic and local circuitry underlying dopamine prediction errors. Nature 2015, 525, 243–246. [Google Scholar] [CrossRef]
- Lalive, A.L.; Rudolph, U.; Lüscher, C.; Tan, K.R. Is there a way to curb benzodiazepine addiction? Swiss Med. Wkly. 2011, 141, w13277. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.S.; Watson, C.H.; Leroux, B.G.; Prall, C.W.; Kaiyala, K.J. Conditioned place aversion and self-administration of nitrous oxide in rats. Pharmacol. Biochem. Behav. 2003, 74, 623–633. [Google Scholar] [CrossRef]
- Tracy, M.E.; Slavova-Hernandez, G.G.; Shelton, K.L. Assessment of reinforcement enhancing effects of toluene vapor and nitrous oxide in intracranial self-stimulation. Psychopharmacology 2014, 231, 1339–1350. [Google Scholar] [CrossRef]
- Lambertenghi-Deliliers, G.; Orazi, A.; Luksch, R.; Annaloro, C.; Soligo, D. Myelodysplastic syndrome with increased marrow fibrosis: A distinct clinico-pathological entity. Br. J. Haematol. 1991, 78, 161–166. [Google Scholar] [CrossRef]
- Yang, T.; Yue, G.; Ge, Y.; Zhang, Y.; Xu, P.; Wang, Y.; Li, B.; Di, B. SCH 23390 inhibits the acquisition of nitrous oxide-induced conditioned place preference and the changes in ERK phosphorylation expression in nucleus accumbens of mice. Neurosci. Lett. 2022, 781, 136674. [Google Scholar] [CrossRef]
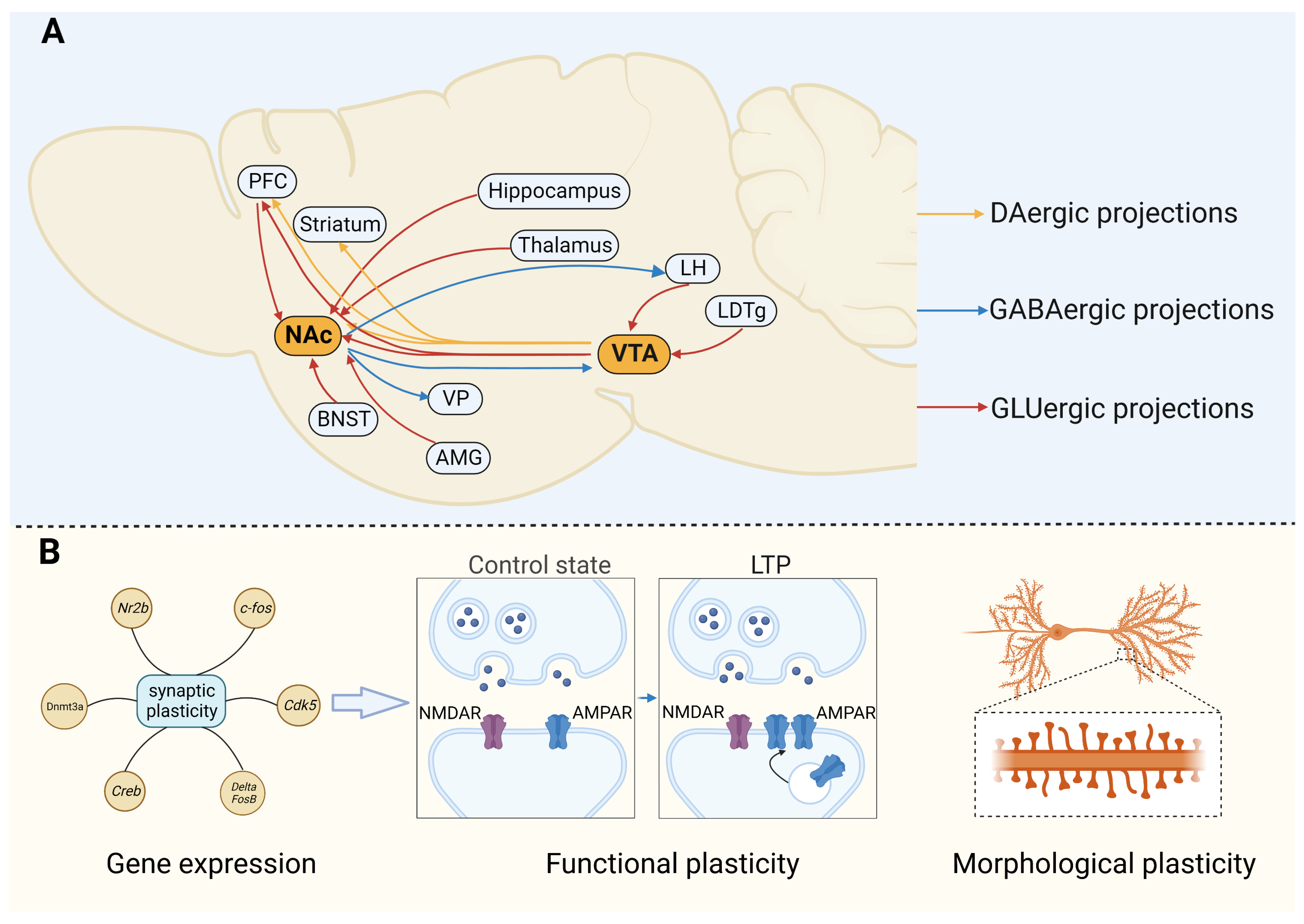
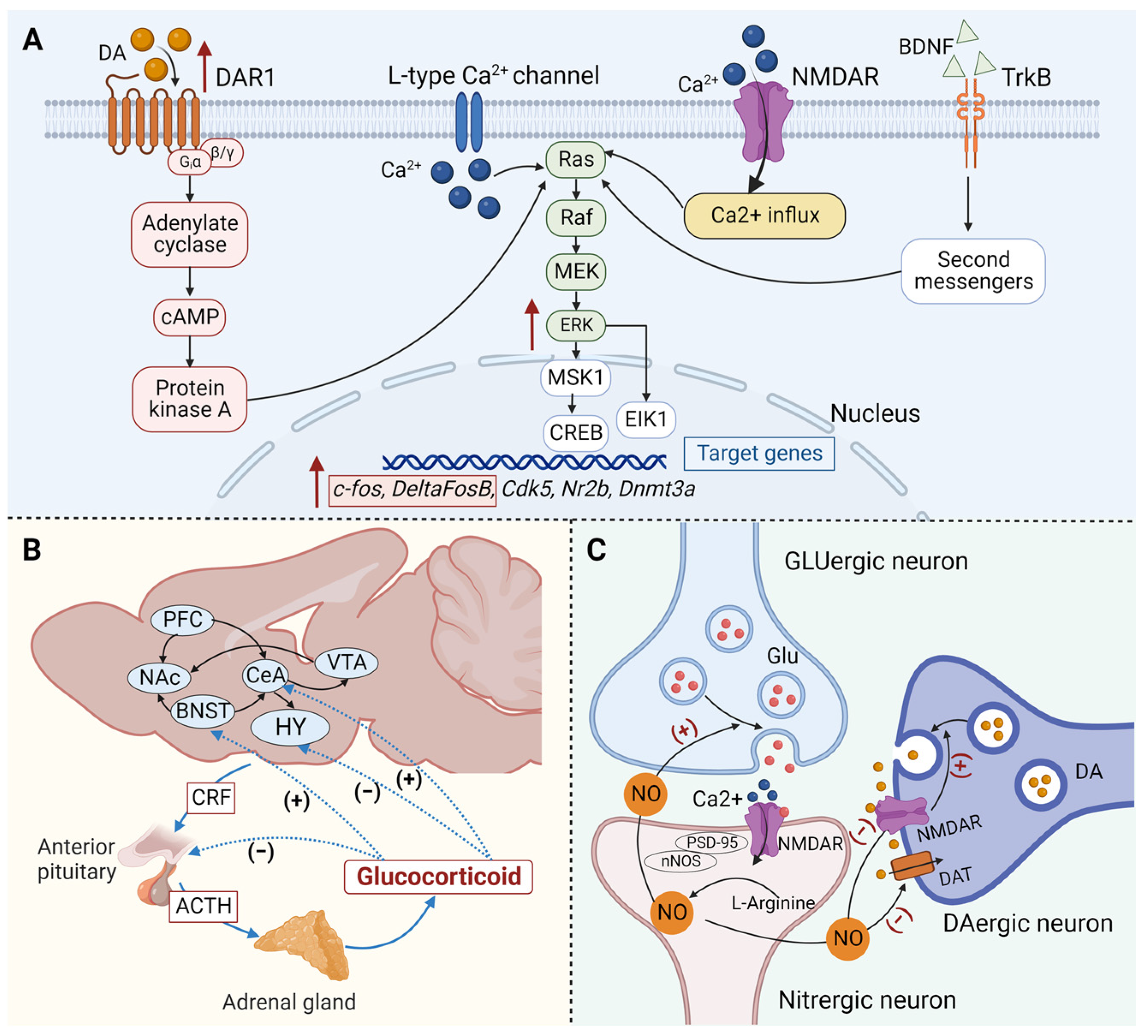
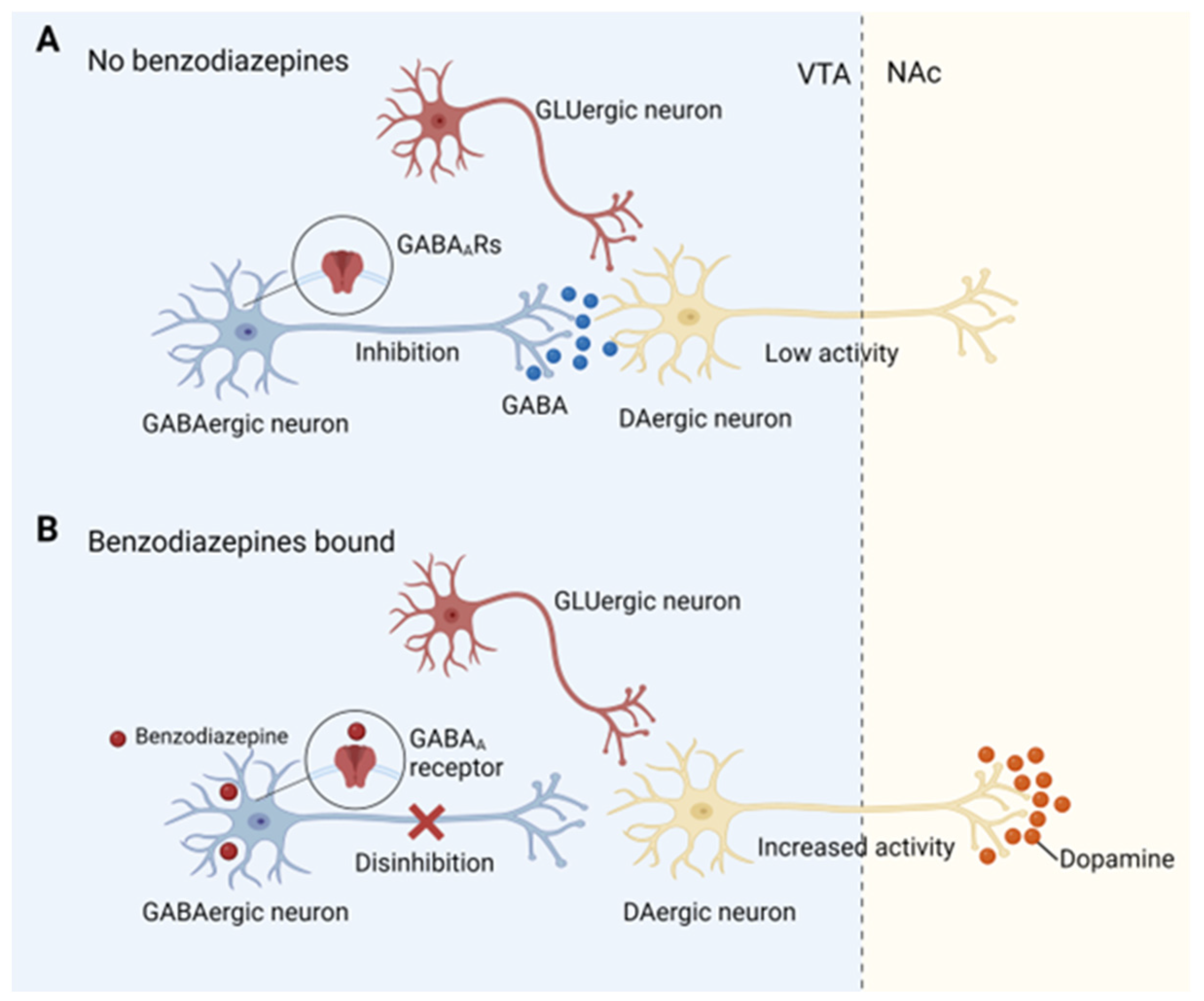
| Target | Brain Region | Paradigm | Manipulation | Results | Species | Ref. |
|---|---|---|---|---|---|---|
| ERK | NAc | SA | MEK inhibitor | Impaired propofol-maintained SA | Rat | [54] |
| ERK | Nac | SA | MEK inhibitor | Decreased SA | Rat | [58] |
| DeltaFosB | NAc | / | WB, PCR | Increased DeltaFosB expression | Rat | [59] |
| CRF1R | Systemic | SA | CRF1R antagonist | Inhibited acquisition | Rat | [53] |
| GR | Systemic | SA | GR agonist or antagonist | Inhibited reward-enhancing effect | Rat | [60] |
| GR | NAc | SA | GR agonist | Increased SA | Rat | [61] |
| CNS | Systemic | LA | NO inhibitor | Inhibited LA | Rat | [62] |
| NO | Systemic | CPP | NOS inhibitor | Abolished CPP | Rat | [55] |
| NO | Systemic | LA | NOS inhibitor | Inhibited LA | Rat | [63] |
| GABAAR | Systemic | Nose-poke discrimination | GABAARs agonist | discriminative stimulus effects | Rat | [64] |
| NMDAR | Systemic | SA | NDMAR antagonist | Inhibited acquisition | Rat | [65] |
| A2AR | NAc | SA | A2AR agonist or antagonist | Agonist inhibited and antagonist promoted SA | Rat | [52] |
| NA system | LC | CPP | Chemogenetic inhibition | Abolished CPP | Rat | [56] |
| Target | Brain Region | Paradigm | Manipulation | Results | Species | Ref. |
|---|---|---|---|---|---|---|
| NMDA | VTA | SA, CPP | NR1 knockout | Confined addiction liability | Mouse | [88] |
| GluN2D | Systemic | / | GluN2D knockout | No changes in sEPSC frequency | Mouse | [89] |
| GluA2/3 | Systemic | / | Rhy | Suppressed glua2/3 and glun1 expression | Rat | [90] |
| GluN2D | Systemic | LA | GluN2D knockout | Inhibited locomotor sensitization | Mouse | [91] |
| DAR2, DAT | PFC | / | / | Decreased in DAR2 and DAT expression | Monkey | [92] |
| 5HT2, DAR2 | Systemic | LA | 5HT2, DAR2 antagonist | Inhibited locomotor sensitization | Mouse | [93] |
| DA | NAc | / | Pentobarbital | Inhibited ketamine-induced dopamine | Rat | [94] |
| ERK, CREB | Systemic | CPP | l-THP | Abolished CPP | Rat | [95] |
| CREB, Nurr1, BDNF | Systemic | CPP | Rhy | Abolished CPP | Rat | [96] |
| TH | TH | / | rsfMRI | Decreased TH Connectivity | Human | [97] |
| Cortex | Cortex | / | MRI | Cortical atrophy | Human | [98] |
| GLUergic system | mFPC | / | MRI | Increased GLUergic activity | Human | [99] |
| ACC, PC | ACC, PC | / | fMRI | Increased activation in the ACC and PC | Human | [100] |
| PFC | PFC | / | fMRI | Alterations in the FC of PFC | Human | [101] |
| Striatal | Striatal | / | fMRI | Altered striatal connectivity | Human | [102] |
| miR-331-5p | HIP | CPP | Rhy | Abolished CPP | Rat | [103] |
| αCaMKII | Reward-Related | SA | / | αCaMKII autophosphorylation | Rat | [85] |
| Spine | NAc | CPP | / | Increased in spine density | Rat | [87] |
| GSK-3β | CPU, NAc, and VTA | SA | GSK-3β inhibitor | Inhibited reward-enhancing effect | Rat | [104] |
| Subtypes of GABAARs | Brain Region | Paradigm | Manipulation | Results | Species | Ref. |
|---|---|---|---|---|---|---|
| α1 | VTA | SA | Electrophysiology | Disinhibition | Mouse | [136] |
| α1 | Systemic | OCB | GABAARs agonist | Decreased response rates | Monkey | [137] |
| α2 and α3 | NAc | ICSS | α2 and α3-point-mutant mice | Abolished reward-enhancing effect | Mouse | [138] |
| α2 | NAc | ICSS, TBCD | α2-point-mutant mice | Inhibited reward-enhancing effect | Mouse | [139] |
| α3 | Systemic | SA | GABAARs agonist | Reinforced reward-enhancing effect | Monkey | [140] |
| α2, α3, and α5 | Systemic | SA | GABAARs agonist | Reinforced reward-enhancing effect | Monkey | [141] |
| α2 and α3 | Systemic | SA | GABAARs agonist | Reinforced reward-enhancing effect | Baboons | [142] |
| α5 | HIP | LA | α5-point-mutant mice | Reinforced tolerance | Mouse | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Wu, L.; Gao, R.; Xu, X.; Chen, C.; Liu, J. Non-Opioid Anesthetics Addiction: A Review of Current Situation and Mechanism. Brain Sci. 2023, 13, 1259. https://doi.org/10.3390/brainsci13091259
Deng L, Wu L, Gao R, Xu X, Chen C, Liu J. Non-Opioid Anesthetics Addiction: A Review of Current Situation and Mechanism. Brain Sciences. 2023; 13(9):1259. https://doi.org/10.3390/brainsci13091259
Chicago/Turabian StyleDeng, Liyun, Lining Wu, Rui Gao, Xiaolin Xu, Chan Chen, and Jin Liu. 2023. "Non-Opioid Anesthetics Addiction: A Review of Current Situation and Mechanism" Brain Sciences 13, no. 9: 1259. https://doi.org/10.3390/brainsci13091259
APA StyleDeng, L., Wu, L., Gao, R., Xu, X., Chen, C., & Liu, J. (2023). Non-Opioid Anesthetics Addiction: A Review of Current Situation and Mechanism. Brain Sciences, 13(9), 1259. https://doi.org/10.3390/brainsci13091259






