The Use of Quantitative Electroencephalography (QEEG) to Assess Post-COVID-19 Concentration Disorders in Professional Pilots: An Initial Concept
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. QEEG Analysis
2.3. Statistical Analysis
3. Results
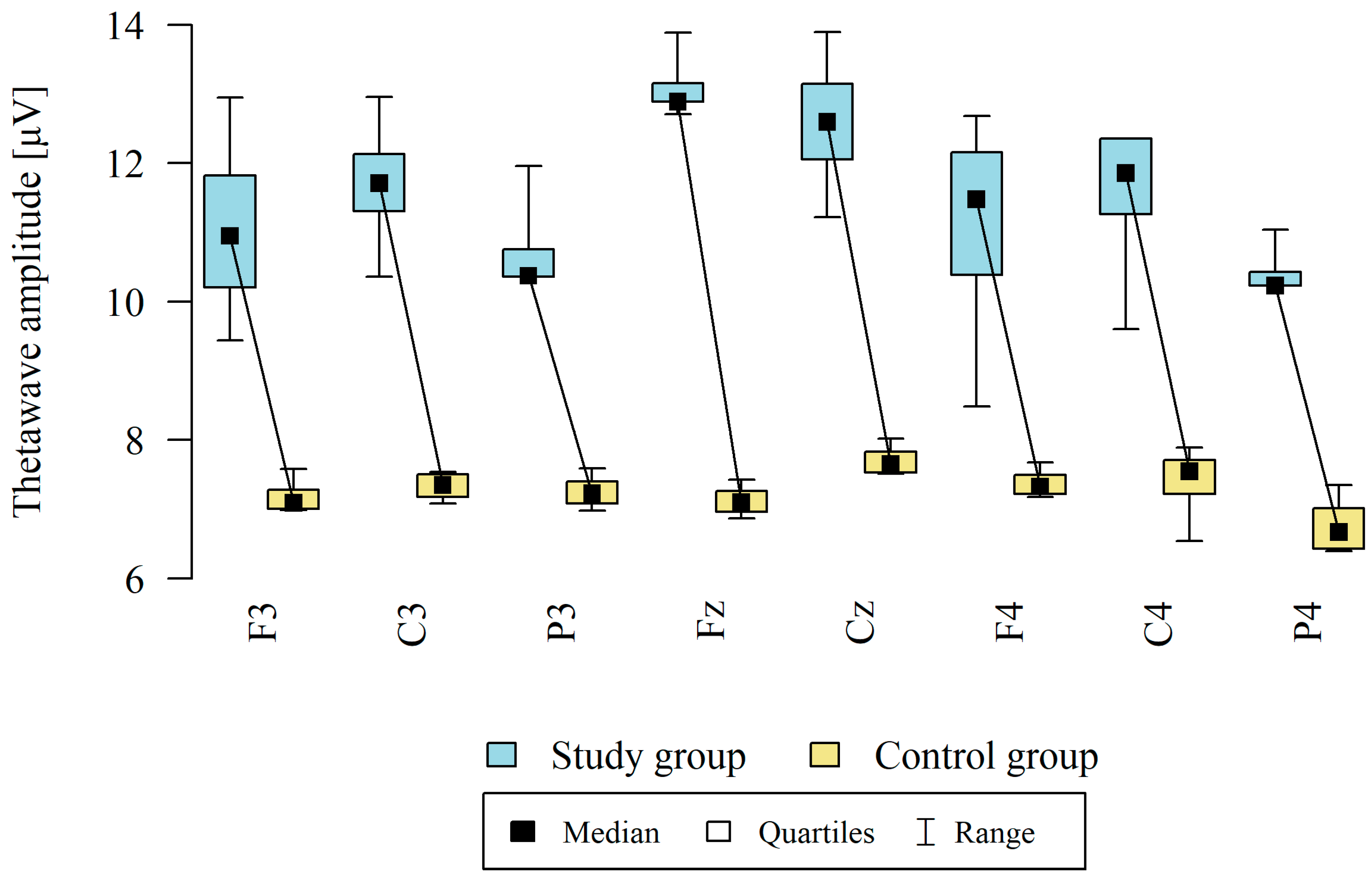
3.1. Theta Waves
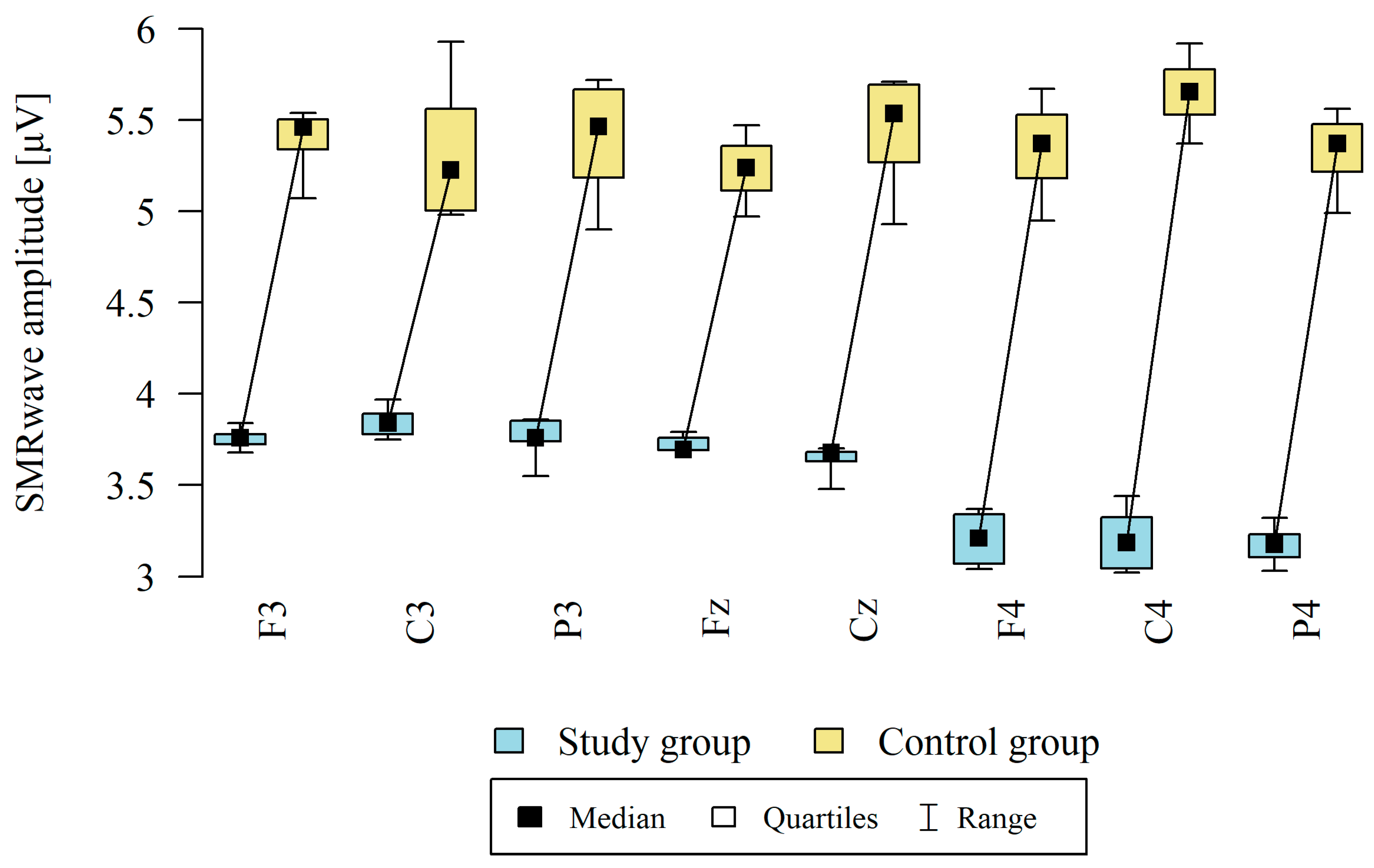
3.2. Sensorimotor Waves (SMR)
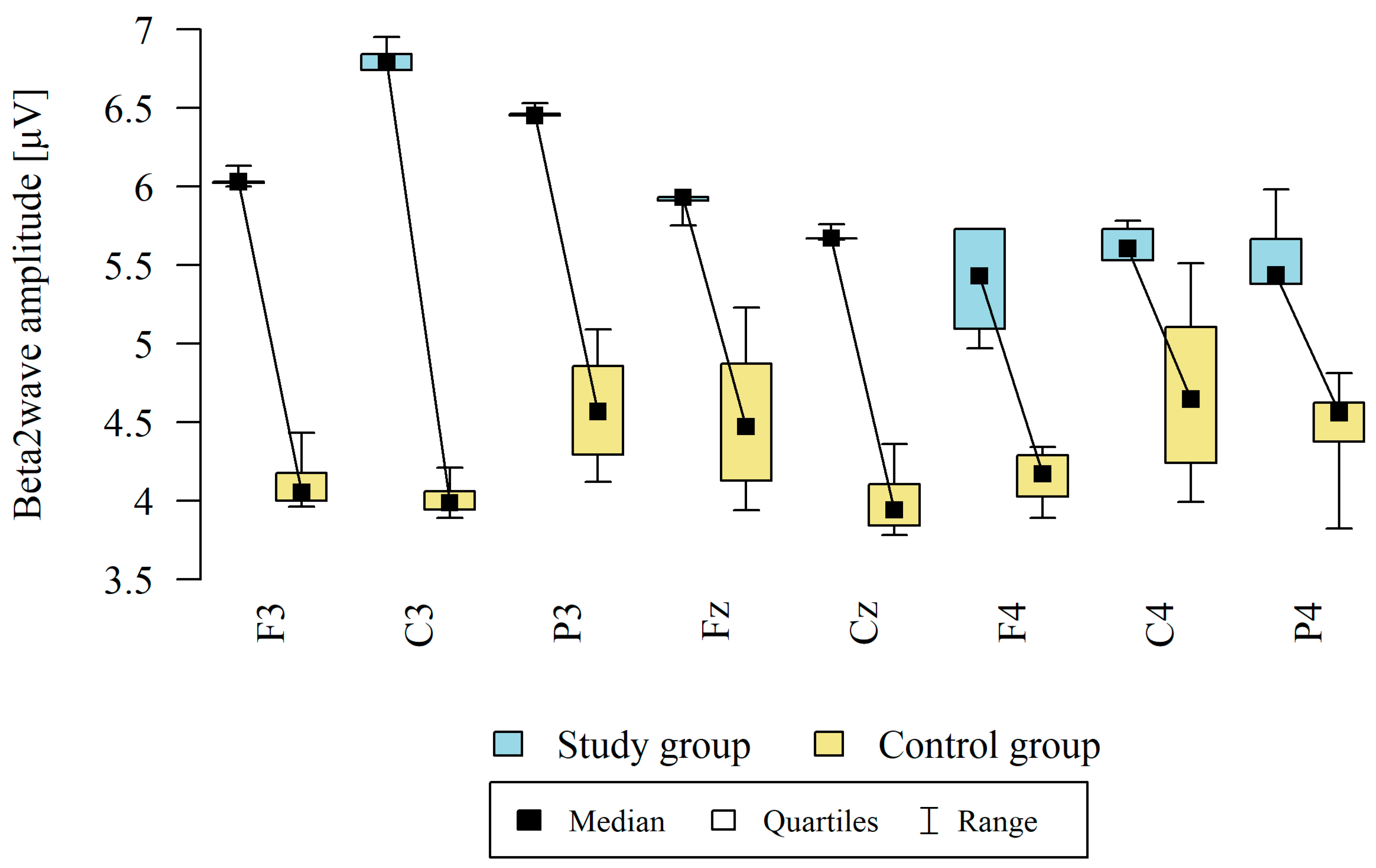
3.3. Beta2 Waves
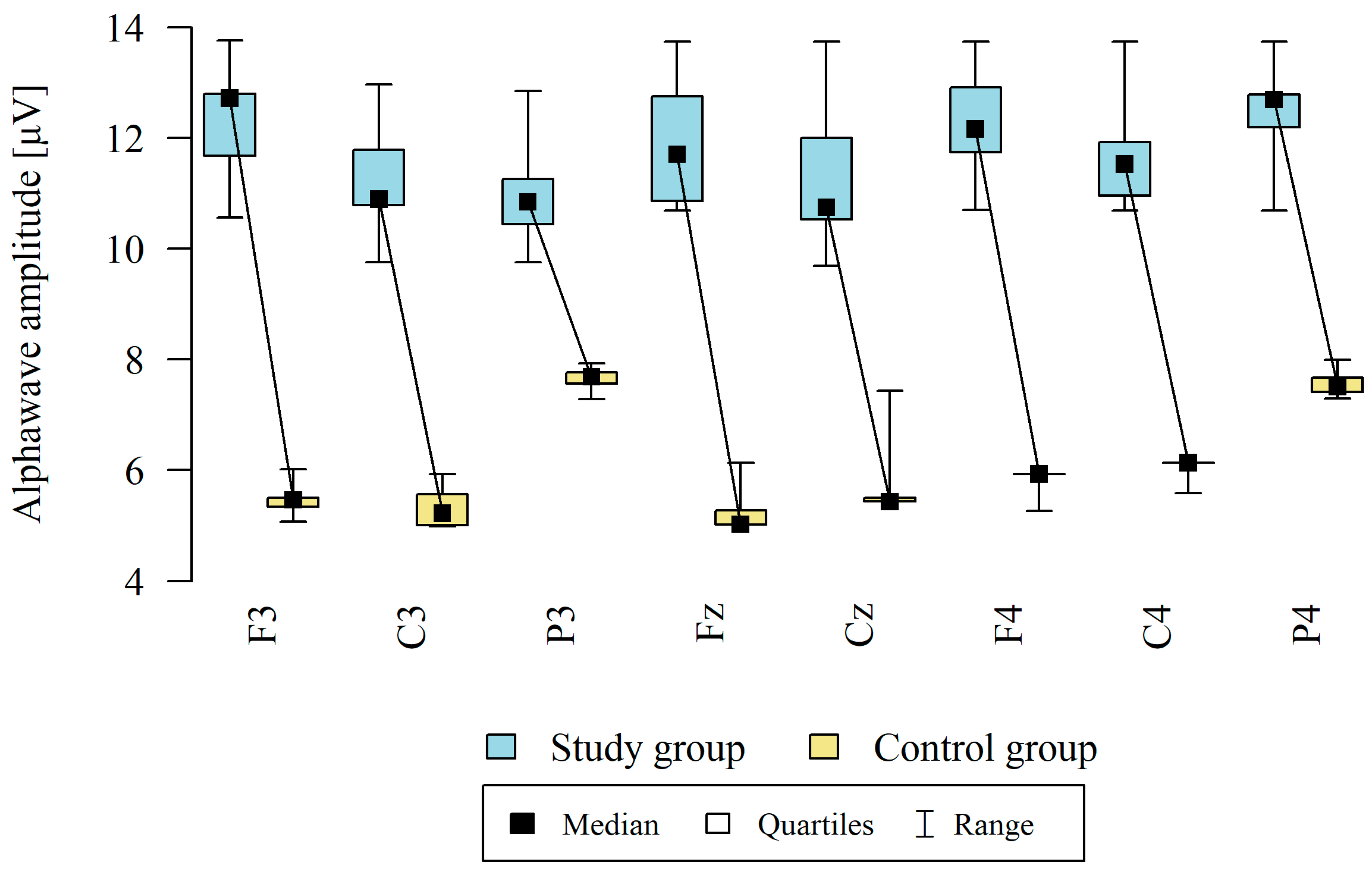
3.4. Alpha Waves
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological Complications of Coronavirus Disease COVID-19: Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Okeagu, C.N.; Pham, A.D.; Silva, R.A.; Hurley, J.J.; Arron, B.L.; Sarfraz, N.; Lee, H.N.; Ghali, G.E.; Gamble, J.W.; et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract. Res. Clin. Anaesthesiol. 2021, 35, 293–306. [Google Scholar] [CrossRef]
- Ahmad, T.; Haroon, H.; Baig, M.; Hui, J. Coronavirus Disease 2019 (COVID-19) Pandemic and Economic Impact. Pak. J. Med. Sci. 2020, 36, S73. [Google Scholar] [CrossRef]
- Xie, J.; Ding, C.; Li, J.; Wang, Y.; Guo, H.; Lu, Z.; Wang, J.; Zheng, C.; Jin, T.; Gao, Y.; et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J. Med. Virol. 2020, 92, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-COV-2. Turk. J. Med. Sci. 2020, 50, 549–556. [Google Scholar] [CrossRef]
- Camargo-Martínez, W.; Lozada-Martínez, I.; Escobar-Collazos, A.; Navarro-Coronado, A.; Moscote-Salazar, L.; Pacheco-Hernández, A.; Janjua, T.; Bosque-Varela, P. Post-COVID 19 neurological syndrome: Implications for sequelae’s treatment. J. Clin. Neurosci. 2021, 88, 219–225. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Nienhaus, A.; Stranzinger, J.; Kozak, A. COVID-19 as an Occupational Disease—Temporal Trends in the Number and Severity of Claims in Germany. Int. J. Environ. Res. Public Health 2023, 20, 1182. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Neurological complications of coronavirus and COVID-19. Rev. Neurol. 2020, 70, 311–322. [Google Scholar] [PubMed]
- Wiśniewska, M.; Gmitrowicz, A.; Pawełczyk, N. Zastosowanie QEEG w psychiatrii z uwzględnieniem populacji rozwojowej. Psychiatr. Psychol. Klin. 2016, 16, 188–193. [Google Scholar] [CrossRef]
- Kopańska, M.; Dejnowicz-Velitchkov, A.; Bartman, P.; Szczygielski, J. MiniQEEG and Neurofeedback in Diagnosis and Treatment of COVID-19-Related Panic Attacks: A Case Report. Brain Sci. 2022, 12, 1541. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, P. Atlas EEG i QEEG. Podręcznik ilościowej elektroencefalografii i jej zastosowanie w planowaniu neurofeedbacku. Wydaw. Biomed Neurotechnol. 2017, 8393427037, 9788393427031. [Google Scholar]
- Grassmann, M.; Vlemincx, E.; von Leupoldt, A.; Van den Bergh, O. Individual differences in cardiorespiratory measures of mental workload: An investigation of negative affectivity and cognitive avoidant coping in pilot candidates. Appl. Ergon. 2017, 59, 274–282. [Google Scholar] [CrossRef]
- Kopańska, M.; Banaś-Ząbczyk, A.; Łagowska, A.; Kuduk, B.; Szczygielski, J. Changes in EEG Recordings in COVID-19 Patients as a Basis for More Accurate QEEG Diagnostics and EEG Neurofeedback Therapy: A Systematic Review. J. Clin. Med. 2021, 10, 1300. [Google Scholar] [CrossRef]
- Xiaoru, W.; Damin, Z.; Shasha, L. Effects of perceptual load related to flight task on auditory ERPs. Acta Sci. Nat. Univ. Pekin. 2011, 47, 995–1002. [Google Scholar]
- Ahmad, S.J.; Feigen, C.M.; Vazquez, J.P.; Kobets, A.J.; Altschul, D.J. Neurological Sequelae of COVID-19. J. Integr. Neurosci. 2022, 21, 77. [Google Scholar] [CrossRef]
- Rydzika, Ł.; Pałka, T.; Sobiło-Rydzik, E.; Totę, Ł.; Ambroży, D.; Ambrożego, T.; Ruzbarski, P.; Czarnego, W.; Kopańska, M. An Attempt to Develop a Model of Brain Waves Using Quantitative Electroencephalography with Closed Eyes in K1 Kickboxing Athletes—Initial Concept. Sensors 2023, 23, 4136. [Google Scholar] [CrossRef]
- Matławska, I. Ginkgo biloba w łagodzeniu objawów neurologicznych po przebyciu COVID-19. Postępy Fitoter. 2022, 23–32. [Google Scholar] [CrossRef]
- Najt, P.; Richards, H.L.; Fortune, D.G. Brain imaging in patients with COVID-19: A systematic review. Brain Behav. Immun.-Health 2021, 16, 100290. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 28 July 2023).
- Wu, Z.; Yang, D. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur. J. Med. Res. 2020, 25, 54. [Google Scholar] [CrossRef] [PubMed]
- Prauzner, T.; Prauzner, K.; Prauzner, M. Aktywność pracy mózgu w procesie dydaktycznym w ujęciu badań elektroencefalograficznych. Eduk.-Tech.-Inform. 2019, 10, 312–317. [Google Scholar] [CrossRef]
- Barr, E.; Peper, E.; Swatzyna, R. Slouched Posture, Sleep Deprivation, and Mood Disorders: Interconnection and Modulation by Theta Brain Waves. NeuroRegulation 2019, 6, 181–189. [Google Scholar] [CrossRef]
- Bland, B.H.; Oddie, S.D. Theta band oscillation and synchrony in the hippocampal formation and associated structures: The case for its role in sensorimotor integration. Behav. Brain Res. 2001, 127, 119–136. [Google Scholar] [CrossRef]
- Campos da Paz, V.K.; Garcia, A.; Campos da Paz Neto, A.; Tomaz, C. SMR Neurofeedback Training Facilitates Working Memory Performance in Healthy Older Adults: A Behavioral and EEG Study. Front. Behav. Neurosci. 2018, 12, 321. [Google Scholar] [CrossRef]
- Nath, A. Long-Haul COVID. Neurology 2020, 95, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Ochojska, D.; Dejnowicz-Velitchkov, A.; Banaś-Ząbczyk, A. Quantitative Electroencephalography (QEEG) as an Innovative Diagnostic Tool in Mental Disorders. Int. J. Environ. Res. Public Health 2022, 19, 2465. [Google Scholar] [CrossRef]
- Öksüz, Ö.; Günver, M.G.; Arıkan, M.K. Ilościowe wyniki elektroencefalografii u pacjentów z cukrzycą. Klin. EEG Neuronauka 2022, 53, 248–255. [Google Scholar] [CrossRef]
- Massey, S.L.; Topjian, A.A. PICU Bedside Quantitative Electroencephalography: Ready for “Real-Time”*. Pediatr. Crit. Care Med. 2020, 21, 592–593. [Google Scholar] [CrossRef]
- Yeung, A.; Garudadri, H.; Van Toen, C.; Mercier, P.; Balkan, O.; Makeig, S.; Virji-Babul, N. Comparison of foam-based and spring-loaded dry EEG electrodes with wet electrodes in resting and moving conditions. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 7131–7134. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Temko, A.; Bocchino, A.; O’Mahony, C.; Boylan, G.; Popovici, E. Analysis of a Low-Cost EEG Monitoring System and Dry Electrodes toward Clinical Use in the Neonatal ICU. Sensors 2019, 19, 2637. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Thompson, L. Neurofeedback—Wprowadzenie do Podstawowych Koncepcji Psychofizjologii Stosowanej. 2013. Available online: https://biomed.org.pl/publikacje/neurofeedback/ (accessed on 28 July 2023).
- Han, S.H.; Chul Youn, Y. Quantitative electroencephalography changes in patients with mild cognitive impairment after choline alphoscerate administration. J. Clin. Neurosci. 2022, 102, 42–48. [Google Scholar] [CrossRef]
- Saretzki, C.; Bergmann, O.; Dahmann, P.; Janser, F.; Keimer, J.; Machado, P.; Morrison, A.; Page, H.; Pluta, E.; Stübing, F.; et al. Are small airplanes safe with regards to COVID-19 transmission? J. Travel. Med. 2021, 28, taab105. [Google Scholar] [CrossRef]
- Mohanavelu, K.; Poonguzhali, S.; Adalarasu, K.; Ravi, D.; Vijayakumar, C.; Vinutha, S.; Ramachandran, K.; Srinivasan, J. Dynamic cognitive workload assessment for fighter pilots in simulated fighter aircraft environment using EEG. Biomed. Signal Process. Control 2020, 61, 102018. [Google Scholar] [CrossRef]
- Mohanavelu, K.; Poonguzhali, S.; Janani, A.; Vinutha, S. Machine learning-based approach for identifying mental workload of pilots. Biomed. Signal Process. Control 2022, 75, 103623. [Google Scholar] [CrossRef]
- Wenbin, L.; Shan, C.; Hang, W.; Yaoming, C. EEG microstate changes according to mental fatigue induced by aircraft piloting simulation: An exploratory study. Behav. Brain Res. 2023, 438, 114203. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, W.; Ren, Z.; Zhu, H. EEG-based analysis for pilots’ at-risk cognitive competency identification using RF-CNN algorithm. Front. Neurosci. 2023, 17, 1172103. [Google Scholar] [CrossRef]
- Klaproth, O.W.; Vernaleken, C.; Krol, L.R.; Halbruegge, M.; Zander, T.O.; Russwinkel, N. Tracing Pilots’ Situation Assessment by Neuroadaptive Cognitive Modeling. Front. Neurosci. 2020, 14, 795. [Google Scholar] [CrossRef]
- Friedman, N.; Fekete, T.; Gal, K.; Shriki, O. EEG-Based Prediction of Cognitive Load in Intelligence Tests. Front. Hum. Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef]
- Sestito, M.; Harel, A.; Nador, J.; Flach, J. Investigating Neural Sensorimotor Mechanisms Underlying Flight Expertise in Pilots: Preliminary Data From an EEG Study. Front. Hum. Neurosci. 2018, 12, 489. [Google Scholar] [CrossRef]
- Binias, B.; Myszor, D.; Binias, S.; Cyran, K.A. Analysis of Relation between Brainwave Activity and Reaction Time of Short-Haul Pilots Based on EEG Data. Sensors 2023, 23, 6470. [Google Scholar] [CrossRef]
- Klaproth, O.W.; Halbrügge, M.; Krol, L.R.; Vernaleken, C.; Zander, T.O.; Russwinkel, N. A Neuroadaptive Cognitive Model for Dealing With Uncertainty in Tracing Pilots’ Cognitive State. Top. Cogn. Sci. 2020, 12, 1012–1029. [Google Scholar] [CrossRef]
- Hebbar, P.A.; Bhattacharya, K.; Prabhakar, G.; Pashilkar, A.A.; Biswas, P. Correlation Between Physiological and Performance-Based Metrics to Estimate Pilots’ Cognitive Workload. Front. Psychol. 2021, 12, 555446. [Google Scholar] [CrossRef]
- Taheri Gorji, H.; Wilson, N.; VanBree, J.; Hoffmann, B.; Petros, T.; Tavakolian, K. Using machine learning methods and EEG to discriminate aircraft pilot cognitive workload during flight. Sci. Rep. 2023, 13, 2507. [Google Scholar] [CrossRef]
- Dehais, F.; Duprès, A.; Blum, S.; Drougard, N.; Scannella, S.; Roy, R.N.; Lotte, F. Monitoring Pilot’s Mental Workload Using ERPs and Spectral Power with a Six-Dry-Electrode EEG System in Real Flight Conditions. Sensors 2019, 19, 1324. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Yu, B.W.; Lee, D.H.; Lee, S.W. Classification of Drowsiness Levels Based on a Deep Spatio-Temporal Convolutional Bidirectional LSTM Network Using Electroencephalography Signals. Brain Sci. 2019, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Giraudet, L.; St-Louis, M.E.; Scannella, S.; Causse, M. P300 event-related potential as an indicator of inattentional deafness? PLoS ONE 2015, 10, e0118556. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, C.; Sun, L.; Liu, K.; Liu, H.; Zhu, W.; Jiang, C. Detection of Pilot’s Mental Workload Using a Wireless EEG Headset in Airfield Traffic Pattern Tasks. Entropy 2023, 25, 1035. [Google Scholar] [CrossRef]
- Hamann, A.; Carstengerdes, N. Investigating mental workload-induced changes in cortical oxygenation and frontal theta activity during simulated flights. Sci. Rep. 2022, 12, 6449. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Ochojska, D.; Muchacka, R.; Dejnowicz-Velitchkov, A.; Banaś-Ząbczyk, A.; Szczygielski, J. Comparison of QEEG Findings before and after Onset of Post-COVID-19 Brain Fog Symptoms. Sensors 2022, 22 Pt b, 6606. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Kuduk, B.; Łagowska, A.; Mytych, W.; Muchacka, R.; Banaś-Za̧bczyk, A. Quantitative electroencephalography interpretation of human brain activity after COVID-19 before and after Sudarshan Kriya Yoga. Front. Hum. Neurosci. 2022, 16 Pt a, 988021. [Google Scholar] [CrossRef] [PubMed]
- Kopańska, M.; Ochojska, D.; Mytych, W.; Lis, M.W.; Banaś-Ząbczyk, A. Development of a brain wave model based on the quantitative analysis of EEG and EEG biofeedback therapy in patients with panic attacks during the COVID-19 pandemic. Sci. Rep. 2022, 12 Pt c, 14908. [Google Scholar] [CrossRef]
- Rydzik, Ł.; Ambroży, T.; Pałka, T.; Wąsacz, W.; Spieszny, M.; Perliński, J.; Król, P.; Kopańska, M. Preliminary Development of a Brainwave Model for K1 Kickboxers Using Quantitative Electroencephalography (QEEG) with Open Eyes. Int. J. Mol. Sci. 2023, 24, 8882. [Google Scholar] [CrossRef]
- Kober, S.E.; Witte, M.; Ninaus, M.; Neuper, C.; Wood, G. Learning to modulate one’s own brain activity: The effect of spontaneous mental strategies. Front. Hum. Neurosci. 2013, 7, 695. [Google Scholar] [CrossRef]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psychophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Long COVID syndrome-associated brain fog. J. Med. Virol. 2022, 94, 979–984. [Google Scholar] [CrossRef]
- Kamiński, J.; Brzezicka, A.; Wróbel, A. Aktywność EEG w paśmie alfa podczas wykonywania zadań angażujących uwagę wzrokową. Przegląd Psychol. 2008, 51, 135–148. [Google Scholar]
- Radziszewska, M.; Kaźmierski, J.; Sobów, T. EEG and QEEG biomarkers as predictors of antidepressant treatment response. Psychiatr. Psychol. Klin. 2015, 15, 19–25. [Google Scholar] [CrossRef]
- Crivelli, L.; Palmer, K.; Calandri, I.; Guekht, A.; Beghi, E.; Carroll, W.; Frontera, J.; García-Azorín, D.; Westenberg, E.; Winkler, A.S.; et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. 2022, 18, 1047–1066. [Google Scholar] [CrossRef]
- Duggal, P.; Pensona, T.; Manley, H.N.; Vergara, C.; Munday, R.M.; Duchena, D.; Linton, E.A.; Zurn, B.; Keruly, J.C.; Mehta, S.H.; et al. Post-sequelae symptoms and comorbidities after COVID-19. J. Med. Virol. 2022, 94, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, M.K. Neuroimaging findings of brain MRI and CT in patients with COVID-19: A systematic review and meta-analysis. Eur. J. Radiol. 2020, 133, 109393. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Laurent, S.; Onur, O.A.; Kleineberg, N.N.; Fink, G.R.; Schweitzer, F.; Warnke, C. A systematic review of neurological symptoms and complications of COVID-19. J. Neurol. 2021, 268, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, K.J.; Kung, J.C.; Senetar, A.J.; Mendoza, D.; Bonnin, D.A.; Purtlebaugh, W.L.; Cabatbat, R.M.; Dickens, R.; Echevarria, F.D.; Kariyawasam, V.; et al. Post-COVID seizure: A new feature of “long-COVID”. eNeurologicalSci 2021, 23, 100340. [Google Scholar] [CrossRef] [PubMed]
- Flamand, M.; Perron, A.; Buron, Y.; Szurhaj, W. Pay more attention to EEG in COVID-19 pandemic. Clin. Neurophysiol. 2020, 131, 2062–2064. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Vabanesi, M.; Canu, E.; Fanelli, G.; Barbieri, A.; Bernasconi, M.P.; Lazzarin, S.; Impellizzeri, M.; Giacalone, G.; et al. Prospective eeg cortical sources and connectivity evaluation in patients with recent COVID-19 and cognitive disturbances: An eLORETA study. J. Neurol. 2021, 429, 117786. [Google Scholar] [CrossRef]
- Furlanis, G.; Stella, A.B.; Biaduzzini, F.; Bellavita, G.; Frezza, N.A.; Olivo, S.; Menichelli, A.; Lunardelli, A.; Ajčević, M.; Manganotti, P. Cognitive deficit in post-acute COVID-19: An opportunity for EEG evaluation? Neurol. Sci. 2023, 44, 1491–1498. [Google Scholar] [CrossRef]
- Gulyaev, S.A. EEG Microstate Analysis and the EEG Inverse Problem Solution as a Tool for Diagnosing Cognitive Dysfunctions in Individuals Who Have Had a Mild Form of COVID-19. Hum. Physiol. 2022, 48, 587–597. [Google Scholar] [CrossRef]
- Berkowicz, K.; Obmiński, Z.; Michnik, K.; Czaja, R. The impact of covid-19 lockdown on aerobic capacity in female soccer players. J. Kinesiol. Exerc. Sci. 2021, 31, 49–53. [Google Scholar] [CrossRef]
- Vespignani, H.; Colas, D.; Lavin, B.S.; Soufflet, C.; Maillard, L.; Pourcher, V.; Paccoud, O.; Medjebar, S.; Frouin, P. Report on Electroencephalographic Findings in Critically Ill Patients with COVID-19. Ann. Neurol. 2020, 88, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Rubega, M.; Ciringione, L.; Bertuccelli, M.; Paramento, M.; Sparacino, G.; Vianello, A.; Masiero, S.; Vallesi, A.; Formaggio, E.; Felice, A.D. High-density EEG sleep correlates of cognitive and affective impairment at 12-month follow-up after COVID-19. Clin. Neurophysiol. 2022, 140, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Roberto, K.T.; Espiritu, A.I.; Fernandez, M.L.L.; Gutierrez, J.C. Electroencephalographic findings in COVID-19 patients: A systematic review. Seizure 2020, 82, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, G.; Agosta, F.; Canu, E.; Basaia, S.; Barbieri, A.; Cardamone, R.; Bernasconi, M.P.; Castelnovo, V.; Cividini, C.; Cursi, M.; et al. Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J. Neurol. 2022, 269, 3400–3412. [Google Scholar] [CrossRef]
- Pastor, J.; Vega-Zelaya, L.; Martín Abad, E. Specific EEG Encephalopathy Pattern in SARS-CoV-2 Patients. J. Clin. Med. 2020, 9, 1545. [Google Scholar] [CrossRef]
- Nisreen, A.A.; Luke, J. Defining long COVID: Going back to the start. Med 2021, 2, 501–504. [Google Scholar] [CrossRef]
- Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; Hees, H.W.H.; Helvoort, H.; Boogaard, M.; Hoeven, H.; et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, 1089–1098. [Google Scholar] [CrossRef]
- Pihlaja, R.E.; Kauhanen, L.L.S.; Ollila, H.S.; Tuulio-Henriksson, A.S.; Koskinen, S.K.; Tiainen, M.; Salmela, V.R.; Hästbacka, J.; Hokkanen, L.S.; Tiainen, M.; et al. Associations of subjective and objective cognitive functioning after COVID-19: A six-month follow-up of ICU, ward, and home-isolated patients. Brain Behav. Immun.-Health 2023, 27, 100587. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Pedersen, J.K.; Gunnarsson, D.V.; Roikjer, T.K.; Podlekareva, D.; Hansen, H.; Dall, C.H.; Johnsen, S. Cognitive impairments among patients in a long-COVID clinic: Prevalence, pattern and relation to illness severity, work function and quality of life. J. Affect. Disord. 2023, 324, 162–169. [Google Scholar] [CrossRef]
- Sun, J.C.-Y.; Yeh, K.P.-C. The effects of attention monitoring with EEG biofeedback on university students’ attention and self-efficacy: The case of anti-phishing instructional materials. Comput. Educ. 2017, 106, 73–82. [Google Scholar] [CrossRef]
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age | 27.00 | 1.11 | 26.00 | 29.00 |
| Body Height | 183.75 | 6.60 | 176.00 | 191.00 |
| Body Mass | 87.00 | 3.91 | 83.00 | 92.00 |
| Pilot Number | Score/Result |
|---|---|
| 1 | 24 |
| 2 | 19 |
| 3 | 23 |
| 4 | 20 |
| 5 | 22 |
| 6 | 18 |
| 7 | 25 |
| 8 | 21 |
| 9 | 23 |
| 10 | 19 |
| 11 | 25 |
| 12 | 22 |
| Point | Group | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| F3 | Study group | 12 | 11.07 | 1.35 | 10.95 | 9.44 | 12.95 | 10.20 | 11.82 | p < 0.001 * |
| Control group | 8 | 7.19 | 0.25 | 7.10 | 6.99 | 7.58 | 7.01 | 7.28 | ||
| C3 | Study group | 12 | 11.72 | 0.92 | 11.71 | 10.36 | 12.96 | 11.31 | 12.13 | p < 0.001 * |
| Control group | 8 | 7.33 | 0.20 | 7.35 | 7.08 | 7.54 | 7.18 | 7.50 | ||
| P3 | Study group | 12 | 10.76 | 0.70 | 10.38 | 10.36 | 11.96 | 10.36 | 10.76 | p < 0.001 * |
| Control group | 8 | 7.26 | 0.25 | 7.23 | 6.98 | 7.59 | 7.08 | 7.40 | ||
| Fz | Study group | 12 | 13.13 | 0.46 | 12.89 | 12.71 | 13.89 | 12.89 | 13.16 | p < 0.001 * |
| Control group | 8 | 7.12 | 0.23 | 7.10 | 6.87 | 7.43 | 6.96 | 7.26 | ||
| Cz | Study group | 12 | 12.59 | 1.00 | 12.60 | 11.22 | 13.90 | 12.06 | 13.15 | p < 0.001 * |
| Control group | 8 | 7.71 | 0.22 | 7.65 | 7.51 | 8.02 | 7.53 | 7.83 | ||
| F4 | Study group | 12 | 11.05 | 1.63 | 11.48 | 8.49 | 12.68 | 10.38 | 12.15 | p < 0.001 * |
| Control group | 8 | 7.38 | 0.21 | 7.33 | 7.18 | 7.68 | 7.22 | 7.49 | ||
| C4 | Study group | 12 | 11.53 | 1.04 | 11.86 | 9.60 | 12.36 | 11.26 | 12.36 | p < 0.001 * |
| Control group | 8 | 7.38 | 0.55 | 7.55 | 6.54 | 7.89 | 7.22 | 7.71 | ||
| P4 | Study group | 12 | 10.43 | 0.37 | 10.23 | 10.23 | 11.04 | 10.23 | 10.43 | p < 0.001 * |
| Control group | 8 | 6.77 | 0.41 | 6.67 | 6.40 | 7.35 | 6.43 | 7.01 |
| Point | Group | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| F3 | Study group | 12 | 3.76 | 0.06 | 3.76 | 3.68 | 3.84 | 3.72 | 3.78 | p < 0.001 * |
| Control group | 8 | 5.38 | 0.20 | 5.46 | 5.07 | 5.54 | 5.34 | 5.50 | ||
| C3 | Study group | 12 | 3.84 | 0.08 | 3.84 | 3.75 | 3.97 | 3.78 | 3.89 | p < 0.001 * |
| Control group | 8 | 5.34 | 0.41 | 5.22 | 4.98 | 5.93 | 5.00 | 5.56 | ||
| P3 | Study group | 12 | 3.78 | 0.09 | 3.76 | 3.55 | 3.86 | 3.74 | 3.85 | p < 0.001 * |
| Control group | 8 | 5.39 | 0.35 | 5.46 | 4.90 | 5.72 | 5.19 | 5.67 | ||
| Fz | Study group | 12 | 3.72 | 0.04 | 3.70 | 3.69 | 3.79 | 3.69 | 3.76 | p < 0.001 * |
| Control group | 8 | 5.23 | 0.20 | 5.24 | 4.97 | 5.47 | 5.11 | 5.36 | ||
| Cz | Study group | 12 | 3.63 | 0.09 | 3.68 | 3.48 | 3.70 | 3.63 | 3.68 | p < 0.001 * |
| Control group | 8 | 5.43 | 0.34 | 5.54 | 4.93 | 5.71 | 5.27 | 5.70 | ||
| F4 | Study group | 12 | 3.20 | 0.15 | 3.21 | 3.04 | 3.37 | 3.07 | 3.34 | p < 0.001 * |
| Control group | 8 | 5.34 | 0.29 | 5.37 | 4.95 | 5.67 | 5.18 | 5.53 | ||
| C4 | Study group | 12 | 3.20 | 0.18 | 3.18 | 3.02 | 3.44 | 3.04 | 3.32 | p < 0.001 * |
| Control group | 8 | 5.65 | 0.22 | 5.66 | 5.37 | 5.92 | 5.53 | 5.78 | ||
| P4 | Study group | 12 | 3.16 | 0.10 | 3.17 | 3.03 | 3.32 | 3.10 | 3.23 | p < 0.001 * |
| Control group | 8 | 5.32 | 0.23 | 5.37 | 4.99 | 5.56 | 5.22 | 5.48 |
| Point | Group | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| F3 | Study group | 12 | 6.03 | 0.03 | 6.03 | 6.00 | 6.13 | 6.02 | 6.03 | p < 0.001 * |
| Control group | 8 | 4.12 | 0.20 | 4.05 | 3.96 | 4.43 | 4.00 | 4.18 | ||
| C3 | Study group | 12 | 6.80 | 0.07 | 6.79 | 6.74 | 6.95 | 6.74 | 6.84 | p < 0.001 * |
| Control group | 8 | 4.02 | 0.13 | 3.98 | 3.89 | 4.21 | 3.94 | 4.06 | ||
| P3 | Study group | 12 | 6.46 | 0.03 | 6.45 | 6.45 | 6.53 | 6.45 | 6.46 | p < 0.001 * |
| Control group | 8 | 4.58 | 0.40 | 4.56 | 4.12 | 5.09 | 4.29 | 4.86 | ||
| Fz | Study group | 12 | 5.90 | 0.06 | 5.93 | 5.75 | 5.93 | 5.91 | 5.93 | p < 0.001 * |
| Control group | 8 | 4.53 | 0.54 | 4.47 | 3.94 | 5.23 | 4.13 | 4.87 | ||
| Cz | Study group | 12 | 5.68 | 0.03 | 5.67 | 5.66 | 5.76 | 5.67 | 5.67 | p < 0.001 * |
| Control group | 8 | 4.00 | 0.24 | 3.94 | 3.78 | 4.36 | 3.84 | 4.10 | ||
| F4 | Study group | 12 | 5.39 | 0.36 | 5.43 | 4.97 | 5.73 | 5.09 | 5.73 | p < 0.001 * |
| Control group | 8 | 4.14 | 0.19 | 4.17 | 3.89 | 4.34 | 4.03 | 4.29 | ||
| C4 | Study group | 12 | 5.63 | 0.11 | 5.61 | 5.53 | 5.78 | 5.53 | 5.73 | p < 0.001 * |
| Control group | 8 | 4.70 | 0.63 | 4.64 | 3.99 | 5.51 | 4.24 | 5.11 | ||
| P4 | Study group | 12 | 5.57 | 0.26 | 5.44 | 5.38 | 5.98 | 5.38 | 5.66 | p < 0.001 * |
| Control group | 8 | 4.44 | 0.40 | 4.56 | 3.82 | 4.81 | 4.38 | 4.62 |
| Point | Group | N | Mean | SD | Median | Min | Max | Q1 | Q3 | p |
|---|---|---|---|---|---|---|---|---|---|---|
| F3 | Study group | 12 | 12.32 | 1.03 | 12.72 | 10.56 | 13.76 | 11.68 | 12.79 | p < 0.001 * |
| Control group | 8 | 5.44 | 0.30 | 5.46 | 5.07 | 6.02 | 5.34 | 5.50 | ||
| C3 | Study group | 12 | 11.30 | 0.97 | 10.89 | 9.75 | 12.97 | 10.79 | 11.79 | p < 0.001 * |
| Control group | 8 | 5.34 | 0.41 | 5.22 | 4.98 | 5.93 | 5.00 | 5.56 | ||
| P3 | Study group | 12 | 11.00 | 0.93 | 10.84 | 9.75 | 12.85 | 10.45 | 11.26 | p < 0.001 * |
| Control group | 8 | 7.64 | 0.25 | 7.69 | 7.28 | 7.93 | 7.56 | 7.77 | ||
| Fz | Study group | 12 | 11.89 | 1.02 | 11.70 | 10.69 | 13.74 | 10.87 | 12.75 | p < 0.001 * |
| Control group | 8 | 5.28 | 0.49 | 5.02 | 5.02 | 6.13 | 5.02 | 5.27 | ||
| Cz | Study group | 12 | 11.39 | 1.37 | 10.74 | 9.69 | 13.74 | 10.53 | 12.00 | p < 0.001 * |
| Control group | 8 | 5.72 | 0.70 | 5.43 | 5.43 | 7.43 | 5.43 | 5.50 | ||
| F4 | Study group | 12 | 12.20 | 0.93 | 12.16 | 10.70 | 13.74 | 11.74 | 12.92 | p < 0.001 * |
| Control group | 8 | 5.85 | 0.24 | 5.93 | 5.26 | 5.93 | 5.93 | 5.93 | ||
| C4 | Study group | 12 | 11.65 | 0.90 | 11.53 | 10.69 | 13.74 | 10.96 | 11.92 | p < 0.001 * |
| Control group | 8 | 6.06 | 0.19 | 6.13 | 5.58 | 6.13 | 6.13 | 6.13 | ||
| P4 | Study group | 12 | 12.47 | 0.77 | 12.70 | 10.69 | 13.74 | 12.20 | 12.79 | p < 0.001 * |
| Control group | 8 | 7.57 | 0.28 | 7.50 | 7.29 | 7.99 | 7.41 | 7.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopańska, M.; Rydzik, Ł.; Błajda, J.; Sarzyńska, I.; Jachymek, K.; Pałka, T.; Ambroży, T.; Szczygielski, J. The Use of Quantitative Electroencephalography (QEEG) to Assess Post-COVID-19 Concentration Disorders in Professional Pilots: An Initial Concept. Brain Sci. 2023, 13, 1264. https://doi.org/10.3390/brainsci13091264
Kopańska M, Rydzik Ł, Błajda J, Sarzyńska I, Jachymek K, Pałka T, Ambroży T, Szczygielski J. The Use of Quantitative Electroencephalography (QEEG) to Assess Post-COVID-19 Concentration Disorders in Professional Pilots: An Initial Concept. Brain Sciences. 2023; 13(9):1264. https://doi.org/10.3390/brainsci13091264
Chicago/Turabian StyleKopańska, Marta, Łukasz Rydzik, Joanna Błajda, Izabela Sarzyńska, Katarzyna Jachymek, Tomasz Pałka, Tadeusz Ambroży, and Jacek Szczygielski. 2023. "The Use of Quantitative Electroencephalography (QEEG) to Assess Post-COVID-19 Concentration Disorders in Professional Pilots: An Initial Concept" Brain Sciences 13, no. 9: 1264. https://doi.org/10.3390/brainsci13091264
APA StyleKopańska, M., Rydzik, Ł., Błajda, J., Sarzyńska, I., Jachymek, K., Pałka, T., Ambroży, T., & Szczygielski, J. (2023). The Use of Quantitative Electroencephalography (QEEG) to Assess Post-COVID-19 Concentration Disorders in Professional Pilots: An Initial Concept. Brain Sciences, 13(9), 1264. https://doi.org/10.3390/brainsci13091264











