Explanted Skull Flaps after Decompressive Hemicraniectomy Demonstrate Relevant Bone Avitality-Is Their Reimplantation Worth the Risk?
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Material Collection, Patterns of Storage, Histological and Statistical Analysis
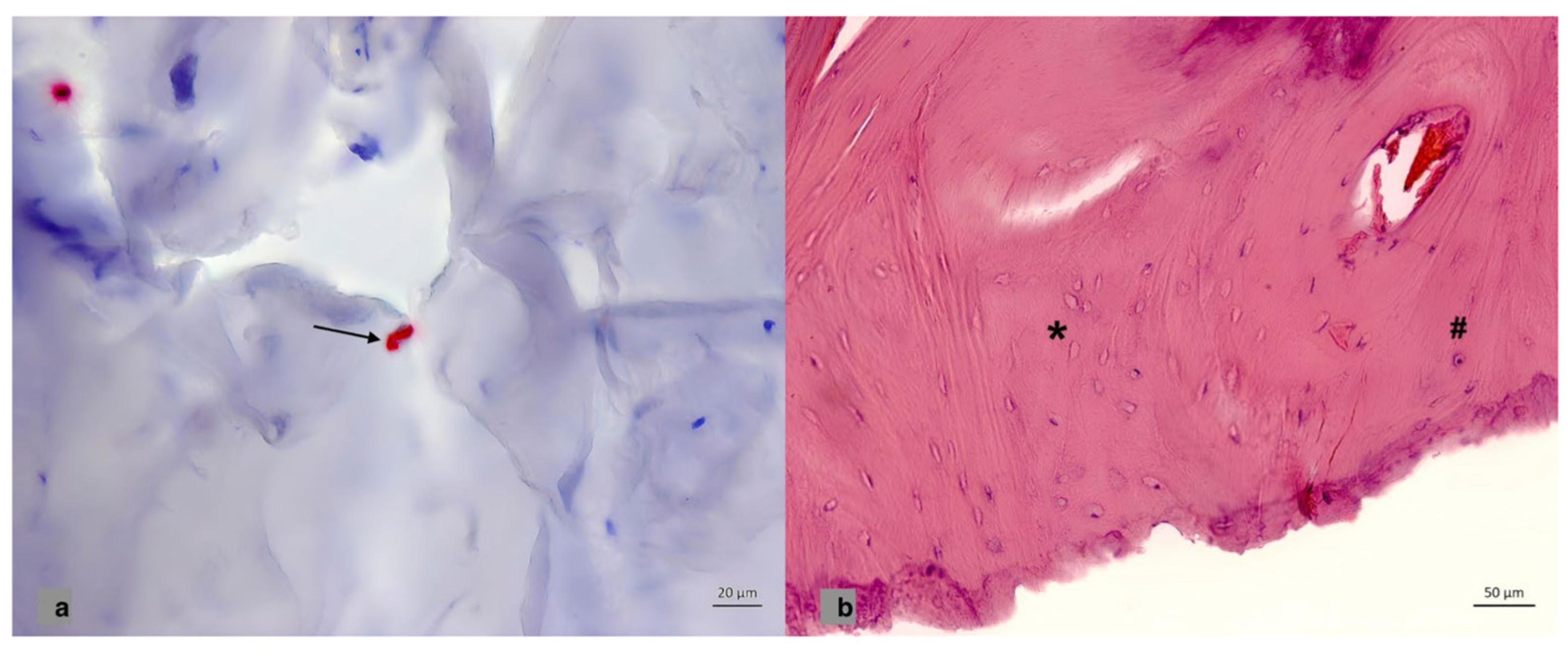
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbrevations
| CP | Cranioplasty |
| DH | Decompressive hemicraniectomies |
| BFR | Bone flap resorption |
| DRKS | Deutsches Register Klinischer Studien (German Clinical Trials Register) |
| PTH1 | Parathyroid hormone 1 receptor |
| OPG | Osteoprotegerin |
| H&E | Hematoxylin and Eosin |
| SF | Synthetic flap |
| ABF | Autologous bone flap |
| CT | Computer tomography |
| °C | Celsius |
| sTBI | Severe traumatic brain injury |
| ACM | Arteria cerebri media |
| FFPE | Formalin-fixed paraffin-embedded |
| HPF | High power field |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| TRAIL | Tumor necrosis factor related apotosis-inducing ligand |
References
- Mee, H.; Anwar, F.; Timofeev, I.; Owens, N.; Grieve, K.; Whiting, G.; Alexander, K.; Kendrick, K.; Helmy, A.; Hutchinson, P.; et al. Cranioplasty: A Multidisciplinary Approach. Front. Surg. 2022, 9, 864385. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibary, A.; Alharbi, A.; Alnefaie, N.; Oqalaa Almubarak, A.; Aloraidi, A.; Khairy, S. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects, and Complications. World Neurosurg. 2020, 139, 445–452. [Google Scholar] [CrossRef]
- Signorelli, F.; Giordano, M.; Caccavella, V.M.; Ioannoni, E.; Gelormini, C.; Caricato, A.; Olivi, A.; Montano, N. A systematic review and meta-analysis of factors involved in bone flap resorption after decompressive craniectomy. Neurosurg. Rev. 2022, 45, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Shepetovsky, D.; Mezzini, G.; Magrassi, L. Complications of cranioplasty in relationship to traumatic brain injury: A systematic review and meta-analysis. Neurosurg. Rev. 2021, 44, 3125–3142. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.; Lu, J.; Palzer, E.F.; Cramer, S.W.; Huling, J.D.; Johnson, R.A.; Zhu, P.; Jean, J.N.; Howard, M.A.; Sabal, L.T.; et al. Rates of operative intervention for infection after synthetic or autologous cranioplasty: A National Readmissions Database analysis. J. Neurosurg. 2022, 138, 514–521. [Google Scholar] [CrossRef]
- Gerstl, J.V.E.; Rendon, L.F.; Burke, S.M.; Doucette, J.; Mekary, R.A.; Smith, T.R. Complications and cosmetic outcomes of materials used in cranioplasty following decompressive craniectomy-a systematic review, pairwise meta-analysis, and network meta-analysis. Acta Neurochir. 2022, 164, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Honeybul, S.; Ho, K.M. Cranioplasty: Morbidity and failure. Br. J. Neurosurg. 2016, 30, 523–528. [Google Scholar] [CrossRef]
- Malcolm, J.G.; Mahmooth, Z.; Rindler, R.S.; Allen, J.W.; Grossberg, J.A.; Pradilla, G.; Ahmad, F.U. Autologous Cranioplasty is Associated with Increased Reoperation Rate: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, 60–68. [Google Scholar] [CrossRef]
- Lee, J.H.; Chough, C.K.; Choi, H.J.; Ko, J.K.; Cho, W.H.; Cha, S.H.; Choi, C.H.; Kim, Y.H. Bone Flap Changes after Cranioplasty Using Frozen Autologous Bone Flaps: A Three-Dimensional Volumetric Reconstruction Study. Yonsei Med. J. 2019, 60, 1067–1073. [Google Scholar] [CrossRef]
- Korhonen, T.K.; Salokorpi, N.; Niinimaki, J.; Serlo, W.; Lehenkari, P.; Tetri, S. Quantitative and qualitative analysis of bone flap resorption in patients undergoing cranioplasty after decompressive craniectomy. J. Neurosurg. 2018, 130, 312–321. [Google Scholar] [CrossRef]
- Spake, C.S.; Goli, R.; Beqiri, D.; Crozier, J.W.; Cielo, D.J.; Klinge, P.M.; Svokos, K.; Woo, A.S. Evidence of Linear Bone Flap Resorption in Patients Undergoing Autologous Cranioplasty Following Decompressive Craniectomy: A 3D Slicer Segmented Analysis of Serial Computed Tomography Images. World Neurosurg. 2022, 164, e799–e807. [Google Scholar] [CrossRef] [PubMed]
- Brommeland, T.; Rydning, P.N.; Pripp, A.H.; Helseth, E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Dunisch, P.; Walter, J.; Sakr, Y.; Kalff, R.; Ewald, C. Cranioplasty after decompressive craniectomy: Is there a rationale for an initial artificial bone-substitute implant? A single-center experience after 631 procedures. J. Neurosurg. 2016, 124, 710–715. [Google Scholar] [CrossRef]
- Agrawal, R.; Rompf, C.; Pranada, A.B.; Vollmar, P.; De Lorenzo, A.; Hoyer, A.; Gousias, K. Microbiological profile and infection potential of different cryopreserved skull flaps after decompressive hemicraniectomy. Is cryopreservation at −80 better? BMC Res. Notes 2022, 15, 167. [Google Scholar] [CrossRef]
- Melin, S.; Haase, I.; Nilsson, M.; Claesson, C.; Ostholm Balkhed, A.; Tobieson, L. Cryopreservation of autologous bone flaps following decompressive craniectomy: A new method reduced positive cultures without increase in post-cranioplasty infection rate. Brain Spine 2022, 2, 100919. [Google Scholar] [CrossRef] [PubMed]
- Schuss, P.; Vatter, H.; Oszvald, Á.; Marquardt, G.; Imöhl, L.; Seifert, V.; Güresir, E. Bone flap resorption: Risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J. Neurotrauma 2013, 30, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Dobran, M.; Nasi, D.; Polonara, G.; Paracino, R.; Mancini, F.; Della Costanza, M.; Jonis, G.; Campa, S.; Lattanzi, S.; Iacoangeli, M. Clinical and radiological risk factors of autograft cranioplasty resorption after decompressive craniectomy for traumatic brain injury. Clin. Neurol. Neurosurg. 2020, 196, 105979. [Google Scholar] [CrossRef]
- Bhaskar, I.P.; Yusheng, L.; Zheng, M.; Lee, G.Y. Autogenous skull flaps stored frozen for more than 6 months: Do they remain viable? J. Clin. Neurosci. 2011, 18, 1690–1693. [Google Scholar] [CrossRef]
- Mirabet, V.; Garcia, D.; Yague, N.; Larrea, L.R.; Arbona, C.; Botella, C. The storage of skull bone flaps for autologous cranioplasty: Literature review. Cell Tissue Bank. 2021, 22, 355–367. [Google Scholar] [CrossRef]
- Inamasu, J.; Kuramae, T.; Nakatsukasa, M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J. Trauma 2010, 68, 183–187; discussion 187. [Google Scholar] [CrossRef]
- Deutsches Register Klinischer Studien. Available online: https://drks.de/search/de/results (accessed on 19 January 2023).
- Beri, A., Jr.; Pisulkar, S.G.; Bansod, A.V.; Dahihandekar, C. Paradigm Shift in Materials for Skull Reconstruction Facilitated by Science and Technological Integration. Cureus 2022, 14, e28731. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.T.; Lohmeier, S.J.; Langdell, H.C.; Pyfer, B.J.; Komisarow, J.; Powers, D.B.; Erdmann, D. Current Concepts in Cranial Reconstruction: Review of Alloplastic Materials. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4466. [Google Scholar] [CrossRef] [PubMed]
- Akdag, U.B.; Ogut, E.; Barut, C. Intraforaminal Dural Septations of the Jugular Foramen: A Cadaveric Study. World Neurosurg. 2020, 141, e718–e727. [Google Scholar] [CrossRef] [PubMed]
- Ogut, E.; Armagan, K.; Barut, C. Reappraisal of the types of trigeminal porus and importance in surgical applications. Surg. Radiol. Anat. 2021, 43, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Rohringer, C.R.; Rohringer, T.J.; Jhas, S.; Shahideh, M. Sinking skin flap syndrome in a patient with bone resorption after cranioplasty and ventriculoperitoneal shunt placement: Illustrative case. J. Neurosurg. Case Lessons 2021, 2, CASE21359. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Yamazaki, Y.; Moriyama, K.; Sugimoto, T.; Kumazawa, K.; Baba, K.; Sone, Y.; Takeda, A. Differentiation and proliferation potencies of human bone tissue-derived mesenchymal stromal cells (hBT-MSCs) after long-term cryopreservation Comparison among cells stored for 1, 5, 10, 15, and 20 years. Regen. Ther. 2021, 18, 363–371. [Google Scholar] [CrossRef]
- Hernández-Tapia, L.G.; Fohlerová, Z.; Žídek, J.; Alvarez-Perez, M.A.; Čelko, L.; Kaiser, J.; Montufar, E.B. Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts. Materials 2020, 13, 1966. [Google Scholar] [CrossRef]
- Pegg, D.E. Principles of cryopreservation. Methods Mol. Biol. 2015, 1257, 3–19. [Google Scholar] [CrossRef]
- Chan, D.Y.C.; Mok, Y.T.; Lam, P.K.; Tong, C.S.W.; Ng, S.C.P.; Sun, T.F.D.; Poon, W.S. Cryostored autologous skull bone for cranioplasty? A study on cranial bone flaps’ viability and microbial contamination after deep-frozen storage at −80 °C. J. Clin. Neurosci. 2017, 42, 81–83. [Google Scholar] [CrossRef]
- Cho, T.G.; Kang, S.H.; Cho, Y.J.; Choi, H.J.; Jeon, J.P.; Yang, J.S. Osteoblast and Bacterial Culture from Cryopreserved Skull Flap after Craniectomy: Laboratory Study. J. Korean Neurosurg. Soc. 2017, 60, 397–403. [Google Scholar] [CrossRef]
- Gunsser, J.; Hermann, R.; Roth, A.; Lupp, A. Comprehensive assessment of tissue and serum parameters of bone metabolism in a series of orthopaedic patients. PLoS ONE 2019, 14, e0227133. [Google Scholar] [CrossRef]
- Mirabet, V.; García, D.; Roca, A.; Quiroz, A.R.; Antón, J.; Rodríguez-Cadarso, M.; Ocete, D.; Aranda, L.; Melero, A.; Guillot, A.J.; et al. Cranioplasty with Autologous Bone Flaps Cryopreserved with Dimethylsulphoxide: Does Tissue Processing Matter. World Neurosurg. 2021, 149, e582–e591. [Google Scholar] [CrossRef]
- Andrade, M.G.; Sa, C.N.; Marchionni, A.M.; dos Santos Calmon de Bittencourt, T.C.; Sadigursky, M. Effects of freezing on bone histological morphology. Cell Tissue Bank. 2008, 9, 279–287. [Google Scholar] [CrossRef]
- Shaw, J.M.; Hunter, S.A.; Gayton, J.C.; Boivin, G.P.; Prayson, M.J. Repeated freeze-thaw cycles do not alter the biomechanical properties of fibular allograft bone. Clin. Orthop. Relat. Res. 2012, 470, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Göttsche, J.; Mende, K.C.; Schram, A.; Westphal, M.; Amling, M.; Regelsberger, J.; Sauvigny, T.; Hahn, M. Cranial bone flap resorption-pathological features and their implications for clinical treatment. Neurosurg. Rev. 2021, 44, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.M.; Valdez, S.; Gomez, L.; Malicky, P.; White, F.A.; Subler, M.A.; Windle, J.J.; Bidwell, J.P.; Bruzzaniti, A.; Plotkin, L.I. High mobility group box 1 protein regulates osteoclastogenesis through direct actions on osteocytes and osteoclasts in vitro. J. Cell Biochem. 2019, 120, 16741–16749. [Google Scholar] [CrossRef]
- Plotkin, L.I. Apoptotic osteocytes and the control of targeted bone resorption. Curr. Osteoporos. Rep. 2014, 12, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef]
- Verborgt, O.; Gibson, G.J.; Schaffler, M.B. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J. Bone Miner. Res. 2000, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
| Variable | −23 °C | −80 °C | p-Value |
|---|---|---|---|
| Median, Quartiles (25th–75th Percentiles) | Median, Quartiles (25th–75th Percentiles) | ||
| PTH1R | 1.61% (0.91–4.46) | 2.34% (0.99–4.76) | p = 0.923 |
| OPG | 6.91% (3.88–12.53) | 1.32% (0.99–2.44) | p = 0.039 |
| Avital areas | 2.51% (1.19–4.36) | 0.03% (0.00–0.09) | p = 0.008 |
| Avital areas (repeated assays after 6 months) | 13.16% (6.86–16.68) | 8.34% (5.22–15.36) | p = 0.470 |
| Variable | Regression Coefficients β (95% Confidence Interval) | exp(β) (95% Confidence Interval) | p-Value |
|---|---|---|---|
| Group (−23 °C vs. −80 °C) | 2.77 (0.93; 4.60) | 15.96 (2.53; 99.48) | 0.006 |
| Time (initial time point vs. 6 months later) | −4.26 (−6.09; −2.43) | 0.01 (0.00; 0.09) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gousias, K.; Stricker, I.; Hoyer, A.; Theocharous, T.; Rompf, C.; Pranada, A.B.; Tannapfel, A.; Agrawal, R.; Tischoff, I. Explanted Skull Flaps after Decompressive Hemicraniectomy Demonstrate Relevant Bone Avitality-Is Their Reimplantation Worth the Risk? Brain Sci. 2023, 13, 1277. https://doi.org/10.3390/brainsci13091277
Gousias K, Stricker I, Hoyer A, Theocharous T, Rompf C, Pranada AB, Tannapfel A, Agrawal R, Tischoff I. Explanted Skull Flaps after Decompressive Hemicraniectomy Demonstrate Relevant Bone Avitality-Is Their Reimplantation Worth the Risk? Brain Sciences. 2023; 13(9):1277. https://doi.org/10.3390/brainsci13091277
Chicago/Turabian StyleGousias, Konstantinos, Ingo Stricker, Annika Hoyer, Theocharis Theocharous, Csilla Rompf, Arthur B. Pranada, Andrea Tannapfel, Rachit Agrawal, and Iris Tischoff. 2023. "Explanted Skull Flaps after Decompressive Hemicraniectomy Demonstrate Relevant Bone Avitality-Is Their Reimplantation Worth the Risk?" Brain Sciences 13, no. 9: 1277. https://doi.org/10.3390/brainsci13091277





